ABSTRACT
Objective
This study aims to investigate the efficacy and safety of hyperbaric oxygen therapy (HBOT) in the treatment of late-onset hemorrhagic cystitis after allogeneic hematopoietic stem cell transplantation.
Methods
This retrospective analysis included 16 patients with late-onset hemorrhagic cystitis after allogeneic hematopoietic stem cell transplantation between 2016 and 2022. Among them, 8 patients received HBOT in addition to conventional treatment, while the other 8 received only conventional treatment. The clinical efficacy and safety of HBOT were evaluated by comparing the Numeric Rating Scale pain scores and clinical grades of hematuria before and after treatment, reflecting the patients’ urinary pain and hematuria status.
Results
The patients were divided into two groups based on whether they received HBOT. The group that received HBOT (n = 8) had a shorter duration of illness compared to the non-HBOT group (n = 8) (p < 0.05). The time for the NRS to decrease to below 2 was also shorter in the HBOT group. Furthermore, the patients who received HBOT did not experience any significant adverse reactions.
Conclusion
The combination of conventional treatment and hyperbaric oxygen therapy (HBOT) has been shown to improve symptoms such as urinary pain, frequency, urgency, and hematuria in patients with late-onset hemorrhagic cystitis after transplantation. This approach has been proven to be safe and effective.
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is an effective, and in some cases, the only curative treatment for hematological diseases, particularly certain malignancies. However, it is often accompanied by various complications. Hemorrhagic cystitis (HC) is a common complication characterized by varying degrees of hematuria and bladder irritative symptoms. In severe cases, it can lead to urinary obstruction and even acute renal failure [Citation1]. Based on the timing of occurrence, HC can be classified into early-onset HC and late-onset HC (LOHC). The former occurs during the pre-transplant conditioning period and within 72 hours after conditioning, with a transient and self-limiting duration. The use of prophylactic medications has led to a lower incidence of early-onset HC. On the other hand, late-onset HC occurs after 72 hours following the completion of conditioning and may be associated with acute graft-versus-host disease (aGVHD) and various viral infections such as BKV, CMV, and adenovirus [Citation1–4]. HC typically has a self-limiting nature, and after resolution, there are usually no other complications. However, severe cases of HC can persist and not improve due to worsening GVHD or opportunistic infections, which can significantly impact the patient’s quality of life. Currently, the main treatment strategies for HC include hydration, alkalization, fluid replacement, antiviral therapy, diuresis, and bladder irrigation. However, these measures have limitations, particularly in improving urinary irritative symptoms such as frequency, urgency, and pain. Therefore, it is imperative to explore new treatment approaches for LOHC.
Hyperbaric oxygen therapy (HBOT) is a primary or alternative treatment option used for the management of radiation-induced HC. Additionally, research has shown that HBOT can be effective in treating HC patients following allo-HSCT. HBOT has been found to be safe, with minimal adverse reactions, and does not require any invasive procedures. Furthermore, studies have reported a response rate as high as 94% in HC patients who underwent allo-HSCT and received HBOT [Citation5]. However, there is a paucity of literature regarding the improvement of urinary irritative symptoms and hematuria in late-onset HC following the combination of conventional treatment and HBOT. Further research is needed to evaluate the effectiveness of combining HBOT with conventional therapies for managing late-onset HC and its associated urinary symptoms. In this study, we conducted a retrospective analysis of 16 patients who developed HC after undergoing HSCT at our hospital between 2016 and 2022. Among these patients, 8 received a combination of conventional treatment and HBOT. The aim of this analysis was to investigate the efficacy and safety of combining HBOT with conventional treatment for late-onset HC following transplantation.
Patients & methods
Patients
Among the 16 patients with late-onset HC, 8 received only conventional treatment (HBO (−)), while the other 8 patients received a combination of conventional treatment and HBOT (HBO (+)). All patients had achieved complete remission (CR) of their underlying diseases during the onset and treatment of late-onset HC. Prior to HSCT, all patients underwent modified busulfan (BU) + cyclophosphamide (CY) + cytarabine (AraC) + simustine or total body irradiation (TBI) + fludarabine (FLU) + CY + AraC as conditioning regimens. Additionally, preventive measures such as anti-thymocyte globulin (ATG), methotrexate (MTX), cyclosporine (CsA), and mycophenolate mofetil (MMF) were administered based on the patients’ primary diseases and individual conditions to prevent graft-versus-host disease (GVHD). The specific clinical characteristics, including the primary diseases and sources of allogeneic HSCT, are detailed in . All patients involved in the study provided informed consent in accordance with the ethical protocol of the Ethics Committee of Qingdao Central Hospital.
Table 1. General clinical data of HC patients.
Treatment
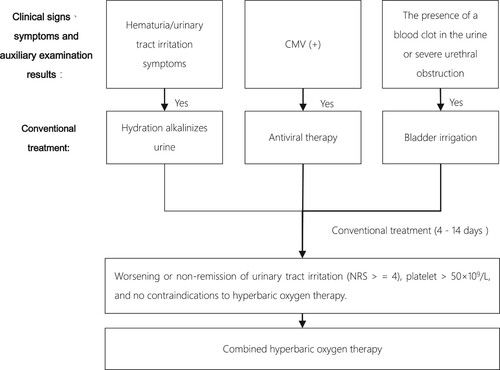
Conventional treatment methods for late-onset HC include hydration, alkalization, antiviral therapy for virus-positive patients, antispasmodic and diuretic treatment, bladder irrigation for gross hematuria or clot obstruction, and cystoscopic intervention surgery for severe cases [Citation7], as illustrated in . After 4–14 days of conventional treatment, if urinary irritative symptoms (frequency, dysuria, urgency, difficulty urinating, etc.) are not relieved, platelet count is above 50 × 10^9/L, and there are no contraindications to hyperbaric oxygen therapy (HBOT), HBOT is administered (NRS ≥4). The HBOT protocol involves treatment at a pressure of 1.8–2.0 ATA for a total treatment time of 100 minutes, with pressurization for 20 min, stable oxygen inhalation for 50 min, rest for 5 min × 2, and decompression oxygen inhalation for 20 minutes. This treatment is administered once daily until the patient’s clinical symptoms improve.
Virus detection
All patients underwent serum CMV-DNA testing (using real-time quantitative polymerase chain reaction, RQ-PCR) after transplantation. Testing was performed 1–2 times per week post-transplantation. Additionally, urine CMV-DNA and BKV-DNA were tested when HC occurred.
Evaluation of therapeutic efficacy in HC
Based on the relief of the urinary system, the outcomes of HC patients are classified into four categories: complete remission (CR), partial remission (PR), non-remission (NR), and recurrence. CR is defined as the disappearance of hematuria and urinary tract irritation during long-term follow-up without recurrence. PR is defined as a significant reduction in hematuria, no need for blood transfusion, or asymptomatic persistent microscopic hematuria, and a reduction in urinary tract irritation. NR is defined as no improvement or relief of urinary tract irritation or hematuria. Recurrence is defined as the reappearance of hematuria and urinary tract irritation one week after their disappearance [Citation8]. The criterion for ending treatment is when the patient’s HC condition reaches the PR or CR state.
The numeric rating scale (NRS) [Citation9]
NRS provides a numerical value ranging from 0 to 10, where patients verbally express the number that best represents the severity of their pain. Patients are instructed to ‘Please tell me your current level of pain, from 0 to 10, with 0 indicating no pain and 10 indicating the worst possible pain. Tell me the number that corresponds to your current level of pain.’ NRS scoring can be used to assess the clinical symptoms of HC patients after transplantation, providing a clearer and more specific reflection of the pain experienced during urination. It can also reflect the improvement of clinical symptoms in HC patients before and after conventional treatment, and combined with HBOT to better guide HC treatment.
Statistical analysis
The data were analyzed using SPSS 25.0. Based on the distribution characteristics of the sample data, the Mann-Whitney U test was used to compare the characteristics and treatment time of the two groups of patients. GraphPad Prism 8.0.1 was used to analyze the time required for NR <= 2 and post-transplant survival time, and to plot the changes in NRS and HC grade during the patient’s illness. Quantitative data that did not conform to a normal distribution were expressed as M (Qmin, Qmax).
Results
Patients’ characteristics
This study included a total of 16 patients, 9 males and 7 females, with a median age of 33.5 (19, 51) years. Among them, 6 cases had acute lymphocytic leukemia (ALL), 6 cases had acute myeloid leukemia (AML), 1 case had non-Hodgkin’s lymphoma (NHL), 2 cases had myelodysplastic syndrome (MDS), and 1 case had T-cell lymphoblastic lymphoma (T-LBL). Of the 16 patients, 10 received HLA-haploidentical HSCT, and 6 received HLA-matched allogeneic HSCT. The time of onset of LOHC in all patients was 33 (21, 52) days after transplantation. The pre-treatment NRS for HBOT (+) patients was 5.0 (3.0, 8.0), and the post-treatment NRS was 0.0 (0.0, 1.0). The pre-treatment NRS for HBOT (−) patients was 5.0 (3.0, 7.0), and the post-treatment NRS was 1.5 (0.0, 6.0). All patients had HC grades of 1-3. There were no significant differences in gender, age, GVHD grade, pre-treatment HC grade, and NRS score between patients who received and did not receive HOBT, as shown in .
Table 2. Comparison of Patient characteristic data before HBOT in two groups.
The efficacy of HBOT
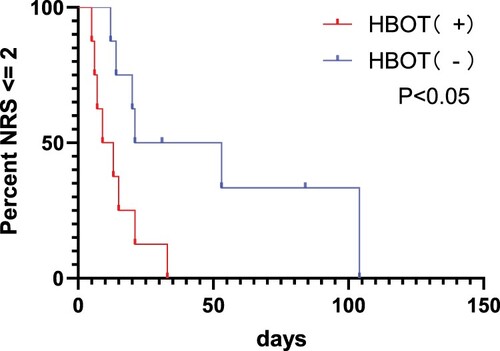
Among the 8 HBOT (−) patients, the median treatment time was 28.5 (22.5, 74.5) days, while among the 8 HBOT (+) patients, the median treatment time was 15.5 (12.0, 23.5) days. Compared to HBOT (−) patients, HBOT (+) patients had a significantly shorter median treatment time, as shown in . Analysis of the two groups of indicators revealed a statistically significant difference in treatment time between the two groups of HC patients, with a p-value of 0.015 < 0.05. Additionally, analyzing the changes in NRS for patients, as shown in , HBOT (+) patients achieved a significant reduction in NRS below 2 in a shorter time compared to HBOT (−) patients, with a p-value of 0.0175 < 0.05, indicating that HBOT can alleviate urinary pain symptoms more quickly.
Table 3. The treatment time of the two groups.
Patients who received HBOT (+) treatment experienced a median resolution of hematuria after 20 days (range: 4–41), whereas patients who did not receive HBOT (+) had a median resolution of hematuria symptoms after 23.5 days (range: 8–142). Analysis of the data using the Mann-Whitney test revealed no significant difference in the time required for hematuria resolution between the two groups (p > 0.05). However, it remains unclear whether HBOT (+) is responsible for the resolution of hematuria or if antiviral medications play a role in this process. Further research is needed to understand the impact of HBOT (+) on the improvement of hematuria symptoms. Following routine supportive treatments such as antiviral therapy, all patients showed a decrease in CMV-DNA levels in both serum and urine, with positive BKV results.
No severe adverse reactions related to HBOT were observed in the 8 patients who received the treatment.
Follow-up outcomes after transplantation
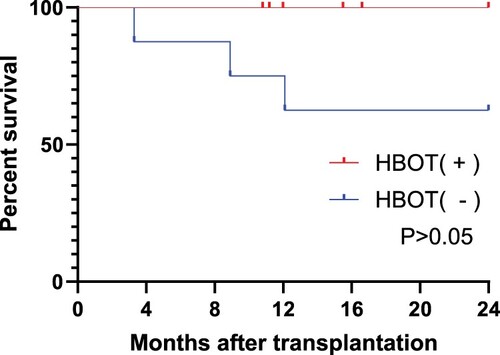
As of 1st December 2022, this study analyzed the survival rate of 16 patients who were followed up for 2 years after transplantation. All 8 patients who received HBOT (+) treatment survived. Among the 8 patients who did not receive HBOT (+) treatment, Case10 died due to AML relapse in the 9th month after transplantation, while Case11 and Case14 died from transplantation-related complications in the 4th and 13th months after transplantation, respectively. As shown in , survival analysis revealed that the combined use of HBOT did not significantly affect the survival time of post-transplant patients. There was no significant difference in the long-term survival rate between the two groups of patients.
Discussion
Allo-HSCT is the most effective approach for treating malignant hematological disorders, immunodeficiency diseases, and certain genetic metabolic disorders. LOHC, a common complication following allo-HSCT, involves inflammation, injury, and diffuse bleeding of the bladder mucosa and superficial blood vessels. The pathogenesis of LOHC remains unclear, but several studies have suggested associations with viruses (such as CMV and BKV), aGVHD, and HLA compatibility. Currently, there is no standardized management protocol for LOHC patients. Conventional treatments mainly include hydration, urine alkalization, antispasmodics, diuretics, hemostasis, and analgesics. Antiviral therapies such as ganciclovir, sodium phosphorus, and cidofovir are administered for patients with viral infections such as BKV and CMV [Citation10]. However, conventional treatments have been found to be slow in improving clinical symptoms and unsatisfactory in terms of efficacy.
HBOT was initially introduced for the improvement of radiation-induced hemorrhagic cystitis, demonstrating favorable efficacy and minimal adverse reactions [Citation11–14]. In 1985, Weiss [Citation15], et al. first discovered the effectiveness of hyperbaric oxygen therapy (HBOT) in treating radiation-induced hemorrhagic cystitis. Since then, HBOT has gained popularity in the treatment of this condition. In 2001, Hattori et al. [Citation16] in Japan began applying HBOT for the treatment of post-transplantation hemorrhagic cystitis, achieving remarkable results. In 2014, Qian et al. initiated the clinical application of HBOT for post-transplantation hemorrhagic cystitis in China, achieving significant therapeutic effects. HBOT plays a crucial role in correcting the hypoxic state of bladder mucosal epithelial cells by increasing the oxygen tension in the blood. This therapy is beneficial in promoting the vitality and division of vascular fibroblasts, collagen fiber formation, and improvement of microcirculation. It facilitates the repair of bladder mucosal tissue and enhances aerobic capacity by promoting collagen protein formation, fibroblast growth, angiogenesis, and accelerating bladder wound healing [Citation11, Citation17].
In recent years, some studies have indicated the effectiveness of HBOT in post-transplantation hemorrhagic cystitis. However, there is limited research on the adjunctive treatment effects of hyperbaric oxygen therapy for hemorrhagic cystitis. During our study, we utilized the Numeric Rating Scale (NRS) to measure the pain experienced by patients with hemorrhagic cystitis, providing a more intuitive assessment. If the NRS score remained above 4 after 4–14 days of conventional treatment, we introduced HBOT as supplementary therapy. The results demonstrated that combined treatment significantly reduced the overall treatment duration compared to conventional supportive therapy alone. HBOT (+) patients showed a significant reduction in the time required to achieve an NRS <= 2 compared to HBOT (−) patients, indicating the effective pain relief provided by HBOT. Furthermore, we found that HBOT effectively alleviated pain compared to conventional supportive therapy alone but did not alleviate hemorrhagic cystitis. There was no significant difference in long-term survival rates between the two groups, although further data is needed for validation.
The association between HC and aGVHD remains unclear. Studies by Lunde et al. [Citation18] and Uhm et al. [Citation19] have identified aGVHD as a high-risk factor for HC occurrence, with grades 3–4 aGVHD showing a significant correlation with HC. Peterson et al.’s [Citation20] retrospective evaluation of allo-HSCT patients revealed a higher prevalence of aGVHD in evident HC cases. Numerous studies both domestically and internationally have indicated a close relationship between aGVHD and HC occurrence [Citation3, Citation19, Citation21]. In our study, we found that 13 out of 16 HC patients (81.25%) exhibited symptoms of aGVHD (grades I to III). However, it is still under investigation whether GVHD targeting bladder epithelial cells manifests as HC, and whether aGVHD and immunosuppression induced by steroids are related to HC and its severity [Citation19, Citation22].
The efficacy of combined HBOT in clearing CMV virus remains unclear. According to the data in , post-treatment, 2/8 patients in the HBOT(+) group showed seroconversion of CMV in serum, and 1/8 patients showed seroconversion of CMV in urine. In the HBOT(−) group, 2/8 patients showed seroconversion of CMV in serum, 1/8 patients had a decrease in CMV levels in serum, and 1/8 patients showed seroconversion of CMV in urine. There was no significant difference between the two groups. Urine BKV-DNA testing was conducted in the early stages of patients presenting with genitourinary symptoms. The testing was outsourced to the Hematology Research Institute of the Chinese Academy of Medical Sciences, and all tested patients had positive results. The specific values could not be traced, and subsequent seroconversion of BKV-DNA in urine was not tested. It is also unclear whether conventional antiviral treatment may have influenced these results, making it difficult to determine the impact of HBOT on CMV and BKV in blood and urine. The role of CMV viremia as a high-risk factor for HC occurrence is currently debated, with more literature domestically and internationally suggesting that BKV is an independent risk factor for HC occurrence. One study [Citation17] suggests that CMV viremia is not correlated with HC. However, this virus may promote the amplification of BKV-DNA, leading to the development of HC. Further research is needed to better understand the role of CMV and BKV in the occurrence of HC.
Table 4. Type of viruses.
This retrospective study has several limitations. Currently, there are few patients who receive HBOT as a treatment for HC after transplantation, resulting in a small sample size in this study. The NRS pain score was used to evaluate patients’ subjective feelings, which may be highly subjective. The improvement in viral load cannot exclude the influence of antiviral drugs. In addition, the conventional treatment given to each patient varied. In the next phase, we plan to collect more cases and data to enable a more definitive analysis of the impact of HBOT.
In conclusion, hyperbaric oxygen therapy (HBOT) as an adjunctive treatment can shorten the duration of illness in patients with hemorrhagic cystitis (HC). It can provide faster and safer relief of clinical symptoms in HC patients, leading to an improved post-transplantation survival experience. Therefore, HBOT can be considered as an effective and novel approach in the management of HC.
Acknowledgments
Li Ying performed the research. Qu Yiwen, Qiao Xiansen, Ding Xiaojie, and Wang Ling offered detailed materials for this manuscript. Zhao Peng and Qu Yiwen guided statistical methods. Qu Yiwen wrote the manuscript. All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Bing Z, Chenyuan H, Di Y, et al. Multivariate analysis influencing recovery from hemorrhagic cystitis after allogeneic hematopoietic stem cell transplantation. Chin J Exp Hematol. 2019;27(3):976–982.
- Qian W, Fang Z, Ningxia S, et al. Clinical features and risk factors of hemorrhagic cystitis after allogeneic hematopoietic stem cell transplantation. Chin J Hematol. 2019;(3):187–190.
- Xiong Y, Cai D, Chen J, et al. Risk factors for delayed hemorrhagic cystitis after allogeneic hematopoietic stem cell transplantation in 227 cases. J Third Milit Med Univ. 2021;43(21):2321–2330.
- Ying L, Jun Y, Xiaoqian X, et al. Risk factors for hemorrhagic cystitis after allogeneic hematopoietic stem cell transplantation. Theory Practice Internal Med. 2020;15(5):326–331.
- Hosokawa K, Aoki G, Ohata K, et al. Effectiveness of hyperbaric oxygen therapy for virus-associated hemorrhagic cystitis after allogeneic hematopoietic stem cell transplantation. Int J Hematol. 2021;114(1):109–115. doi:10.1007/s12185-021-03120-y
- Savva-Bordalo J, Pinho Vaz C, Sousa M, et al. Clinical effectiveness of hyperbaric oxygen therapy for BK-virus-associated hemorrhagic cystitis after allogeneic bone marrow transplantation. Bone Marrow Transplant. 2012;47(8):1095–1098. doi:10.1038/bmt.2011.228
- Zhenghua J, Youyuan L, Weiqing H, et al. Clinical study of a 5-step sequential approach in the treatment of radiation cystitis bleeding. Chin J Urol. 2018;39(12):890–894.
- Liu P, Zhang Z, Bai K, et al. Analysis of early symptoms and surgical treatment of hemorrhagic cystitis after allogeneic hematopoietic stem cell transplantation in children. J Shanghai Jiaotong Univ (Medical Science). 2022;42(7):958–963.
- Myrvik MP, Yan K. A comparison of pain assessment measures in pediatric sickle cell disease: visual analog scale versus numeric rating scale. J Pediatr Hematol Oncol. 2015;37(3).
- Mo X-D, Zhang X-H, Xu L-P, et al. Treatment of late-onset hemorrhagic cystitis after allogeneic hematopoietic stem cell transplantation: the role of corticosteroids. Ann Hematol. 2018;97(7):1209–1217. doi:10.1007/s00277-018-3290-0
- Jiawen W, Yang Y. Observation of the efficacy of hyperbaric oxygen in the comprehensive treatment of hemorrhagic radiation cystitis in 56 cases. Chin J Nautical Med Hyperbaric Med. 2016;23(4):323–325.
- Li Z, Meng Y, Li J, et al. Clinical application of hyperbaric oxygen therapy in radiation injury of tumors. Chin J Nautical Med Hyperbaric Med. 2021;28(5):552–561.
- Edwards ML. Hyperbaric oxygen therapy. Part 2: application in disease: hyperbaric oxygen therapy: part 2. J Vet Emerg Crit Care. 2010;20(3):289–297. doi:10.1111/j.1476-4431.2010.00535_1.x
- Thom SR. Oxidative stress is fundamental to hyperbaric oxygen therapy. J Appl Physiol (1985). 2009;106(3):988–995.
- Weiss JP, Boland FP, Mori H, et al. Treatment of radiation-induced cystitis with hyperbaric oxygen. J Urol. 1985;134(2):352–354. doi:10.1016/S0022-5347(17)47166-7
- Qian L, Shen J, Zhao D, et al. Successful treatment of hemorrhagic cystitis after HLA-mismatched allogeneic hematopoietic stem cell transplantation by hyperbaric oxygen. Transplantation. 2014;97(7):e41–42. doi:10.1097/00007890-201407151-00137
- Lele Y, Dexin Y. Clinical efficacy of hyperbaric oxygen adjuvant therapy in patients undergoing minimally invasive surgery for bleeding radiation cystitis. Chin J Nautical Med Hyperbaric Med. 2019;(2):152–154.
- Lunde LE, Dasaraju S, Cao Q, et al. Hemorrhagic cystitis after allogeneic hematopoietic cell transplantation: risk factors, graft source and survival. Bone Marrow Transplant. 2015;50(11):1432–1437. doi:10.1038/bmt.2015.162
- Uhm J, Hamad N, Michelis FV, et al. The risk of polyomavirus BK-associated hemorrhagic cystitis after allogeneic hematopoietic SCT is associated with myeloablative conditioning, CMV viremia and severe acute GVHD. Bone Marrow Transplant. 2014;49(12):1528–1534. doi:10.1038/bmt.2014.181
- Peterson L, Ostermann H, Fiegl M, et al. Reactivation of polyomavirus in the genitourinary tract is significantly associated with severe GvHD and oral mucositis following allogeneic stem cell transplantation. Infection. 2016;44(4):483–490. doi:10.1007/s15010-016-0872-4
- Seber A, Shu XO, Defor T, Sencer S, Ramsay N. Risk factors for severe hemorrhagic cystitis following BMT. Bone Marrow Transplant. 1999;23(1):35–40. doi:10.1038/sj.bmt.1701523
- Lee G-W, Lee J-H, Choi S-J, et al. Hemorrhagic cystitis following allogeneic hematopoietic cell transplantation. J Korean Med Sci. 2003;18(2):191–195. doi:10.3346/jkms.2003.18.2.191