ABSTRACT
Myelodysplastic syndromes (MDS) patients with DEAD-box helicase 41 (DDX41) mutations have been reported to be treated effectively with lenalidomide; however, there are no randomized studies to prove it. Venetoclax and azacitidine are safe and effective in high-risk MDS/AML. In this study, we evaluated the efficacy of venetoclax and azacitidine combination therapy in eight consecutive MDS patients with DDX41 mutations at our centre from March 2021 to November 2023. We retrospectively analyzed the genetic features and clinical characteristics of these patients. Our findings suggest that MDS patients with DDX41 mutation may benefit from the therapy, for six subjects received this regimen as initial therapy and five of the six subjects achieved complete remission.
Germline mutations in the DEAD-box helicase 41 (DDX41) promote the onset of myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML) [Citation1]. Characteristics of DDX41-associated myeloid neoplasms include male predominance, cytopenias, additional somatic DDX41 mutations and favorable prognosis [Citation2–5]. Until now, there is no standard therapy for DDX41-mutated (mDDX41) MDS patients. Lenalidomide (LEN) has been previously reported to be effective in the treatment of mDDX41 MDS patients with normal karyotypes [Citation1,Citation6–8], Nyquist et al. found that mDDX41 MDS patients show 100% response rate to low-dose melpanlan [Citation9]. But there are no randomized studies with large sample sizes to prove those findings, thus further explorations are required.
Venetoclax and azacitidine are safe and effective in high-risk MDS/AML [Citation3,Citation10,Citation11]. The current study, we aimed to retrospectively evaluate the efficacy of venetoclax and azacitidine combination therapy in eight consecutive MDS patients with DDX41 mutations at our center.
This was a single-center retrospective cohort study. We identified 14 MDS with excess blasts-1 (MDS-EB-1) patients and 38 MDS-EB-2 patients received venetoclax and azacitidine at our center between March 2021 and November 2023. Patients with DDX41 mutations were included in this study. Diagnosis was based on the 2016 World Health Organization (WHO) criteria [Citation12]. Prognosis risk was stratified according to the Revised International Prognostic Scoring System (IPSS-R) [Citation13] and the molecular IPSS (IPSS-M) [Citation14]. Response was evaluated according to the 2006 IWG MDS criteria [Citation15]. Subjects gave written informed consent in compliance with precepts of the Declaration of Helsinki.
In eight patients, five were male. Median age was 61 years (range, 38–73 years; ). One subject had MDS-EB-1 and 7, MDS-EB-2. Three subjects were IPSS-R intermediate-risk, 3, high-risk and 2, very high-risk. The three subjects in the IPSS-R intermediate risk category were up-staged into higher-risk categories (moderately high/high) in the IPSS-M. IPSS-M risk categories were moderately high-, 1, high-, 3 and very high-risk, 4. Median bone marrow blast percentage was 11.5% (range, 8.0–18.0%). Median hemoglobin concentration was 85 g/L (range, 54–133 g/L). Seven subjects had normal cytogenetics and 1, a complex karyotype. Two subjects were previously treated.
Table 1. Subjects’ co-variates.
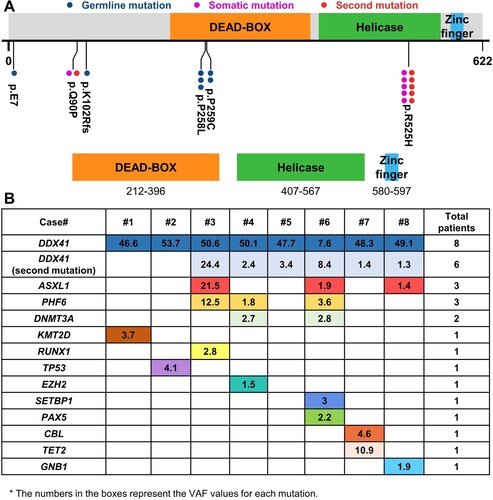
The most common mutation was mis-sense in exon8. Median DDX41 variant allele frequency (VAF) was 48.7% (range, 8.4–53.7%). The most common variant was p.R525H (5, 63%) and the second, p.P258L (3, 38%; A). Two subjects had only 1 DDX41 mutation and 6, 2 DDX41 mutations. DDX41 germline mutations were in 7. One subject had two somatic mutations with VAF values of 8% one of which was an intronic c.1400-1G > T mutation. 12 other mutations were detected and the most common of which were ASXL1(n = 3) and PHF6 (n = 3; B).
Figure 1. Characteristics in mDDX41 myelodysplastic syndromes. (A) Detection and positioning of DDX41 variants on the DDX41 protein, including its functional domains, with representation of both germline and somatic statuses. (B) Patterns of the co-mutations identified in the cases and respective VAF values.
All patients received at least one cycle of venetoclax and azacitidine. Azacitidine was administered at a dose of 75 mg/m2 for 1–7 consecutive days, and venetoclax 400 mg daily (or 100 mg/day if combined with azole antifungal agents) on days 1–14. Median number of cycles of venetoclax and azacitidine was 3 (range, 1–8). Median number of cycles to achieve complete remission (CR) was 1.5 (range, 1–3). Six subjects received this regimen as initial therapy and five of the six subjects achieved complete remission. Other two subjects were previously treated before starting venetoclax/azacitidine combination therapy. One subject received five cycles of decitabine and then one cycle venetoclax/azacitidine, transformed to AML after failure of these treatments. Another subject initially received three cycles of azacitidine and later venetoclax was added, after three cycles venetoclax/azacitidine achieved hematological improvement in neutrophil and erythroid. Median follow-up is 18 months (range, 2–63 months). Median survival is 55 months (95% confidence interval, 42–67 months).
Currently, there are many studies on the treatment of mDDX41 patients. Single or small series subjects have been reported to have unique responsiveness to LEN [Citation1,Citation6,Citation7]. Low-dose melphalan showed a 100% response rate in six mDDX41 MDS-EB patients [Citation9]. A systematic review by Wan et al. found a 70% overall response rate (ORR) to hypomethylating agents(HMAs) for DDX41-mutated MDS patients [Citation16]. An Australian group has found that favorable results can be achieved by treating DDX41-mutated MDS with azacitidine alone or in combination with lenalidomide [Citation17]. Nanaa et al. reported the real-world experience of treating three mDDX41 MDS patients with HMA+VEN, all of them achieved CR [Citation11]. However, there is still no consensus on how mDDX41 patients should be treated, further research is still required.
We investigated the genetic features and clinical characteristics of mDDX41 MDS patients in our cohort, they were predominantly male, with only one case of complex karyotype, the most common somatic mutation was p.R525H, and were predominantly high-risk MDS patients, which was consistent with previous studies about DDX41. More importantly, we explored possible therapy for MDS patients with DDX41 mutations. Five of the eight mDDX41 MDS patients achieved CR after receiving venetoclax and azacitidine, and the ORR was 75%. Especially in six subjects who received this regimen as initial therapy, the ORR was 83.3%. According to Ahmad Nanaa et al. [Citation11], AML/MDS patients showing an isolated DDX41 molecular signature are more likely to benefit from the current hypomethylating agent regimen combined with VEN. However, most of our MDS patients exhibit double mutations in DDX41, suggesting that patients with double mutations might similarly have better outcomes.
In conclusion, our results indicate that for MDS patients with DDX41 mutation, the venetoclax/azacytidine combination may offer an effective treatment alternative. However, there are some limitations of our study, such as, this is a retrospective study from our single center, and the small sample size makes this study unable to provide long-term outcomes and draw definite conclusions, which need to be confirmed by prospective clinical trials with expanded sample size.
Author contributions
Zhijian Xiao designed the study. Xin Wang acquired the data, analyzed, and interpreted the data, performed statistical analysis; Tiejun Qin, Zefeng Xu, Shiqiang Qu, Bing Li, Lijuan Pan, Qingyan Gao, Meng Jiao participated in clinical information collection. Xin Wang, Robert Peter Gale, and Zhijian Xiao drafted the typescript. All authors approved the typescript, accept responsibility for the content and agreed to submit for publication.
Ethics approval
Ethical approval was approved by the Ethical Committee on Medical Research at Institute of Hematology and Blood Disease Hospital.
Acknowledgement
RPG acknowledges support from the UK National Institute of Health Research (NIHR) Biomedical Research Centre and the Ministry of Science and Technology of China (84000-51200002).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Available from the corresponding authour upon reasonable request and compliant with the laws of China.
Additional information
Funding
References
- Polprasert C, Schulze I, Sekeres MA, et al. Inherited and somatic defects in DDX41 in myeloid neoplasms. Cancer Cell. 2015;27(5):658–670. doi:10.1016/j.ccell.2015.03.017
- Sebert M, Passet M, Raimbault A, et al. Germline DDX41 mutations define a significant entity within adult MDS/AML patients. Blood. 2019;134(17):1441–1444. doi:10.1182/blood.2019000909
- Al-Kali A, Nanaa A, Viswanatha D, et al. Observation and treatment in DDX41-mutated acute myeloid leukemia and myelodysplastic syndrome. Blood Cancer Journal. 2023;13(1):49. doi:10.1038/s41408-023-00818-6
- Gangat N, Karrar O, Iftikhar M, et al. Venetoclax and hypomethylating agent combination therapy in newly diagnosed acute myeloid leukemia: genotype signatures for response and survival among 301 consecutive patients. Am J Hematol. 2024;99(2):193–202. doi:10.1002/ajh.27138
- Gangat N, Komrokji RS, Tefferi A. Venetoclax and hypomethylating agent therapy in myelodysplastic syndromes: Big picture perspective. Am J Hematol. 2023;98(2):225–228. doi:10.1002/ajh.26781
- Dalle I A, Kantarjian H, Bannon SA, et al. Successful lenalidomide treatment in high risk myelodysplastic syndrome with germline DDX41 mutation. Am J Hematol. 2020;95(2):227–229. doi:10.1002/ajh.25610
- Negoro E, Radivoyevitch T, Polprasert C, et al. Molecular predictors of response in patients with myeloid neoplasms treated with lenalidomide. Leukemia. 2016;30(12):2405–2409. doi:10.1038/leu.2016.228
- Qu S, Li B, Qin T, et al. Molecular and clinical features of myeloid neoplasms with somatic DDX41 mutations. Br J Haematol. 2021;192(6):1006–1010. doi:10.1111/bjh.16668
- Nyquist OE, Dalgaard J, Spetalen S, et al. Pathogenic DDX41 variants, possible response predictors to low-dose melphalan in hypo- and normocellular MDS and AML. Br J Haematol. 2024;204(2):724–729. doi:10.1111/bjh.19226
- Bazinet A, Darbaniyan F, Jabbour E, et al. Azacitidine plus venetoclax in patients with high-risk myelodysplastic syndromes or chronic myelomonocytic leukaemia: phase 1 results of a single-centre, dose-escalation, dose-expansion, phase 1-2 study. Lancet Haematol. 2022;9(10):e756–e765. doi:10.1016/S2352-3026(22)00216-2
- Nanaa A, He R, Foran JM, et al. Venetoclax plus hypomethylating agents in DDX41-mutated acute myeloid leukaemia and myelodysplastic syndrome: Mayo Clinic series on 12 patients. Br J Haematol. 2024;204(1):171–176. doi:10.1111/bjh.19105
- Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. doi:10.1182/blood-2016-03-643544
- Greenberg PL, Tuechler H, Schanz J, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120(12):2454–2465. doi:10.1182/blood-2012-03-420489
- Bernard E, Tuechler H, Greenberg PL, et al. Molecular international prognostic scoring system for myelodysplastic syndromes. NEJM Evidence. 2022;1(7):1–14. doi:10.1056/EVIDoa2200008
- Cheson BD, Greenberg PL, Bennett JM, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108(2):419–425. doi:10.1182/blood-2005-10-4149
- Wan Z, Han B. Clinical features of DDX41 mutation-related diseases: a systematic review with individual patient data. Ther Adv Hematol. 2021;12:20406207211032433. doi:10.1177/20406207211032433
- Tiong IS, Stevenson WS, Wall M, et al. Favorable outcomes of DDX41-mutated myelodysplastic syndrome and low blast count acute myeloid leukemia treated with azacitidine +/- lenalidomide. EJHaem. 2023;4(4):1212–1215. doi:10.1002/jha2.767
