ABSTRACT
Introduction:
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare, acquired, non-malignant hematologic disease characterized by complement-mediated hemolysis (with or without hemoglobinuria), fatigue, increased susceptibility to thrombosis, and bone marrow dysfunction. The development of complement inhibitors has transformed outcomes for patients with PNH, but patients may still experience pharmacodynamic breakthrough hemolysis (BTH), which can be caused by exposure to a complement amplifying condition (CAC), such as vaccination, infection, or surgery.
Materials and methods:
A 13-member expert panel used a validated methodology (a RAND/UCLA modified Delphi panel) to develop consensus on how to classify pharmacodynamic BTH in patients with complement-inhibitor treated PNH. Physicians reviewed literature, rated the appropriateness of over 400 scenarios, and discussed the ratings at an in-person meeting.
Results:
After the meeting, the panel agreed on 77% of scenarios. Here, we present the group’s agreed-upon recommendations on how to manage BTH caused by a CAC, as well as provide a severity classification system for BTH and strategies to mitigate risk of BTH in special circumstances (e.g. vaccination, planned or unplanned surgery, and pregnancy).
Discussion:
In general, as severity of BTH increased, experts agreed more interventions to manage the BTH were appropriate. These recommendations are based on clinical experience and opinion. Without clear data from randomized trials to guide the management of BTH, expert opinion can be useful to support patient care.
Plain language summary
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare blood disorder characterized by the premature breakdown of red blood cells due to the ineffective inhibition of complement, a central part of the immune system. Patients may experience a wide range of symptoms due to PNH – from the unnoticeable or minor to severe, potentially life threatening, symptoms with multiple complications. The development of complement inhibitors has dramatically changed the treatment landscape for patients with PNH. However, many patients may still experience the destruction of red blood cells despite treatment, called breakthrough hemolysis (BTH).
This study convened a 13-member expert panel to develop consensus on how to treat BTH in patients with PNH treated with complement inhibitors. Physicians reviewed literature, rated different patient scenarios, and discussed their ratings at an in-person meeting.
This paper presents the group’s agreed-upon recommendations on how to manage BTH, as well as provides a severity classification system for BTH, and strategies to mitigate risk of BTH in special circumstances. In general, as severity of BTH increased, experts agreed more interventions to manage the BTH were appropriate. These recommendations can be useful to support patient care.
Introduction
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare, acquired, non-malignant hematologic disease characterized by complement-mediated hemolysis (with or without hemoglobinuria), fatigue, increased susceptibility to thrombosis, and bone marrow dysfunction [Citation1,Citation2]. The development of targeted complement component C5 inhibitors (e.g. eculizumab, ravulizumab) has transformed outcomes for patients with PNH [Citation3–7]. More recently, newer complement inhibitors have been developed, including the C3 inhibitor pegcetacoplan [Citation8–10] (approved by the FDA in 2021), and danicopan and iptacopan (both currently in Phase III clinical trials). The latter two act by blocking either factor D or factor B in the alternative complement pathway, respectively.
Despite available treatments, patients may experience breakthrough hemolysis (BTH), which may be identified by signs and symptoms of intravascular hemolysis, including hemoglobinuria, a marked increase in serum lactate dehydrogenase (LDH), and a sharp decrease in the hemoglobin level [Citation11–13]. Different definitions and reporting of BTH have been used in complement inhibitor trials, [Citation11,Citation13] and no agreed upon definition exists. The severity of BTH episodes may vary depending on the extent to which complement pathway inhibition is incomplete, which may further depend in part on which component is therapeutically targeted, the precise mechanism of inhibition, red blood cell (RBC) clone size, and individual patient characteristics [Citation12].
Published estimates of BTH events vary in how they are reported in clinical trials. Combined estimates include 19.9–21.5 events per 100-patient years for patients treated with eculizumab and 6.8 events per 100-patient years for patients treated with ravulizumab [Citation13]. There were no BTH events reported in one recent phase 3 clinical trial for iptacopan [Citation14] and fewer events than eculizumab and ravulizumab (3.2% versus 17.1%, respectively) in another phase 3 clinical trial [Citation15]. In the extension phase of two studies of patients with PNH receiving ravulizumab, the proportion who experienced BTH was low (5.8% and 6.2%) [Citation16] In a phase 2 trial of danicopan monotherapy, two out of ten patients experienced a BTH event [Citation17]. In a phase 3 trial comparing pegcetacoplan to eculizumab, 10% of patients treated with pegcetacoplan experienced BTH (compared to 23% on eculizumab) [Citation16,Citation17]. However, some argue that patients treated with pegcetacoplan have the potential for more severe BTH [Citation8,Citation12,Citation18,Citation19].
The etiologies of BTH can be primarily pharmacokinetic, due to low or inadequate drug levels with suboptimal complement inhibition [Citation20], or pharmacodynamic, as when infection or other inflammatory states elicit strong complement activation that (generally transiently) overcomes drug induced C5 blockade [Citation21,Citation22]. BTH while taking C3, factor D, or factor B inhibitors has not yet been fully characterized.
Given the minimal reported experience with these events, optimal management remains to be determined, as does whether there are identifiable risk factors that can predict patients at risk for severe breakthrough events [Citation23]. A few groups have recently published expert opinions on how to manage BTH [Citation24–26], but none have included detailed guidance on how to manage pharmacodynamic BTH (caused by a complement amplifying condition [CAC]) and none have defined BTH severity. We sought to develop this guidance using a standardized method of soliciting expert opinion.
The RAND/UCLA modified Delphi panel method is a formal group consensus process that systematically and quantitatively combines expert opinion and evidence by asking panelists to rate, discuss, and then re-rate items [Citation27]. Such panels have been used to develop medical society guidelines [Citation28], other practice guidelines [Citation29–33], and quality improvement interventions [Citation34]. We used this method to develop guidance on how to manage various levels of BTH severity caused by a CAC in patients with treated PNH.
Materials and methods
Our panel included 13 hematologists with an average of 23 years (range 10–45 years) of clinical experience and extensive experience treating patients with PNH (seeing an average of 40 patients with hemolytic PNH and 44 patients with PNH with bone marrow failure per year). Nine were from the United States (three from the East, four from the Midwest, two from the West) and four were from Europe (two Germany, one England, one France). We were compensated for our time by Novartis Pharmaceutical Corporation as part of their ongoing research on iptacopan for PNH. Novartis provided input on the composition of the panel but did not provide input on the methodology or results of the panel. Modified Delphi panels do not involve human subjects as defined in 45 CFR part 46, thus institutional review board approval was not required.
We reviewed a summary of the relevant literature on the management of BTH in patients with treated PNH primarily informed by 16 reviews and 18 clinical studies. A reference list of all studies included in the literature review is provided as a Supplementary Appendix. We did not formally appraise the quality of evidence.
Through individual phone interviews, we collaboratively developed a rating form for panelists to complete prior to an in-person meeting. The final form included 8 interventions physicians may perform to manage BTH (). We considered each intervention in 54 scenarios that varied by patient characteristics outlined in , including decrease in hemoglobin, symptom presentation, CAC status, and type of complement inhibitor. We also included questions about how to minimize the risk of BTH in the setting of a vaccine, planned or unplanned surgery, or trauma (i.e. CACs), as well as during pregnancy and the postpartum period.
Table 1. Potential interventions to manage BTH included in rating forms.
Table 2. Definitions of characteristics included in rating forms.
For each scenario, we first rated the appropriateness of each intervention on a 1–9 scale, where 1 = highly inappropriate (risks outweigh benefits) and 9 = highly appropriate (benefits outweigh risks), and then rated the overall severity of that BTH event, where 1 = mild, 5 = moderate, 9 = severe. All 13 members of the panel completed 458 ratings before an in-person meeting and 12 completed 404 ratings after an in-person meeting (54 questions asking about asymptomatic patients with a 2.5–4 g/dL hemoglobin decrease were excluded in the second-round because experts felt this scenario was not realistic; one panelist was unable to attend the in-person meeting and did not complete the second round ratings).
During an in-person meeting in August 2023, we were provided with a document showing our own rating, the group median, and the range of ratings. As is typical in the RAND/UCLA modified Delphi panel method, we defined disagreement as two or more ratings of 1–3 and two or more ratings of 7–9 [Citation35]. Items without disagreement were grouped into three categories based on their median (1–3, 4–6, 7–9). During the professionally moderated group discussion, we shared our rationale for our ratings and discussed definitions and categories used in the scenarios. We were not asked to reach consensus at the meeting. Instead, following this discussion, we completed the ratings a second time. These second-round ratings were analyzed in the same way as the first round. We developed statements summarizing the consensus found in the second-round ratings, circulated them among the group, and made changes for clarity. These are summarized in the results below.
Results
We agreed on 77% of the second-round ratings, compared to 50% in the first round. The statements presented in summarize our recommendations on how to manage BTH caused by a known CAC in complement-inhibitor treated PNH. In , we present our recommended severity classification system for BTH events. In (and illustrated in ), we provide our recommendations on how to manage complement inhibitor dosing. In , we present strategies to mitigate risk of BTH in special circumstances (e.g. vaccination, planned or unplanned surgery, and pregnancy).
Figure 1. Illustration of expert consensus on complement inhibitor dosing.
Note: CAC: complement amplifying condition; SOB: shortness of breath * Includes giving eculizumab if the patient is on ravulizumab. † Symptoms other than SOB, chest pain, or abdominal pain. ‡ E.g. no improvement in signs and/or symptoms of hemolysis in 24–48 h. At the time of publication, there is a lack of safety data for this recommendation, especially in cases of repeated use. § Unless the patient has symptoms other than SOB, chest pain, or abdominal pain, and the CAC is stabilizing or improving, in which case it may be considered if clinically indicated. # Unless the patient is early in their dosing interval of a terminal complement inhibitor and the CAC is stabilizing or improving, in which case it may be considered if clinically indicated.
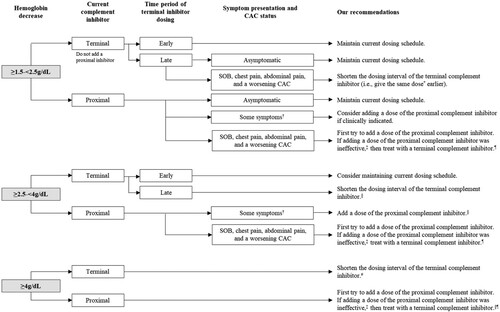
Table 3. Expert consensus on severity classification of BTH events.
Table 4. Expert consensus on complement inhibitor dosing.
Table 5. Expert consensus on strategies to mitigate risk of BTH in special circumstances.
Our intention was for these recommendations to be only relevant to patients experiencing a BTH event due to a CAC (pharmacodynamic breakthrough) and not as the result of underdosing (pharmacokinetic breakthrough), although we recognize it is not always possible to distinguish the two. They are intended to support the management of a BTH event and are only relevant during the BTH event. Changes to medication dosing should be made until signs and/or symptoms of hemolysis have resolved (i.e. patients should be switched back to their original medication and dosing after the BTH event due to a CAC).
Every clinical situation is different, with its own set of complex characteristics. In practice, many other clinical and non-clinical factors beyond those addressed below will affect how to manage BTH in PNH. For instance, to keep the guidance general, we do not consider absolute hemoglobin levels, and instead focus on the hemoglobin change, symptoms, and other factors.
Severity classification
Our definition of BTH was informed by the position paper from the Severe Aplastic Anemia Working Party of the European Group for Bone Marrow Transplantation [Citation36] and our opinion. We defined BTH as an acute drop of hemoglobin of ≥1.5 g/dL (compared to the patient’s latest assessment) in the presence of newly elevated lactate dehydrogenase (LDH) > 1.5 × upper limit of normal (ULN), known to be caused by a CAC (e.g. a recent vaccination, infection, surgery).
To broadly guide management strategies, we agreed on a severity classification of BTH events using both symptoms and hemoglobin drop (). Mild BTH is when a patient has a drop in hemoglobin of ≥1.5–<2.5 g/dL, who either remains asymptomatic, or has symptoms other than shortness of breath (SOB), chest pain, or abdominal pain, and the CAC is stabilizing or improving. Severe BTH is when a patient has a drop in hemoglobin of ≥4 g/dL; or has a drop in hemoglobin of ≥2.5–<4 g/dL and has SOB, chest pain, or abdominal pain. Moderate BTH is anything not classified as mild or severe; and includes, for example, a patient with a drop in hemoglobin of ≥2.5–<4 g/dL and has symptoms other than SOB, chest pain, or abdominal pain; or has a drop in hemoglobin of ≥1.5–<2.5 g/dL and has SOB, chest pain, or abdominal pain. Patients on complement inhibitors that result in improved PNH RBC survival and thus larger RBC clone size may have the potential for a more severe BTH event.
Complement inhibitor dosing
In and illustrated in , we provide our recommendations on how to manage complement inhibitor dosing based on drop in hemoglobin and type of complement inhibitor. There are times when we recommend shortening the dosing interval of patients on a terminal complement inhibitor. For those on ravulizumab, we understand that shortening the dosing interval by more than a couple of weeks is unlikely due to cost and insurance constraint. Further, ravulizumab is not included on most hospital formularies and, therefore, is not available to many inpatients. In these cases, adding a dose of eculizumab is a part of this recommendation.
Other interventions
Transfusions should be given per clinician decision-making and consistent with institutional guidelines. In general, in cases of severe BTH, we recommend RBC transfusion; in cases of mild BTH, we usually do not recommend RBC transfusion. In cases of moderate BTH, transfusion may be considered if clinically indicated.
We recommend ordering laboratory tests (e.g. hemoglobin, LDH, reticulocyte count, bilirubin, complement levels) with increasing frequency for more severe BTH. For example, we recommend daily laboratory tests in patients with a drop of hemoglobin of ≥2.5 g/dL and those in an inpatient setting. We also recommend educating patients on recognizing their symptoms, particularly if they switch from one type of complement inhibitor to another, and encouraging them to return to the office for more laboratory tests if they do not feel well.
Unfortunately, we did not reach consensus on whether to treat with corticosteroids or begin prophylactic anticoagulation. For patients already receiving anticoagulation, continue as clinically indicated. Additional data are needed to make a recommendation.
Strategies to mitigate risk of BTH in special circumstances
In , we present strategies to mitigate risk of BTH in special circumstances, including in the case of vaccination, elective surgery, unplanned surgery or trauma, and pregnancy.
Discussion
In the current study, 13 expert hematologists reviewed published evidence, incorporated our clinical opinion, and independently rated 404 questions on defining BTH severity, how to manage pharmacodynamic BTH events, and how to mitigate the risk of BTH. In general, as severity of BTH increased, we agreed that more interventions were appropriate. The statements are intended only as general guidance for experienced clinicians who treat patients with PNH. They are in no way intended to supersede individual physician and patient decision-making.
As there are few randomized controlled trials to inform the management of pharmacodynamic BTH, our recommendations are based on our clinical experience and opinion. However, we used a validated method (the RAND/UCLA modified Delphi panel method) to develop these statements. The method has been used extensively to guide clinical care [Citation28–34]. Further, guidelines developed using this method have content, construct, and predictive validity. Results of modified Delphi panels conducted using the same evidence base produce similar results, and patients treated according to the resulting guidelines have been shown to have improved outcomes [Citation37,Citation38].
BTH was first described in the pivotal eculizumab trial [Citation39]. A position paper from the Severe Aplastic Anemia Working Party of the European Group for Bone Marrow Transplantation suggested a working definition of BTH and defined clinical versus subclinical presentations [Citation11]. Specifically, they defined clinical breakthrough as a hemoglobin drop of ≥2 g/dL or the development of clinical signs or symptoms of hemolysis, in combination with an increase in LDH of >1.5× ULN; and sub-clinical breakthrough as an increase in LDH of >1.5× ULN with a hemoglobin drop of <2 g/dL and no signs or symptoms. This is similar to the definition we used, which was based on our opinion and discussion. While others have used a higher threshold of LDH increase (>2.0 ULN) [Citation4], we chose a slightly lower threshold to define a minimum meaningful laboratory finding. This may result in a higher estimated incidence of mild BTH, as well as potentially increased awareness of BTH. To create more specific guidance, we also defined three levels of hemoglobin drop (≥1.5-<2.5 g/dL, ≥ 2.5-<4 g/dL, ≥ 4 g/dL).
We recommend classifying a BTH event into one of three levels of severity (mild, moderate, or severe). This classification may help broadly inform interventions. For example, we generally do not recommend transfusion in the case of mild BTH, and we recommend ordering laboratory tests with increasing frequency for more severe BTH. However, this system is only intended as a guide. We did not use real patient data to inform these definitions nor validate whether cases of more severe BTH are associated with more severe outcomes.
To fill a gap in the literature, we chose to focus on only BTH caused by a CAC. To date, there is limited published guidance on this topic. One study that also relied on expert opinion recommended the following: ‘for sporadic pharmacodynamic BTH (e.g. pregnancy, infection and major surgery), do not switch therapy and treat the triggering condition’ [Citation25]. Our recommendations add to the literature by providing more detailed guidance. However, it is not always possible to identify the etiology of the BTH event and confirm it is caused by a CAC. For example, a patient may present with severe BTH without any recent dosing changes and a physician may only be able to assume it is caused by a CAC that has not been identified. In particular, it may be difficult to distinguish pharmacodynamic and pharmacokinetic BTH in patients on pegcetacoplan, which is not dosed by weight [Citation40]. Physician expertise on whether to follow our recommendations in this case would be needed.
We note that patients on complement inhibitors that result in improved PNH RBC survival and thus larger RBC clone size may potentially have more severe BTH events. Notaro and Luzzatto [Citation12] noted this and theorized that patients on pegcetacoplan may have more severe BTH for two other reasons: The half-life of pegcetacoplan is relatively short, so plasma levels may decrease below the efficacy threshold simply because of a missed dose or injection-related issues. A recent study shows that exposure to a higher and sustained dose of pegcetacoplan can minimize this risk [Citation41] Additionally, incomplete inhibition may result in different hemolytic potential. With C5 inhibition (e.g. eculizumab), if inhibition is incomplete, only one membrane attack complex (MAC) will be formed for each molecule of C5 that escapes inhibition. However, with incomplete pathway inhibition on pegcetacoplan, each molecule of C5 convertase can catalyze the cleavage of several molecules of C5 leading to the assembly of multiple MACs, thus resulting in the potential for massive BTH.
We did not reach consensus on whether to treat BTH with corticosteroids. While some panelists prefer a short course of corticosteroids [Citation42], others found it ineffective [Citation43]. American Society of Hematology guidelines note that for patients with continued symptoms of extravascular hemolysis while on eculizumab, ‘splenectomy or corticosteroids may ameliorate the hemolysis in symptomatic or transfusion-dependent patients by removing or inhibiting the function of phagocytic cells’ [Citation44]. However, these guidelines also acknowledge that ‘long-term use of corticosteroids is associated with significant toxicity.’ Additional data are needed to make a recommendation.
Our study has several limitations. First, we did not use randomized controlled trials to develop our guidance because such evidence does not exist. In particular, no systematic data exists to inform the appropriateness of adding a proximal inhibitor to patients being treated with a terminal complement inhibitor, or using corticosteroids, especially in cases of repeated use. While the RAND/UCLA Delphi panel method has been shown to be reproducible, it is more reproducible when there is a stronger evidence base [Citation45]. It is possible that other experts would have come to different conclusions. Second, our guidance is not intended for patients experiencing pharmacokinetic BTH (BTH due to inadequate dosing). Expert guidance on how to manage this type of BTH is published elsewhere [Citation24–26]. Further, we did not provide detailed guidance on interventions that should be individualized due to many patient characteristics and institutional policies, such as transfusions. Lastly, to simplify the discussion, we used broad categories of patient characteristics and excluded items that may impact physician care. For example, we grouped terminal inhibitors and proximal inhibitors respectively, and did not consider absolute hemoglobin levels. Users of this guidance should consider each patient’s therapy and hemoglobin, which may impact the severity of the BTH event and interventions to manage it.
The guidance summarized here reflects the areas of greatest agreement among a panel of hematologists using a methodologically sound process. Our hope is that they can support physicians treating patients with PNH. As new treatments are approved and more is learned about the severity and frequency of BTH on these therapies, our recommendations should be revisited.
Authorship contributions
All authors have met ICMJE authorship criteria.
Supplemental Material
Download Zip (791.9 KB)Disclosure statement
All authors completed ICMJE forms. All authors declare the following: support for the present manuscript (e.g. funding, provision of study materials, medical writing, article processing charges, etc.): Novartis.
The authors declare the additional following potential conflicts of interest:
DD: Grants or contracts from any entity: K36 Therapeutics. Consulting fees: Alexion/Astra Zeneca, Bristol Myers Squibb, Janssen, Novartis, Apellis, Sanofi, Takeda, Argenx, Legend Biotech. Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: Alexion, Sanofi. Support for attending meetings and/or travel: Novartis. Participation on a Data Safety Monitoring Board or Advisory Board: Sorrento Therapeutics.
CMD: Consulting fees: Alexion Pharmaceuticals, Apellis Pharmaceuticals, Omeros, Bristol Myers Squibb, Genentech. Consulting fees: Biocryst. Support for attending meetings and/or travel: Novartis. Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: Alexion Pharmaceuticals, Apellis Pharmaceuticals, Omeros, Bristol Myers Squibb, Genentech. Support for attending meetings and/or travel: Novartis. Participation on a Data Safety Monitoring Board or Advisory Board: Regeneron. Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid: AA-MDS Foundation Medical Board of Directors.
JLK: Consulting fees: Apellis, Alexion, GSK 2023 Myelofibrosis Community Physician Strategic Council. Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: Alexion, Apellis, Amgen, Jazz, CTI, BMS, Abbvie, DSMC: Georgetown/John Theurer Cancer Center joint DSMC. Support for attending meetings and/or travel: Novartis.
AGK: Consulting fees: Novartis, Alexion/AstraZeneca, Amgen, Roche, Pfizer, SOBI, Regeneron, Arrowhead, Silence Therapeutics. Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: SOBI, Alexion/AstraZeneca, Amgen, Roche, Novartis, Celgene/BMS, Janssen. Support for attending meetings and/or travel: Novartis, SOBI, Alexion/Astrazeneca, Amgen, Roche, Pfizer. Participation on a Data Safety Monitoring Board or Advisory Board: GERON corporation, Regeneron, Novartis.
JPM: Consulting fees: Novartis. Support for attending meetings and/or travel: Novartis.
BPM: Consulting fees: Apellis. Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: Apellis. Support for attending meetings and/or travel: Novartis.
JPP: All support for the present manuscript (e.g. funding, provision of study materials, medical writing, article processing charges, etc.): Blueprint. Consulting fees: Apellis/SOBI, Alexion, Amgen, Astra Zeneca, Blueprint Medicines, Boehringer Ingelheim, Bristol Myers Squibb, Chugai, Deciphera, Grünenthal, F Hoffmann-La Roche Ltd, MSD, Novartis, Pfizer. Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: Apellis/SOBI, Alexion, Amgen, Biologix, Blueprint Medicines, Boehringer Ingelheim, Bristol Myers Squibb, Chugai, Gilead, F Hoffmann-La Roche Ltd, MSD, Novartis, Pfizer, Swixx Biopharma. Support for attending meetings and/or travel: Novartis. Participation on a Data Safety Monitoring Board or Advisory Board: Apellis/SOBI, Alexion, Blueprint Medicines, Amgen, Gilead, F Hoffmann-La Roche Ltd, MSD, Novartis, Sanofi. Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid: DGHO.
VP: Consulting fees: Novartis. Support for attending meetings and/or travel: Novartis.
Participation on a Data Safety Monitoring Board or Advisory Board: Novartis.
AR: Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: Alexion, Amgen, Apellis, Novartis, Roche, Sanofi, Sobi. Support for attending meetings and/or travel: Novartis, Sobi, Alexion. Participation on a Data Safety Monitoring Board or Advisory Board: Alexion, Amgen, Apellis, Novartis, Roche, Sanofi, Sobi, Samsung.
JS: Grants or contracts from any entity: Alexion, CTI, BMS, Protagonist, Astra Zeneca, Apellis, AbbVie. Consulting fees: Alexion- Astra Zeneca, BMS, Apellis, CTI, GSK, Novartis, Incyte, Sanofi, Sobi, Blueprint. Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: OncLive, PER, Alexion, Apellis, Incyte, Sanofi, Novartis. Support for attending meetings and/or travel: Novartis, Alexion. Participation on a Data Safety Monitoring Board or Advisory Board: NS-BIO, Apellis. Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid: ASH executive committee, MJH board member. Stock or stock options: AbbVie, Baxter.
LT: Consulting fees: SOBI, Alexion AZ, Novartis, Roche. Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: SOBI, Alexion, Novartis. Payment for expert testimony: Roche. Support for attending meetings and/or travel: Novartis, Alexion AZ.
ICW: Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: Alexion Pharmaceuticals. Participation on a Data Safety Monitoring Board or Advisory Board: AAV Duchene. Support for attending meetings and/or travel: Novartis.
IY: Support for attending meetings and/or travel: Novartis. IY is an employee of PHAR, which was paid by Novartis to conduct the research described in the manuscript. PHAR also discloses financial relationships with the following entities outside of the submitted work: Akcea, Amgen, BioMarin Pharmaceuticals, Bristol-Myers Squibb, Celgene, Dompe, Eisai, Genentech, Gilead, Greenwich Biosciences, Ionis, Jazz, Nobelpharma, Pfizer, Recordati, Regeneron. Sanofi US Services, Takeda Pharmaceuticals U.S.A.
SNG: Support for attending meetings and/or travel: Novartis. SNG is an employee of PHAR, which was paid by Novartis to conduct the research described in the manuscript. PHAR also discloses financial relationships with the following entities outside of the submitted work: Akcea, Amgen, BioMarin Pharmaceuticals, Bristol-Myers Squibb, Celgene, Dompe, Eisai, Genentech, Gilead, Greenwich Biosciences, Ionis, Jazz, Nobelpharma, Pfizer, Recordati, Regeneron. Sanofi US Services, Takeda Pharmaceuticals U.S.A.
MSB: Support for attending meetings and/or travel: Novartis. MSB is an employee of PHAR, which was paid by Novartis to conduct the research described in the manuscript. PHAR also discloses financial relationships with the following entities outside of the submitted work: Akcea, Amgen, BioMarin Pharmaceuticals, Bristol-Myers Squibb, Celgene, Dompe, Eisai, Genentech, Gilead, Greenwich Biosciences, Ionis, Jazz, Nobelpharma, Pfizer, Recordati, Regeneron. Sanofi US Services, Takeda Pharmaceuticals U.S.A.
DOB: DOB is an employee of PHAR, which was paid by Novartis to conduct the research described in the manuscript. PHAR also discloses financial relationships with the following entities outside of the submitted work: Akcea, Amgen, BioMarin Pharmaceuticals, Bristol-Myers Squibb, Celgene, Dompe, Eisai, Genentech, Gilead, Greenwich Biosciences, Ionis, Jazz, Nobelpharma, Pfizer, Recordati, Regeneron. Sanofi US Services, Takeda Pharmaceuticals U.S.A.
DJK: Grants or contracts from any entity: Alnylam, Biocryst, Novartis, Rigel, Sanofi (Principia), Takeda (Bioverativ), UCB. Royalties or licenses: Uptodate. Consulting fees: AIRx, Alexion (Syntimmune), Alnylam, Alpine, Amgen, Argenx, BioCryst, Bristol Myers Squibb (BMS), Caremark, Cellularity, Cellphire, Chugai, CRICO, Daiichi Sankyo, Dianthus, Electra Therapeutics, Fuji, Hemopure, Hengrui, Immunovant, Incyte, Inmagenebio, Kezar, Kyowa-Kirin, Merck Sharp Dohme, Momenta, Novartis, Nuvig, Pfizer, Platelet Biogenesis, Platelet Disorder Support Association, Protagonist, Rigel, Sanofi (Bioveratif), Sanofi (Principia), Sanofi (Genzyme), Sobi (Dova), Takeda, UCB, Up-To-Date, Zafgen. Support for attending meetings and/or travel: Novartis. Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid: Platelet Disorder Support Association. Stock or stock options: Rubius.
Additional information
Funding
References
- Hill A, DeZern AE, Kinoshita T, et al. Paroxysmal nocturnal haemoglobinuria. Nat Rev Dis Primer. 2017;3(1):17028, doi:10.1038/nrdp.2017.28
- Socié G, Mary JY, De Gramont A, et al. Paroxysmal nocturnal haemoglobinuria: long-term follow-up and prognostic factors. Lancet. 1996;348(9027):573–577. doi:10.1016/S0140-6736(95)12360-1
- Socié G, Schrezenmeier H, Muus P, et al. Changing prognosis in paroxysmal nocturnal haemoglobinuria disease subcategories: an analysis of the International PNH Registry: Paroxysmal nocturnal haemoglobinuria. Intern Med J. 2016;46(9):1044–1053. doi:10.1111/imj.13160
- Brodsky RA. How I treat paroxysmal nocturnal hemoglobinuria. Blood. 2021;137(10):1304–1309. doi:10.1182/blood.2019003812
- Lee JW, Kulasekararaj AG. Ravulizumab for the treatment of paroxysmal nocturnal hemoglobinuria. Expert Opin Biol Ther. 2020;20(3):227–237. doi:10.1080/14712598.2020.1725468
- Kelly RJ, Hill A, Arnold LM, et al. Long-term treatment with eculizumab in paroxysmal nocturnal hemoglobinuria: sustained efficacy and improved survival. Blood. 2011;117(25):6786–6792. doi:10.1182/blood-2011-02-333997
- Hillmen P, Muus P, Röth A, et al. Long-term safety and efficacy of sustained eculizumab treatment in patients with paroxysmal nocturnal haemoglobinuria. Br J Haematol. 2013;162(1):62–73. doi:10.1111/bjh.12347
- Hillmen P, Szer J, Weitz I, et al. Pegcetacoplan versus eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2021;384(11):1028–1037. doi:10.1056/NEJMoa2029073
- Wong RSM. Safety and efficacy of pegcetacoplan in paroxysmal nocturnal hemoglobinuria. Ther Adv Hematol. 2022;13:1–17. doi:10.1177/20406207221114673
- Castro C, Grossi F, Weitz IC, et al. C3 inhibition with pegcetacoplan in subjects with paroxysmal nocturnal hemoglobinuria treated with eculizumab. Am J Hematol. 2020;95(11):1334–1343. doi:10.1002/ajh.25960
- Risitano AM, Marotta S, Ricci P, et al. Anti-complement treatment for paroxysmal nocturnal hemoglobinuria: time for proximal complement inhibition? A position paper from the SAAWP of the EBMT. Front Immunol. 2019;10:1157, doi:10.3389/fimmu.2019.01157
- Notaro R, Luzzatto L. Breakthrough hemolysis in PNH with proximal or terminal complement inhibition. Longo DL, ed. N Engl J Med. 2022;387(2):160–166. doi:10.1056/NEJMra2201664
- Brodsky RA, De Latour RP, Rottinghaus ST, et al. Characterization of breakthrough hemolysis events observed in the phase 3 randomized studies of ravulizumab versus eculizumab in adults with paroxysmal nocturnal hemoglobinuria. Haematologica. 2020;106(1):230–237. doi:10.3324/haematol.2019.236877
- Risitano AM, Han B, Ueda Y, et al. OS12-06 oral complement factor B inhibitor Iptacopan monotherapy improves hemoglobin to normal/near-normal levels in paroxysmal nocturnal hemoglobinuria patients naïve to complement inhibitors: phase III APPOINT-PNH trial. Presented at: 49th Annual Meeting of the European Society for Blood and Marrow Transplantation (EBMT); April 23, 2023; Paris, France. Accessed October 2, 2023. https://clin.larvol.com/abstract-detail/EBMT%202023/63248157.
- de Latour RP, Roeth A, Kulasekararaj A, et al. Oral monotherapy with Iptacopan, a Proximal Complement Inhibitor of Factor B, Has Superior Efficacy to Intravenous Terminal Complement Inhibition with Standard of Care Eculizumab or Ravulizumab and Favorable Safety in Patients with Paroxysmal Nocturnal Hemoglobinuria and Residual Anemia: results from the Randomized, Active-Comparator-Controlled, Open-Label, Multicenter, Phase III Apply-PNH Study. Blood. 2022;140(Supplement LBA-2): doi:10.1182/blood-2022-171469
- Kulasekararaj AG, Griffin M, Langemeijer S, et al. Long-term safety and efficacy of ravulizumab in patients with paroxysmal nocturnal hemoglobinuria: 2-year results from two pivotal phase 3 studies. Eur J Haematol. 2022;109(3):205–214. doi:10.1111/ejh.13783
- Risitano AM, Kulasekararaj AG, Lee JW, et al. Danicopan: an oral complement factor D inhibitor for paroxysmal nocturnal hemoglobinuria. Haematologica. 2020;106(12):3188–3197. doi:10.3324/haematol.2020.261826
- Gerber GF, Brodsky RA. Pegcetacoplan for paroxysmal nocturnal hemoglobinuria. Blood. 2022;139(23):3361–3365. doi:10.1182/blood.2021014868
- Pegcetacoplan versus Eculizumab in PNH. N Engl J Med. 2021;385(18):1723–1726. doi:10.1056/NEJMc2106424
- Hall C, Richards S, Hillmen P. Primary prophylaxis with warfarin prevents thrombosis in paroxysmal nocturnal hemoglobinuria (PNH). Blood. 2003;102(10):3587–3591. doi:10.1182/blood-2003-02-0593
- Röth A, Hock C, Konik A, et al. Chronic treatment of paroxysmal nocturnal hemoglobinuria patients with eculizumab: safety, efficacy, and unexpected laboratory phenomena. Int J Hematol. 2011;93(6):704–714. doi:10.1007/s12185-011-0867-y
- Sica M, Rondelli T, Ricci P, et al. Eculizumab treatment: stochastic occurrence of C3 binding to individual PNH erythrocytes. J Hematol OncolJ Hematol Oncol. 2017;10(1):126, doi:10.1186/s13045-017-0496-x
- Risitano AM, Peffault De Latour R, Marano L, et al. Discovering C3 targeting therapies for paroxysmal nocturnal hemoglobinuria: achievements and pitfalls. Semin Immunol. 2022;59:101618, doi:10.1016/j.smim.2022.101618
- Bodó I, Amine I, Boban A, et al. Complement Inhibition in Paroxysmal Nocturnal Hemoglobinuria (PNH): a systematic review and expert opinion from Central Europe on special patient populations. Adv Ther. 2023;40(6):2752–2772. doi:10.1007/s12325-023-02510-4
- Risitano AM, Peffault De Latour R. How we(’ll) treat paroxysmal nocturnal haemoglobinuria: diving into the future. Br J Haematol. 2022;196(2):288–303. doi:10.1111/bjh.17753
- Schubert J, Bettelheim P, Brümmendorf TH, et al. Paroxysmale Nächtliche Hämoglobinurie (PNH) [Paroxysmal Nocturnal Hemoglobinuria (PNH)]. Onkopedia. Published June 2023. Accessed August 25, 2023. https://www.onkopedia.com/de/onkopedia/guidelines/paroxysmale-naechtliche-haemoglobinurie-pnh.
- Fink A, Kosecoff J, Chassin M, et al. Consensus methods: characteristics and guidelines for use. Am J Public Health. 1984;74(9):979–983. doi:10.2105/AJPH.74.9.979
- Bickel KE, McNiff K, Buss MK, et al. Defining high-quality palliative care in oncology practice: An American society of clinical oncology/American academy of hospice and palliative medicine guidance statement. J Oncol Pract. 2016;12(9):e828–e838. doi:10.1200/JOP.2016.010686
- Strosberg JR, Fisher GA, Benson AB, et al. Systemic treatment in unresectable metastatic well-differentiated carcinoid tumors: consensus results from a modified Delphi process. Pancreas. 2013;42(3):397–404. doi:10.1097/MPA.0b013e31826d3a17
- Geer EB, Ayala A, Bonert V, et al. Follow-up intervals in patients with Cushing’s disease: recommendations from a panel of experienced pituitary clinicians. Pituitary. 2017;20(4):422–429. doi:10.1007/s11102-017-0801-2
- Herman PM, Hurwitz EL, Shekelle PG, et al. Clinical scenarios for which spinal mobilization and manipulation are considered by an expert panel to be inappropriate (and appropriate) for patients with chronic low back pain. Med Care. 2019;57(5):391–398. doi:10.1097/MLR.0000000000001108
- Cuker A, Despotovic JM, Grace RF, et al. Tapering thrombopoietin receptor agonists in primary immune thrombocytopenia: expert consensus based on the RAND/UCLA modified Delphi panel method. Res Pract Thromb Haemost. 2021;5(1):69–80. doi:10.1002/rth2.12457.
- Hemingway H, Crook AM, Dawson JR, et al. Rating the appropriateness of coronary angiography, coronary angioplasty and coronary artery bypass grafting: the ACRE study. Appropriateness of Coronary Revascularisation Study. J Public Health Med. 1999;21(4):421–429. doi:10.1093/pubmed/21.4.421
- Campbell SM. Research methods used in developing and applying quality indicators in primary care. Qual Saf Health Care. 2002;11(4):358–364. doi:10.1136/qhc.11.4.358
- Fitch K, ed. The rand/UCLA appropriateness method user’s manual. Rand: Publi; 2001.
- Debureaux PE, Kulasekararaj AG, Cacace F, et al. Categorizing hematological response to eculizumab in paroxysmal nocturnal hemoglobinuria: a multicenter real-life study. Bone Marrow Transplant. 2021;56(10):2600–2602. doi:10.1038/s41409-021-01372-0
- Hemingway H, Crook AM, Feder G, et al. Underuse of coronary revascularization procedures in patients considered appropriate candidates for revascularization. N Engl J Med. 2001;344(9):645–654. doi:10.1056/NEJM200103013440906
- Kravitz RL, Laouri M, Kahan JP, et al. Validity of criteria used for detecting underuse of coronary revascularization. JAMA. 1995;274(8):632–638. doi:10.1001/jama.1995.03530080048040
- Brodsky RA, Young NS, Antonioli E, et al. Multicenter phase 3 study of the complement inhibitor Eculizumab for the treatment of patients with paroxysmal nocturnal hemoglobinuria. Blood. 2008;111(4):1840–1847. doi:10.1182/blood-2007-06-094136
- Apellis Pharmaceuticals, Inc. EMPAVELI® (pegcetacoplan) injection, for subcutaneous use [Prescribing information]. Published online September 2023.
- Griffin M, Kelly R, Deeren D, et al. Intensive pegcetacoplan dosing in the management of acute hemolysis as part of the 307 open-label extension study. Blood. 2022;140(Supplement 1):2937–2939. doi:10.1182/blood-2022-163117
- Rosse WF. Treatment of paroxysmal nocturnal hemoglobinuria. Blood. 1982;60(1):20–23. doi:10.1182/blood.V60.1.20.20
- Röth A, Alashkar F, Herich-Terhürne D, et al. Paroxysmal nocturnal haemoglobinuria: to prednisone or not to prednisone? – A case report of a patient previously treated with steroids for 15 yrs and significant response on eculizumab. Eur J Haematol. 2015;95(2):177–180. doi:10.1111/ejh.12480
- Parker CJ. Update on the diagnosis and management of paroxysmal nocturnal hemoglobinuria. Hematology. 2016;2016(1):208–216. doi:10.1182/asheducation-2016.1.208
- Shekelle PG, Kahan JP, Bernstein SJ, et al. The reproducibility of a method to identify the overuse and underuse of medical procedures. N Engl J Med. 1998;338(26):1888–1895. doi:10.1056/NEJM199806253382607
- Carson JL, Guyatt G, Heddle NM, et al. Clinical Practice Guidelines From the AABB: Red Blood Cell Transfusion Thresholds and Storage. JAMA. 2016;316(19):2025, doi:10.1001/jama.2016.9185
