ABSTRACT
Myelodysplastic syndrome (MDS) is characterized by activated inflammatory signaling and affects prognosis. Targeting inflammatory signaling may provide a way to treat the disease. We were curious whether there were changes in A20 in peripheral blood mononuclear cells (PBMC) of MDS patients. Therefore, we conducted a study with 60 clinical samples, including 30 MDS patients and 30 healthy controls. All patients with MDS were diagnosed and classified according to the criteria of the 2016 World Health Organization. The study was performed in accordance with the guidelines of the Declaration of Helsinki. Using Quantitative Real-Time RT–PCR, we discovered that A20 mRNA expression in PBMC of the MDS group was significantly lower than that in the control group (P < 0.001). Additionally, using Luminex Liquid Suspension Chip, we observed elevated plasma levels of pro-inflammatory IL-8 and TNF-α in the MDS group compared to the healthy control group (P < 0.001). We did not find a significant correlation between A20 mRNA and clinical characteristics (age, sex, concentration of hemoglobin, neutrophils count, platelets count, percent of blasts, and WHO classification) of the patients, nor between A20 mRNA and plasma cytokines (data not shown). Our study found down-regulated of A20 and increased levels of pro-inflammatory cytokines in the peripheral blood of MDS patients, providing further evidence for the activation of inflammatory signals in MDS.
Myelodysplastic syndrome (MDS) is characterized by activated inflammatory signaling and affects prognosis [Citation1]. Targeting inflammatory signaling may provide a way to treat the disease. We read a study by Muto et al. published in Nature Immunology in 2020, which investigated the impact of inflammatory milieu on MDS hematopoietic stem and progenitor cells and found that interfering with the noncanonical NF-κB signaling pathway by the anti-inflammatory factor A20 could impede MDS progression [Citation2].
We were curious whether there were changes in A20 in peripheral blood mononuclear cells (PBMC) of MDS patients. Therefore, we conducted a study with 60 clinical samples, including 30 MDS patients and 30 healthy controls. All patients with MDS were diagnosed and classified according to the criteria of the 2016 World Health Organization [Citation3]. All 30 MDS patients had no history of other chronic diseases such as cancer, human immunodeficiency virus infection, and autoimmune diseases. In order to eliminate the influence of infection and immunosuppressive drugs on A20, all peripheral blood samples were obtained from individuals who had not experienced any infections or used immunosuppressants in the past three months. The study was performed in accordance with the guidelines of the Declaration of Helsinki. The baseline data of the patients and the experimental methods used can be found in the Appendix. Using Quantitative Real-Time RT–PCR, we discovered that A20 mRNA expression in PBMC of the MDS group was significantly lower than that in the control group (P < 0.001) (). Additionally, using Luminex Liquid Suspension Chip, we observed elevated plasma levels of pro-inflammatory IL-8 and TNF-α in the MDS group compared to the healthy control group (P < 0.001). However, there were no significant changes in pro-inflammatory IFN-γ, IL-1β, IL-6, and anti-inflammatory TGF-β1 (). We did not find a significant correlation between A20 mRNA and clinical characteristics (age, sex, concentration of hemoglobin, neutrophils count, platelets count, percent of blasts, and WHO classification) of the patients, nor between A20 mRNA and plasma cytokines (data not shown).
Figure 1. The relatively expression levels of A20 mRNA in PBMC from MDS patients (n = 27) and healthy controls (n = 27) by Quantitative Real-Time RT-PCR.
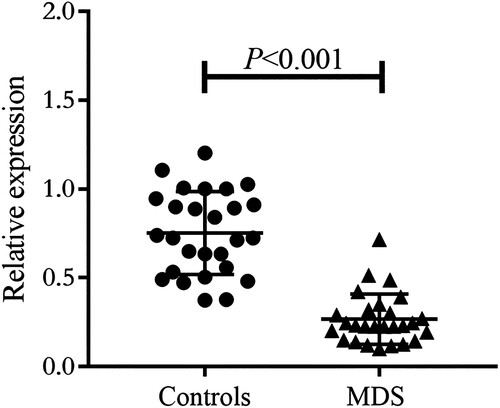
Table 1. The levels of plasma cytokines from MDS patients (n = 30) and healthy controls (n = 30) by Luminex Liquid Suspension Chip.
In 2011, Kristinsson et al. [Citation4] reported that chronic inflammation may play a role in driving MDS. Subsequently, studies have further demonstrated the activation of inflammatory signals in hematopoietic stem cells and the establishment of an inflammatory microenvironment in MDS [Citation5–7]. A20, encoded by the TNFAIP3 gene, is a potent anti-inflammatory molecule. Dysregulated expression of A20 has been associated with various inflammatory diseases, including rheumatoid arthritis [Citation8] and systemic lupus erythematosus [Citation9]. Furthermore, these autoimmune diseases have been linked to the development of MDS [Citation4]. A20 is known to exert its anti-inflammatory effects by inhibiting the NF-κB inflammatory signaling pathway as a ubiquitin editing enzyme. Previous studies have confirmed the upregulation of NF-κB in the peripheral blood and bone marrow of MDS patients [Citation7,Citation10]. Our study found down-regulated of A20 and increased levels of pro-inflammatory cytokines in the peripheral blood of MDS patients, providing further evidence for the activation of inflammatory signals in MDS.
Ethical approval
All the blood samples from the patients and healthy controls were used with informed consent and approval from the Ethics Committee of Yueyang Hospital of Integrated Traditional Chinese and Western Medicine (Approval Number: 2018-114).
Appendix ab.docx
Download MS Word (21.9 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Vegivinti CTR, Keesari PR, Veeraballi S, et al. Role of innate immunological/inflammatory pathways in myelodysplastic syndromes and AML: a narrative review. Exp Hematol Oncol. 2023;12(1):60. doi: 10.1186/s40164-023-00422-1
- Muto T, Walker CS, Choi K, et al. Adaptive response to inflammation contributes to sustained myelopoiesis and confers a competitive advantage in myelodysplastic syndrome HSCs. Nat Immunol. 2020;21(5):535–545. doi: 10.1038/s41590-020-0663-z
- Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. doi: 10.1182/blood-2016-03-643544
- Kristinsson SY, Björkholm M, Hultcrantz M, et al. Chronic immune stimulation might act as a trigger for the development of acute myeloid leukemia or myelodysplastic syndromes. J Clin Oncol. 2011;29(21):2897–2903. doi: 10.1200/JCO.2011.34.8540
- Choudhary GS, Pellagatti A, Agianian B, et al. Activation of targetable inflammatory immune signaling is seen in myelodysplastic syndromes with SF3B1 mutations. Elife. 2022;11:e78136. doi: 10.7554/eLife.78136
- Basiorka AA, McGraw KL, Eksioglu EA, et al. The NLRP3 inflammasome functions as a driver of the myelodysplastic syndrome phenotype. Blood. 2016;128(25):2960–2975. doi: 10.1182/blood-2016-07-730556
- Ping Z, Chen S, Hermans SJF, et al. Activation of NF-κB driven inflammatory programs in mesenchymal elements attenuates hematopoiesis in low-risk myelodysplastic syndromes. Leukemia. 2019;33(2):536–541. doi: 10.1038/s41375-018-0267-x
- Li D, Wang L, Fan Y, et al. Down-regulation of A20 mRNA expression in peripheral blood mononuclear cells from patients with systemic lupus erythematosus. J Clin Immunol. 2012;32(6):1287–1291. doi: 10.1007/s10875-012-9764-2
- Matmati M, Jacques P, Maelfait J, et al. A20 (TNFAIP3) deficiency in myeloid cells triggers erosive polyarthritis resembling rheumatoid arthritis. Nat Genet. 2011;43(9):908–912. doi: 10.1038/ng.874
- de Matos AG, Ribeiro Junior HL, de Paula Borges D, et al. Interleukin-8 and nuclear factor kappa B are increased and positively correlated in myelodysplastic syndrome. Med Oncol. 2017;34(10):168. doi: 10.1007/s12032-017-1023-1
Appendix
Part 1 Demographics and clinical characteristics of included individuals
Part 2 Methods
Quantitative real-time PCR
Peripheral blood mononuclear cells (PBMC) were separated by density gradient centrifugation (1.077 g/mL) from the peripheral blood anticoagulated with EDTA. Total RNA was extracted from PBMC using EZ-press RNA Purification Kit according to the instructions of the manufacturer (EZBioscience, Suzhou, China), then quantified by photometrical measurement. One microgram of RNA was reversely transcribed to cDNA using reverse transcription system kit (TAKARA, Dalian, China) for each sample. The expression of A20 mRNA was evaluated by quantitative real-time PCR in triplicate and the level of β-actin mRNA was also detected as an internal control. Real-time PCR was performed using TB Green® Premix Ex Taq™ PCR kit (TAKARA, Dalian, China) on a LightCycler480 II (Roche, Shanghai, China). The first step of PCR protocol is denaturation process performed for 5 min at 95°C, followed by 45 cycles of 20 s denaturation (95°C), 20 s annealing (60°C) and 20 s extension (72°C) as the second step. The third step of PCR protocol is 95°C for 5 s, 60°C for 1 min and 40°C for 30 s. Primers used were as follows: A20 forward: 5′-GCGTTCAGGACACAGACTTGGTAC-3′, and reverse: 5′-AGCAAAGCCCCGTTTCAACAAATTC-3′; β-actin forward: 5′-CCTGGCACCCAGCACAAT-3′, and reverse: 5′-GGGCCGGACTCGTCATAC-3′.
Luminex liquid suspension chip
Luminex liquid suspension chip detection was performed by Wayen Biotechnologies (Shanghai, China). The Bio-Plex Pro TGF-β Panel and Human High Sensitivity T Cell Magnetic Bead Panel were used in accordance with the manufacturer's instructions, the former for measuring pro-inflammatory mediators (cytokines IL-1β, IL-6, IL-8, IFN-γ, and TNF-a) and the latter for measuring anti-inflammatory cytokine (TGF-β1). In brief, 50μl of serum sample were incubated in 96-wel plates embedded with microbeads for 1 h, and then incubated with detection antibody for 30 min. Subsequently, streptavidin-PE was added into each well for 10 min, and values were read using the Luminex Bio-Plex 200 system (Luminex, Austin, TX, USA). Concentrations below or above the limit of detection were assigned as the respective limit of detection (lowest or highest standard concentration).
