ABSTRACT
Objective
This article conducts a systematic review of eltrombopag combined with immunosuppressive therapy for the treatment of aplastic anemia (AA), to demonstrate the effectiveness and safety of eltrombopag.
Methods
PubMed, Cochrane Library, Embase, OVID, Web of Science, China National Knowledge Infrastructure, and Wanfang databases were searched. Studies that met the inclusion criteria were collected, ranging from the establishment of the database to August 2023. Two reviewers performed meta-analyses using the Cochrane systematic review method and RevMan 5.3 software.
Results
This meta-analysis enrolled 5 studies with a total of 542 AA patients, including 274 in the experimental group and 268 in the control group. Meta-analyses were performed for efficacy and adverse reactions. The endpoint of effects included 6-month complete response (CR), 6-month partial response (PR), and 6-month overall response (OR). Eltrombopag combined with immunotherapy showed significant improvements in 6-month CR (OR: 2.20; 95% CI;1.54–3.12; P < 0.0001) and 6-month OR (OR = 3.66, 95% CI 2.39–5.61, P < 0.001)compared to immunosuppressive therapy for AA patients. In terms of safety, eltrombopag combined with immunosuppressive therapy showed significantly increased pigment deposition and abnormal liver function compared to immunosuppressive therapy alone.
Conclusion
Compared to immunosuppressive therapy alone, eltrombopag combined with immunosuppressive therapy showed significant improvements in 6-month CR and 6-month OR. However, it also resulted in increased pigment deposition and abnormal liver function in terms of safety.
1. Introduction
Aplastic anemia (AA) is a syndrome of bone marrow failure, characterized by peripheral blood cytopenias and decreased bone marrow hematopoietic function [Citation1]. The annual incidence of AA is approximately 2-2.3 per 1,000,000 in Europe and North America, while the incidence in Asia is 7.4 per 1,000,000 [Citation2]. In almost all population-based studies the sex ratio is close to 1:1. There seem to be two main peaks of age incidence, one among young adults (20–25 years) and a second in the elderly [Citation3]. The median age in children and adolescents is 8–9 years [Citation4]. The abnormal activation and overactivity of T lymphocytes are currently believed to be the main factors causing bone marrow dysfunction of acquired aplastic anemia. Based on Camitta criteria [Citation5],
AA is classified as very severe aplastic anemia (VSAA), severe aplastic anemia (SAA), and non-severe aplastic anemia (NSAA). NSAA can be further categorized into transfusion-dependent non-severe aplastic anemia (TD-NSAA) and non-transfusion-dependent non-severe aplastic anemia (NTD-NSAA) depending on the need for blood product transfusion. Patients diagnosed with SAA and TD-NSAA who are ≤40 years old and have HLA-matched sibling donors are recommended to undergo allogeneic hematopoietic stem cell transplantation (allo-HSCT). For patients without HLA-matched sibling donors and those above the age of 40, immune suppressive therapy (IST) [anti-thymocyte/lymphocyte globulin (ATG/ALG) + cyclosporine A (CSA)] in combination with thrombopoietin receptor agonists (TPO-RA) and/or other hematopoietic agents is recommended [Citation6]. CSA in combination with TPO-RA and/or other hematopoietic therapies can be used for NTD-NSAA [Citation6].
Eltrombopag (ELT) is an oral non-peptide thrombopoietin receptor agonist (TPO-RA) that can stimulate the TPO receptor (C-MPL) on hematopoietic stem cells and megakaryocytes, leading to megakaryocyte proliferation and increasing platelet production [Citation7]. ELT was approved to treat primary immune thrombocytopenia (ITP) by U.S. Food and Drug Administration (FDA) in 2008. ELT can also promote recovery of cells in patients with refractory severe aplastic anemia (SAA) [Citation8]. Some clinical studies have shown that the response rate of refractory SAA patients to ELT alone can reach 40-48%.
We performed this systematic review and meta-analysis to examine the beneficial and adverse effects of adding a TPO-receptor agonist to standard treatment for patients with AA.
2. Data and Methods
2.1. Object of study
Randomized controlled trials (RCTs) were included, the control group treated with immunosuppressive therapy, and the Experimental group treated with immunosuppressive therapy eltrombopag and immunosuppressive therapy.
2.2. Data sources
The search was conducted the specified search terms were used in the following databases: PubMed, Cochrane Library, Embase, OVID, Web of Science, CNKI, Wanfang Database. The English search terms were: 1. eltrombopag AND aplastic anemia 2. thrombopoietin receptor agonist AND aplastic anemia. The search was limited to the period from the establishment of the databases until August 2023. In addition, manual searches of conference materials and reference lists of included studies were performed to identify relevant literature.
2.3. Eligibility criteria
The inclusion criteria for the literature were as follows:
Studies published in peer-reviewed journals.
Studies conducted on human subjects.
Studies that focused on the treatment of aplastic anemia (AA) with eltrombopag or other thrombopoietin receptor agonists.
Studies that included patients diagnosed with aplastic anemia (AA) based on established criteria.
Studies that reported relevant outcome measures such as complete remission, partial remission, overall remission rate, and secondary outcomes like hyperpigmentation, abnormal liver function, and gastrointestinal reactions.
Studies that were published in Chinese or English databases.
The exclusion criteria for the literature were as follows:
Studies published in non-peer-reviewed sources.
Animal studies or in vitro experiments.
Studies that did not focus on the use of eltrombopag or other thrombopoietin receptor agonists for the treatment of aplastic anemia (AA).
Studies that did not report relevant outcome measures or secondary outcomes.
Studies that were published in languages other than Chinese or English.
2.4. Data extraction
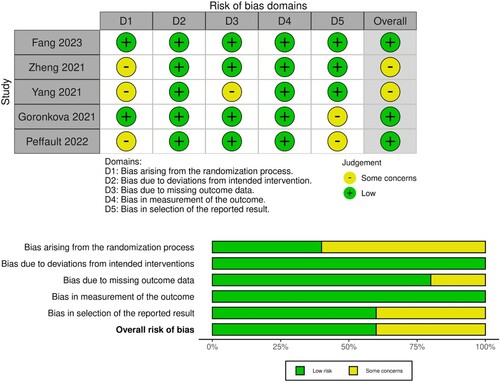
Two researchers independently extracted the relevant data from the literature included in this study, adhering to predefined inclusion and exclusion criteria. Subsequently, they cross-validated their findings and resolved any discrepancies with the assistance of a third researcher, Lei Kang. The extracted information encompassed the title, first author, publication year, number of patients, age, gender, treatment regimen, efficacy, adverse reactions, and other pertinent details. The Cochrane Handbook for Systematic Reviews of Interventions provided guidelines to assess the quality of the studies, considering factors such as random allocation, allocation concealment, participant and personnel blinding, completeness of data, selective reporting, and potential sources of bias.
2.5. Quality assessment
Meta-analysis was conducted using the RevMan 5.3 software provided by the Cochrane Collaboration. Effect sizes (ES) were utilized to assess the outcome measures, with 95% confidence intervals (CI) calculated. The heterogeneity among the included studies was evaluated using the I^2 statistic. Heterogeneity testing was performed by applying the X^2 test, and the level of heterogeneity was determined based on the I^2 value. If I^2 is less than 50%, it suggests low heterogeneity among the studies, indicating that a fixed-effects model can be employed for the meta-analysis. Conversely, if I^2 is 50% or greater, which signifies substantial heterogeneity among the studies, subgroup analysis, sensitivity analysis, or descriptive analysis can be conducted to explore the potential sources of heterogeneity. In such cases, a random-effects model or descriptive analysis may be appropriate.
2.6. Outcomes
For this meta-analysis, CR was defined as peripheral blood ANC ≥1.0 × 109/L,hemoglobin (Hb) ≥ 100 g/L, and platelet count ≥100 × 109/L; PRwas defined as transfusion independence with ANC ≥0.5 × 109/L,Hb ≥85 g/dL, and platelet count ≥30 × 109/L. Overall response(OR) was defined as PR or CR.
3. Results
3.1. Literature search results
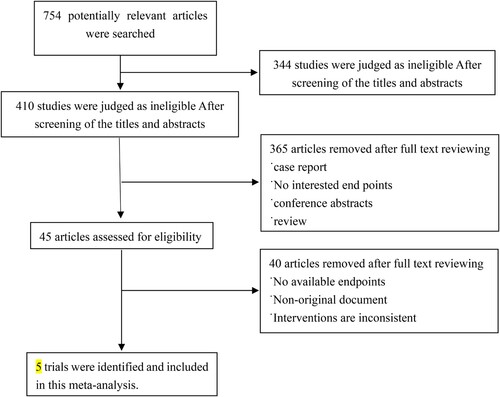
A total of 754 relevant literature articles were obtained through a preliminary screening process. Finally, five articles [Citation9–13] were included after a rigorous selection process. displays the process of literature screening.
3.2. Publication characteristics
The present meta-analysis comprised four studies involving a total of 542 patients diagnosed with AA, with 274 patients assigned to the experimental group and 268 patients assigned to the control group. presents the basic information, while displays the assessment of bias risk.
Table 1. Basic characteristics of the included studies.
3.3. Meta-analysis results
3.3.1. Complete remission rate (CR) at 6 months
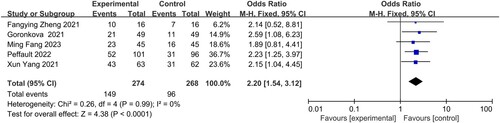
CR was reported in five studies: Fang Min, Zheng Fangying, Yang Xun, Goronkova, and Peffault. No statistical heterogeneity was observed among the five studies (I2 = 0%), which led to the utilization of a fixed-effects model for analysis. The findings revealed a significant difference in CR between the group receiving Eculizumab combined with immunosuppressive therapy and the group receiving immunosuppressive therapy alone for the treatment of AA patients (OR = 2.20, 95% CI 1.54–3.12, P < 0.0001) ().
3.3.2. Partial response rate (PR) at 6 months
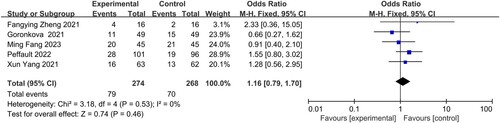
Five studies, conducted by Fang Min, Zheng Fangying, Yang Xun, Goronkova, and Peffault, respectively, reported partial relief (PR). There was no statistical heterogeneity observed among the five studies (I2 = 0%), indicating a consistent trend. Therefore, a fixed-effects model was employed to analyze the data. The results revealed that there was no significant difference in PR between the group receiving eltrombopag combined with immunosuppressive therapy and the group receiving only immunosuppressive therapy in the treatment of patients with AA(OR = 1.16, 95% CI 0.78–1.70, P = 0.46) ().
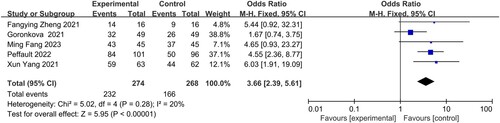
3.3.3. Overall response rate (OR) at 6 months
Five studies, led by Fang Min, Zheng Fangying, Yang Xun, Goronkova, and Peffault, respectively, reported the odds ratio (OR). A slight statistical heterogeneity (I2 = 20%) was observed among the five studies, leading to the use of a fixed-effects model for analysis. The findings showed a significant difference in the odds ratio (OR) between the group receiving eltrombopag combined with immunosuppressive therapy and the group receiving only immunosuppressive therapy for the treatment of patients with AA (OR = 3.66, 95% CI 2.39–5.61, P < 0.00001) ().
3.4. Adverse events
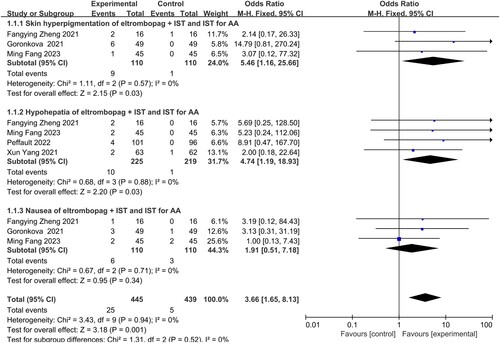
Three studies, conducted by Fang Min, Zheng Fangying, and Goronkova, respectively, reported pigmentation. No statistical heterogeneity was observed among the three studies (I2 = 0%), suggesting consistent findings. Hence, a fixed-effects model was used for the analysis. The results revealed a significant difference in the occurrence of pigmentation adverse reactions between the group receiving eltrombopag combined with immunosuppressive therapy and the group receiving only immunosuppressive therapy for the treatment of AA patients(OR = 5.46, 95% CI 1.16–25.66, P = 0.03) ().
Four studies, conducted by Fang Min, Zheng Fangying, Yang Xun, and Peffault, respectively, reported liver dysfunction. There was no statistical heterogeneity observed among the four studies (I2 = 0%), indicating consistent findings. Consequently, a fixed-effects model was used for analysis. The results revealed a significant difference in adverse reactions related to liver dysfunction between the group receiving eltrombopag combined with immunosuppressive therapy and the group receiving only immunosuppressive therapy in the treatment of patients with AA(OR = 4.74, 95% CI 1.19–18.93, P = 0.03) ().
Fang Min, Zheng Fangying, and Peffault conducted three studies, respectively, reporting gastrointestinal reactions. No statistical heterogeneity was observed among the three studies, demonstrating consistency in the findings. Hence, a fixed-effects model was utilized for the analysis. The results indicated no significant difference in the occurrence of nausea adverse reactions between the group receiving eltrombopag combined with immunosuppressive therapy and the group receiving only immunosuppressive therapy in the treatment of patients with AA(OR = 1.91, 95% CI 0.51–7.18, P = 0.34) ().
4. Discussion
Aplastic anemia (AA) is a condition characterized by the failure of bone marrow. The causes of AA can be classified into acquired and congenital disorders, with acquired disorders further categorized into idiopathic and secondary types. Idiopathic disorders constitute approximately 65% of all cases, and the prevailing belief is that abnormal activation and excessive proliferation of T lymphocytes are the primary factors contributing to the pathogenesis of primary acquired disorders. Secondary disorders result from specific known factors, including biological factors such as bacterial and viral infections, ionizing radiation like X-rays and radioactive isotopes, and chemical drug factors like chemotherapy drugs, benzene, and its derivatives. Congenital disorders, such as Fanconi anemia, congenital keratosis, congenital pure red cell aplasia, and Shwachmann-Diamond syndrome, are relatively rare.
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is recommended for patients diagnosed with severe aplastic anemia (SAA) and non-severe aplastic anemia with trilineage hematopoiesis defect (TD-NSAA) who are ≤40 years old and have an HLA-matched sibling donor. The preferred option is matched sibling donor hematopoietic stem cell transplantation (MSD-HSCT) if there are no active infections or bleeding. Immune suppression therapy (IST), consisting of anti-thymocyte/lymphocyte globulin (ATG/ALG) + cyclosporine A (CsA) along with thrombopoietin receptor agonists (TPO-RA) and/or other hematopoietic stimulating treatments, is recommended for patients over 40 years old or those without an HLA-matched sibling donor. CsA in combination with TPO-RA and/or other hematopoietic stimulating treatments can be employed for TD-NSAA.
The gene encoding the TPO receptor (C-MPL) is situated on chromosome 1p34.2 and encodes a transmembrane protein comprising 635 amino acids [Citation14]. Studies have demonstrated the expression of c-MPL in all hematopoietic stem cells and the majority of progenitor cells [Citation15,Citation16], as well as in megakaryocytes and platelets. A gene situated on chromosome 3q27.1 encodes the ligand for C-MPL, which plays a role in regulating the proliferation and differentiation of megakaryocytes [Citation17]. Thrombopoietin (TPO), a glycoprotein comprising 332 amino acids, is a humoral growth factor synthesized by the liver, kidneys, bone marrow stroma, and other tissues [Citation18]. Binding of TPO to C-MPL triggers the activation of various signaling pathways through cytoplasmic kinase, resulting in the differentiation of hematopoietic stem cells into megakaryocytes and subsequent platelet formation. The initial generation of platelet-stimulating agents involved the use of thrombopoietin receptor agonists (TPO-RAs), such as recombinant human thrombopoietin and pegylated derivatives. Initially, these agents demonstrated favorable efficacy; nevertheless, later on, many patients encountered unusual persistent thrombocytopenia, possibly associated with the generation of endogenous TPO antibodies [Citation19]. The emergence of the newer generation of TPO-RAs, including eltrombopag (ELT) and romiplostim (Rm), has bolstered platelet production. These agents have gained approval from the United States Food and Drug Administration (FDA) for the management of chronic refractory ITP and have exhibited encouraging efficacy in treating thrombocytopenia linked to myelodysplastic syndromes [Citation20,Citation21]. ELT is additionally utilized for the treatment of thrombocytopenia associated with hepatitis C infection [Citation22]. Eltrombopag (ELT) is an orally administered non-peptide thrombopoietin receptor agonist (TPO-RA) capable of stimulating the TPO receptor (C-MPL) on hematopoietic stem cells and megakaryocytes, thereby facilitating the proliferation and differentiation of megakaryocytes and augmenting platelet production.
In 2019, Hong et al. conducted a meta-analysis to investigate the effectiveness and safety of eltrombopag in the treatment of aplastic anemia (AA). Their findings indicated that eltrombopag was effective for both refractory AA and AA that did not respond to ATG treatment [Citation23]. Several studies have also reported on the safety and efficacy of combining eltrombopag with immunosuppressive therapy (IST) compared to IST alone for the treatment of AA [Citation24–27]. The meta-analysis included 5 studies involving a total of 542 AA patients, with 274 in the experimental group and 268 in the control group. It evaluated the efficacy of the treatment and examined adverse reactions, including 6-month CR, 6-month PR and 6-month OR. The combination of eltrombopag with immunotherapy showed higher CR rates at both and 6 months (OR = 2.20, 95% CI 1.54–3.12, P < 0.0001) and 6 months (OR = 3.66, 95% CI 2.39-5.61, P < 0.00001). However, there was no significant difference in 6 months PR (OR = 1.16, 95% CI 0.78–1.70, P = 0.46). The overall reported adverse effects included hemorrhage, hepatitis, liver-enzyme levels elevation, fever, colitis, and hyperbilirubinemia in severe cases. Other gastrointestinal adverse effects included nausea, abdominal pain, dyspepsia diarrhea, and vomiting. Fewer cases showed allergic reaction, muscle cramps, musculoskeletal pain, orthostatic hypotension, chest pain, insomnia, and fatigue.
Eltrombopag combined with immunotherapy had higher occurrence rates of skin pigmentation (OR = 5.46, 95% CI 1.16–25.66, P = 0.03) and liver dysfunction (OR = 4.74, 95% CI 1.19–18.93, P = 0.03).
Does eltrombopag in the treatment of aplastic anemia (AA) induce clonal evolution, thereby increasing the risk of developing myelodysplastic syndrome (MDS) and transforming into myeloid leukemia? Goronkova et al. conducted a study with a median follow-up of 2.3 years, and no instances of clonal evolution to myelodysplastic syndrome, acute myeloid leukemia, or paroxysmal nocturnal hemoglobinuria (PNH) were observed [Citation12]. In a recent study, long-term follow-up of newly diagnosed AA patients who received a combination of eltrombopag and rATG/CsA showed no evidence of clonal evolution [Citation28]. However, in the study by Groarke et al., one patient (2%) in the group receiving immunosuppressive therapy had clinically significant cytogenetic abnormalities, whereas no patients in the eltrombopag + immunosuppressive therapy group exhibited such abnormalities. In contrast, the pediatric cohort had rates of 9% and 13% respectively [Citation29]. Therefore, it is still unclear whether eltrombopag treatment promotes clonal evolution, and additional long-term studies are necessary to provide further clarity on this matter.
This study has several limitations: (1) It had a limited number of included studies, with a total of five studies being incorporated. These comprised two English articles and three Chinese articles, all of which were randomized controlled trials. (2) Insufficient studies were available for certain outcome measures, including the 3-month complete response (CR), 3-month partial response (PR), 3-month overall response (OR), and the occurrence of adverse events. This limitation could potentially impact the results obtained from the meta-analysis. (3) Baseline information among the included studies exhibited inconsistencies. Goronkova's study focused on children, while the studies by Fang Min, Zheng Fangying, Yang Xun, and Peffault focused on adults. Furthermore, treatment regimens differed, with Goronkova, Peffault, and Fang Min employing ATG + CsA + eltrombopag, while Zheng Fangying and Yang Xun utilized CsA + eltrombopag. (4) Some of the included studies did not directly report the necessary outcome measures, such as 3-month complete response (CR), 3-month partial response (PR), 6-month CR, and 6-month PR in Goronkova's study. The extraction of outcome measures from survival curves introduces a potential for error, which could influence the results of this study. (5) The study does not include an analysis of long-term survival indicators.
5. Conclusion
Compared to immunosuppressive therapy alone, eltrombopag combined with immunosuppressive therapy showed significant improvements in 6-month CR and 6-month OR. However, it also resulted in increased pigment deposition and abnormal liver function in terms of safety.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data for this study is available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Wan ZQ, Chen M, Han B. Avatrombopag, a promising novel thrombopoietin receptor agonist for refractory/relapsed/intolerant non-severe aplastic anemia: a phase 2 Single-arm Clinical Trial. Ann Med. 2023;55(1):2224044.
- Young NS, Kaufman DW. The epidemiology of acquired aplastic anemia. Haematologica. 2008;93(4):489–492.
- Furlong E, Carter T. Aplastic anaemia: current concepts in diagnosis and management. J Paediatr Child Health. 2020;56(7):1023–1028.
- Saracco P, Quarello P, Iori AP, et al. Cyclosporin A response and dependence in children with acquired aplastic anaemia: a multicentre retrospective study with long-term observation follow-up. Br J Haematol. 2008;140:197–205. doi: 10.1111/j.1365-2141.2007.06903.x
- Camitta BM, Rappeport JM, Parkman R, et al. Selection of patients for bone marrow transplantation in severe aplastic anemia. Blood. 1975;45(3):355–363. doi: 10.1182/blood.V45.3.355.355
- Red Blood Cell Diseases (Anemia) Group, Hematology Society, Chinese Medical Association. Chinese guidelines for diagnosis and treatment of aplastic anemia (2022 edition). Chin J Hematol. 2022;43(11):881–888.
- Erickson-Miller CL, Delorme E, Tian SS, et al. Preclinical activity of eltrombopag (SB-497115), an oral, nonpeptide thrombopoietin receptor agonist. Stem Cells. 2009;27:424–430. doi: 10.1634/stemcells.2008-0366
- Liu X, Liu S, Feng Q, et al. Thrombopoietin receptor agonists shift the balance of Fcγ receptors toward inhibitory receptor IIb on monocytes in ITP. Blood. 2016;128(6):852–861. doi: 10.1182/blood-2016-01-690727
- Fang M. Effect of Itopopal combined with enhanced immunosuppression in the treatment of patients with aplastic anemia. Chinese Minkang Medicine 2023;35(03):28–30+34.
- Zheng FY, Zhang XL, Huang HY. Observation of curative effect of Itripopa combined with cyclosporine in the treatment of aplastic anemia. Capital Food and Medicine 2021;12:87–89.
- Yang Xun, Yang Xu, Xu CI. Effects of Itripopar combined with cyclosporine A on serum thrombopoietin, granulocyte colony-stimulating factor and granulocyte macrophage colony-stimulating factor in patients with aplastic anemia. World Clinical Drug. 2021;42(12):1108–1112.
- Goronkova O, Novichkova G, Salimova T, et al. Efficacy of combined immunosuppression with or without eltrombopag in children with newly diagnosed aplastic anemia. Blood Advances. 2023;7(6):953–962.
- Peffault de Latour R, Kulasekararaj A, Iacobelli S, et al. Eltrombopag added to immunosuppression in severe aplastic anemia. N Engl J Med. 2022;386(1):11–23.
- Mahat U, Rotz SJ, Hanna R. Use of thrombopoietin receptor agonists in prolonged thrombocytopenia after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2020;26(3):e65–e73.
- Kimura S, Roberts AW, Metcalf D, et al. Hematopoietic stem cell deficiencies in mice lacking c-Mpl, the receptor for thrombopoietin. Proc Natl Acad Sci USA. 1998;95:1195–1200. doi: 10.1073/pnas.95.3.1195
- Huang H, Cantor AB. Common features of megakaryocytes and hematopoietic stem cells: what’s the connection? J Cell Biochem. 2009;107:857–864. doi: 10.1002/jcb.22184
- Gurney AL, Kuang WJ, Xie MH, et al. Genomic structure, chromosomal localization, and conserved alternative splice forms of thrombopoietin. Blood. 1995;85(4):981–988. doi: 10.1182/blood.V85.4.981.bloodjournal854981
- Kaushansky K. Thrombopoietin: a tool for understanding thrombopoiesis. J Thromb Haemost. 2003;1(7):1587–1592. doi: 10.1046/j.1538-7836.2003.00273.x
- Liesveld JL, Phillips GL, Becker M, et al. A phase 1 trial of eltrombopag in patients undergoing stem cell transplantation after total body irradiation. Biol Blood Marrow Transplant. 2013;19(12):1745–1752.
- Mittelman M, Platzbecker U, Afanasyev B, et al. Eltrombopag for advanced myelodysplastic syndromes or acute myeloid leukaemia and severe thrombocytopenia (ASPIRE): a randomised, placebo-controlled, phase 2 trial. The Lancet Haematology. 2018;5(1):e34–e43. doi: 10.1016/S2352-3026(17)30228-4
- Beck JC, Burke MJ, Tolar J. Response of refractory immune thrombocytopenia after bone marrow transplantation to romiplostim. Pediatr Blood Cancer 2010;54(3):490–491. doi: 10.1002/pbc.22332
- McHutchison JG, Dusheiko G, Shiffman ML, et al. Eltrombopag for thrombocytopenia in patients with cirrhosis associated with hepatitis C. N Engl J Med. 2007;357(22):2227–2236. doi: 10.1056/NEJMoa073255
- Hong Y, Li X, Wan B, et al. Efficacy and safety of eltrombopag for aplastic anemia: a systematic review and meta-analysis. Clin Drug Investig. 2019;39(2):141–156. doi: 10.1007/s40261-018-0725-2
- Jie M, Fu L, Li S, et al. Efficacy and safety of eltrombopag in the first-line therapy of severe aplastic anemia in children. Pediatr Hematol Oncol. 2021;38(7):647–657. doi: 10.1080/08880018.2021.1900475
- Patel BA, Groarke EM, Lotter J, et al. Long-term outcomes in patients with severe aplastic anemia treated with immunosuppression and eltrombopag: a phase 2 study. Blood. 2022;139(1):34–43. doi: 10.1182/blood.2021012130
- Fang M, Song H, Zhang J, et al. Efficacy and safety of immunosuppressive therapy with or without eltrombopag in pediatric patients with acquired aplastic anemia: a Chinese retrospective study. Pediatr Hematol Oncol. 2021;38(7):633–646. doi: 10.1080/08880018.2021.1895924
- Lesmana H, Jacobs T, Boals M, et al. Eltrombopag in children with severe aplastic anemia. Pediatr Blood Cancer. 2021;68(8):e29066. doi: 10.1002/pbc.29066
- Imada K, Obara N, Iida H, et al. Eltrombopag in combination with rabbit anti-thymocyte globulin/cyclosporine A in immunosuppressive therapy-naïve patients with aplastic anemia in Japan. Intern Med. 2021;60(8):1159–1168.
- Groarke Emma M, Patel Bhavisha A, Gutierrez-Rodrigues F, et al. Eltrombopag added to immunosuppression for children with treatment-naïve severe aplastic anaemia. Br J Haematol. 2021;192(3):605–614.