ABSTRACT
The outcomes of relapsed Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ALL) resistant to new drugs such as tyrosine kinase inhibitors, inotuzumab ozogamicin (InO) and blinatumomab are dismal. We treated two cases of Ph+ALL resistant to these drugs that achieved long-term survival after treatment with chimeric antigen receptor (CAR)-T cell therapy or a second allogeneic hematopoietic stem cell transplantation (HCT) with a sequential conditioning regimen. Case 1: A 15-year-old boy was diagnosed with Ph+ALL. Despite the second HCT after the treatment of ponatinib and blinatumomab, hematological relapse occurred. InO was ineffective and he was transferred to a CAR-T center. After the CAR-T cell therapy, negative measurable residual disease (MRD) was achieved and maintained for 38 months without maintenance therapy. Case 2: A 21-year-old man was diagnosed with Ph+ALL. Hematological relapse occurred after the first HCT. Despite of the treatment with InO, ponatinib, and blinatumomab, hematological remission was not achieved. The second HCT was performed using a sequential conditioning regimen with clofarabine. Negative MRD was subsequently achieved and maintained for 42 months without maintenance therapy. These strategies are suggestive and helpful to treat Ph+ALL resistant to multiple immunotherapies.
Introduction
The outcomes of Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ALL) have steadily progressed with improvements in tyrosine kinase inhibitors (TKIs) [Citation1–3]. Ponatinib, a third-generation TKI, is effective for relapsed and refractory Ph+ALL, and favorable outcomes can be expected when combined with allogeneic hematopoietic stem cell transplantation (HCT) [Citation4,Citation5]. Furthermore, even in cases of TKI resistance, inotuzumab ozogamicin (InO) and blinatumomab, as off-target effects for BCR-ABL1 tyrosine kinase, in combination with HCT show promise [Citation6–8]. However, resistance to these novel agents limits treatment options, and the survival prognosis in such cases is considered extremely poor [Citation9]. Nevertheless, we experienced two cases of Ph+ALL refractory to all three drugs that achieved long-term survival after treatment with chimeric antigen receptor (CAR)-T cell therapy or a second HCT with a sequential conditioning regimen. These treatment strategies are promising.
Case presentation
Case 1: A 15-year-old boy was diagnosed with Ph+ALL in September 2018. Complete remission (CR) with positive measurable residual disease (MRD), evaluated by polymerase chain reaction methods for BCR-ABL1 mRNA, was achieved with combination chemotherapy with dasatinib. In February 2019, bone marrow transplantation from an HLA-matched unrelated donor was performed following myeloablative conditioning regimen. Disease relapse occurred three months later. Subsequent CR with negative MRD was achieved with ponatinib and one cycle of blinatumomab. In August, cord blood transplantation was performed and five months later, maintenance therapy with ponatinib was initiated because of positive MRD. However, nine months after the second HCT, hematological relapse occurred (positive for the E255 K mutation). One cycle of InO was ineffective and he was transferred to a CAR-T cell therapy center. After ponatinib-combined chemotherapy with methotrexate and cytarabine, CR with positive MRD was obtained. In July 2020, anti-CD19 CAR-T cell therapy, tisagenlecleucel, was performed using conditioning regimen with fludarabine (30 mg/m2, day1-4) plus cyclophosphamide (500 mg/m2, day1,2). Negative MRD was achieved and maintained for 38 months without maintenance therapy at last follow up. At 24 months post-CAR-T cell therapy, CAR-T cells are still detectable by quantitative real-time polymerase-chain-reaction assay.
Case 2: A 21-year-old man was diagnosed with Ph+ALL in October 2018. CR with positive MRD was achieved with combination chemotherapy with dasatinib. In February 2019, cord blood transplantation following myeloablative conditioning regimen was performed; however, graft failure and autologous hematopoiesis occurred. Six months after the HCT, while waiting for salvage transplantation under maintenance therapy with dasatinib, hematological relapse occurred (positive for the V299L mutation). One cycle of InO, ponatinib, and one cycle of blinatumomab were administered sequentially; however, CR was not achieved. As a sequential conditioning regimen including fludarabine (150 mg/m2) melphalan (140 mg/m2) and total body irradiation (4 Gy), clofarabine (Clo; 30 mg/m2 for 1–5 days) was administered at 36 and 22 days prior to HCT with cord blood. At starting conditioning regimen, CR marrow with nuclear cell count of 2000/µL was obtained. Negative MRD was subsequently achieved and maintained for 42 months without maintenance therapy at last follow up.
Overall clinical courses and detailed treatment managements in critical phases are shown in and , respectively.
Figure 1. Overall Clinical courses of two patients with Ph+ALL Dx, diagnosis; uBMT, unrelated bone marrow transplantation; CBT, umbilical cord blood transplantation; CAR-T, chimeric antigen receptor T-cell therapy; Pon, ponatinib; BLN, blinatumomab; InO, inotuzumab ozogamicin; Clo, clofarabine; NR, non-remission, CR, complete remission; MRD, measurable residual disease; CT, chemotherapy.
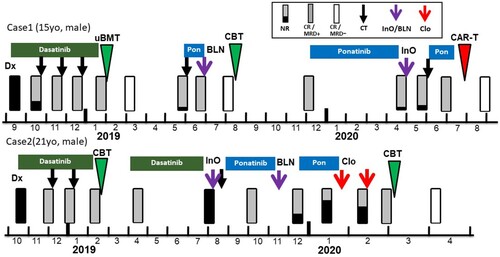
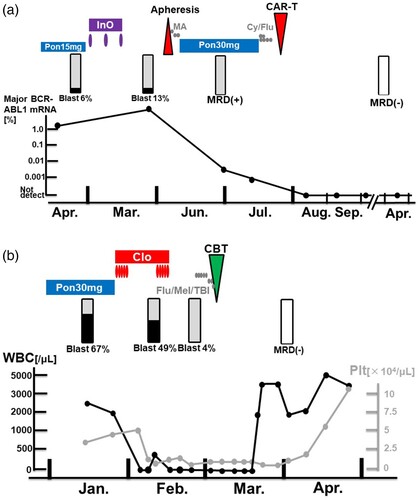
Figure 2. Detailed treatment managements in critical phases for Case 1 (a) and Case 2 (b). CAR-T, chimeric antigen receptor T-cell therapy; Pon, ponatinib; InO, inotuzumab ozogamicin; MA, methotrexate plus cytarabine; Cy, cyclophosphamide; flu, fludarabine; MRD, measurable residual disease for BCR-ABL1 mRNA; Clo, clofarabine; CBT, umbilical cord blood transplantation; Mel, melphalan; TBI, total body irradiation; WBC, white blood cell count; Plt, platelet.
Discussion
We experienced two cases of successful treatment for Ph+ALL relapsing after novel immunotherapies, ponatinib, and HCT. In Case 1, even for the second relapse after the second HCT, CAR-T cell therapy was effective. In Case 2, a sequential conditioning regimen with Clo at HCT was effective.
In Case 1, when starting CAR-T cell therapy, hematological remission with positive MRD was obtained after ponatinib-combined chemotherapy with methotrexate plus cytarabine. The CAR-T cell therapy with good disease control might have contributed to long-term disease-free survival. Two years after CAR-T cells infusion, CAR-T cells are still detectable, which suggests the functional activity of CAR-T cells. Along with the complete donor hematopoiesis, which may have graft-versus-leukemia effect, these factors may have contributed to long-term disease remission. However, CAR-T cell therapy is available only in limited age groups and facilities, and a prompt medical coordination may be another important factor.
Several studies evaluating the outcomes of CAR-T cell therapy for relapsed or refractory Ph+ALL are present. Two studies of CAR-T therapy showed favorable outcomes in Ph+ALL compared to Ph-negative ALL [Citation10,Citation11]. Other two clinical trials showed that CAR-T cell therapy as bridging to the subsequent HCT was associated with better outcomes [Citation12,Citation13]. It was debatable whether the patient should be bridged to a third HCT in this case as well. However, the third HCT was avoided due to high risk of non-relapse mortality. Moreover, TKI maintenance therapy after CAR-T cell therapy was also not offered since the patient had already developed TKI resistance. Finally, only MRD monitoring was performed. The long-term persistence of CAR-T cells can be associated with durable CR, which may partially predict the favorable prognosis of this case [Citation14].
In Case 2, a sequential conditioning regimen with Clo was selected instead of CAR-T cell therapy. Sequential conditioning was defined as any regimen that combines a short, intensive course of salvage chemotherapy to decrease leukemia cell burden followed by transplant conditioning [Citation15]. Sequential conditioning-based HCT, as exemplified by FLAMSA, has long been established as an effective therapy approach for high-risk and relapsed / refractory acute myeloid leukemia [Citation16,Citation17]. While some studies have investigated sequential conditioning in ALL patients [Citation18], very few data are currently available. Bazarbachi AH, et al. identified 115 patients with refractory or relapsed ALL who underwent HCT using sequential conditioning between 2000 and 2017 from the EBMT registry data [Citation19]. They showed that two-year relapse incidence, non-relapse mortality, and overall survival rates were 45%, 41%, and 17%, respectively.
Clofarabine is a second-generation purine nucleoside analog that has been investigated in several phase 1 and 2 trials either as a single agent or in combination for relapsed/refractory acute leukemia in pediatric and adult patients [Citation20–22]. In all studies, a high degree of hematologic toxicity was shown. Our previous studies showed that a bone marrow nuclear cell count of <100,000/µL and preconditioning intervention with intensive chemotherapy may be significant in patients with ALL not in remission [Citation23,Citation24]. The study also suggests that an intensive chemotherapy was associated with low nuclear cell count in bone marrow. Referring to these studies, Clo was selected as a preconditioning intervention to induce bone marrow suppression and as a bridge to a second HCT. The dose and management of Clo was adjusted at 30 mg/m2 (day1–5) for two cycles with 11 days of drug withdrawal. Shown in (A), severe myelosuppression was induced after the use of Clo, but without infectious complications, blast percentage and nuclear cell count in bone marrow was successfully reduced as intended at starting conditioning regimen for cord blood transplantation. As in Case 1, TKI maintenance therapy was also not offered.
Separate treatment strategies were used for the two cases of post-transplant relapse. Case 1, who relapsed after the second HCT and had a post-transplant remission duration of six months, was selected for CAR-T cell therapy due to concerns about the high treatment-related mortality and high relapse rate with a third allogeneic transplant [Citation25]. Case 2 was also eligible for CAR-T cell therapy, but was a recipient hematopoiesis due to graft rejection in the first HCT. Therefore, we opted for a second HCT in anticipation of graft-versus-leukemia effect with donor hematopoiesis. In addition, since the disease status was a non-remission with an active disease control, a sequential conditioning regimen was selected.
Both patients first underwent HCT after failing to achieve MRD-negative status with dasatinib-combined chemotherapy. Accordingly, early disease recurrence was observed. Although new agents, including ponatinib, InO, and blinatumomab, were administered, both cases showed hematological relapse. Treatment options are limited for relapsed and refractory Ph+ALL resistant to immunotherapy, rendering it difficult to induce hematologic remission, let alone achieve MRD negativity. To speculate, its management with low disease control is important [Citation10,Citation23]. Both present cases achieved hematological remission, albeit temporarily, with chemotherapy, which is also an important option. Without missing the appropriate timing, they successfully received definitive post-treatments such as CAR-T cell therapy or a sequential conditioning regimen.
Patients with relapsed or refractory Ph+ALL have received long-term treatment including intensive chemotherapy and HCT. Therefore, they have the risks of treatment-related complications and death [Citation8,Citation25–27]. To reduce them, less intensive intervention with shorter treatment period is recommended. As previously reported, in the case of a second HCT, a reduced conditioning regimen with haploidentical or cord blood transplantation should be considered [Citation4,Citation8]. In summary, sufficient knowledge and experience will be required in the management of second HCT, HCT in non-remission, and CAR-T therapy for these relapsed and refractory cases. In particular, it will be important to properly evaluate the risks and benefits and select the best strategy.
In conclusion, we experienced two cases of relapsed and refractory Ph+ALL resistant to multiple new agents that achieved long-term remission with CAR-T cell therapy or a second HCT with sequential conditioning. In the future, it is important to pursue various possibilities based on evidence and to accumulate cases.
Patient consent
The requirement for approval from an independent ethics committee was waived by the ethics committee. Written informed consents were obtained from the patients for publication of this case report.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Conflict of interest statement
TT reports speakers’ bureaus from Otsuka, Pfizer, MSD, AbbVie, Astellas, and Chugai, outside the submitted work. HN reports honoraria from Novartis and Daiichi-Sankyo, scholarship from Daiichi-Sankyo, Cellgene, Chugai, Nihon-Shinyaku, Astellas, Asahikasei-pharma, Chugai, Takeda, Pfizer, and Eisai, outside the submitted work.
References
- Yanada M, Takeuchi J, Sugiura I, et al. High complete remission rate and promising outcome by combination of imatinib and chemotherapy for newly diagnosed BCR-ABL-positive acute lymphoblastic leukemia: a phase II study by the Japan Adult Leukemia Study Group. J Clin Oncol. 2006;24(3):460–466. doi:10.1200/JCO.2005.03.2177
- Sugiura I, Doki N, Hata T, et al. Dasatinib-based 2-step induction for adults with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood Adv. 2022;6(2):624–636. doi:10.1182/bloodadvances.2021004607
- Jabbour E, Haddad FG, Short NJ, et al. Treatment of adults with Philadelphia chromosome-positive acute lymphoblastic leukemia-from intensive chemotherapy combinations to chemotherapy-free regimens: a review. JAMA Oncol. 2022;8(9):1340–1348. doi:10.1001/jamaoncol.2022.2398
- Tachibana T, Koyama S, Andou T, et al. Salvage and bridging to allogeneic hematopoietic cell transplantation with ponatinib in patients with relapsed or refractory Philadelphia chromosome-positive leukemia. Int J Hematol. 2019;109(2):162–168. doi:10.1007/s12185-018-02571-0
- Nanno S, Matsumoto K, Nakamae M, et al. Effect of prophylactic post-transplant ponatinib administration on outcomes in patients with Philadelphia chromosome-positive acute lymphoblastic Leukemia. Clin Lymphoma Myeloma Leuk. 2020;20(12):813–819.e1. doi:10.1016/j.clml.2020.07.005
- Kantarjian HM, DeAngelo DJ, Stelljes M, et al. Inotuzumab Ozogamicin versus standard therapy for acute lymphoblastic leukemia. N Engl J Med. 2016;375(8):740–753. doi:10.1056/NEJMoa1509277
- Rambaldi A, Ribera JM, Kantarjian HM, et al. Blinatumomab compared with standard of care for the treatment of adult patients with relapsed/refractory Philadelphia chromosome-positive B-precursor acute lymphoblastic leukemia. Cancer. 2020;126(2):304–310. doi:10.1002/cncr.32558
- Tachibana T, Tanaka M, Hagihara M, et al. Outcomes in patients with acute lymphoblastic leukemia who underwent second allogeneic hematopoietic cell transplantation for relapse after first transplantation. Int J Hematol. 2022;116(4):594–602. doi:10.1007/s12185-022-03377-x
- Short NJ, Macaron W, Konopleva M, et al. Dismal outcomes of patients with relapsed/refractory Philadelphia chromosome-negative B-cell acute lymphoblastic leukemia after failure of both inotuzumab ozogamicin and blinatumomab. Am J Hematol. 2022;97(6):E201–E204. doi:10.1002/ajh.26526
- Park JH, Rivière I, Gonen M, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med. 2018;378:449–459. doi:10.1056/NEJMoa1709919
- Leahy AB, Devine KJ, Li Y, et al. Impact of high-risk cytogenetics on outcomes for children and young adults receiving CD19-directed CAR T-cell therapy. Blood. 2022;139:2173–2185. doi:10.1182/blood.2021012727
- Gu B, Shi BY, Zhang X, et al. Allogeneic haematopoietic stem cell transplantation improves outcome of adults with relapsed/refractory Philadelphia chromosome-positive acute lymphoblastic leukemia entering remission following CD19 chimeric antigen receptor T cells. Bone Marrow Transplant. 2021;56(1):91–100. doi:10.1038/s41409-020-0982-6
- Yao Z, Gu B, Zhang Y, et al. CD19 chimeric antigen receptor T-cell therapy as a bridge therapy for allogeneic hematopoietic stem cell transplantation in patients with relapsed Philadelphia chromosome-positive acute lymphoblastic leukemia. Bone Marrow Transplant. 2023;58(1):103–105. doi:10.1038/s41409-022-01845-w
- Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–1517. doi:10.1056/NEJMoa1407222
- Mohty M, Malard F, Blaise D, et al. Sequential regimen of clofarabine, cytosine arabinoside and reduced-intensity conditioned transplantation for primary refractory acute myeloid leukemia. Haematologica. 2017;102:184–191. doi:10.3324/haematol.2016.150326
- Schmid C, Schleuning M, Ledderose G, et al. Sequential regimen of chemotherapy, reduced-intensity conditioning for allogeneic stem-cell transplantation, and prophylactic donor lymphocyte transfusion in high-risk acute myeloid leukemia and myelodysplastic syndrome. J Clin Oncol. 2005;23(24):5675–5687. doi:10.1200/JCO.2005.07.061
- Kolb HJ, Schmid C. The FLAMSA concept-past and future. Ann Hematol. 2020;99(9):1979–1988. doi:10.1007/s00277-020-04131-1
- Arita K, Kondo T, Sugita J, et al. Sequential chemotherapy and myeloablative allogeneic hematopoietic stem cell transplantation for refractory acute lymphoblastic leukemia. Int J Hematol. 2011;94(3):291–295. doi:10.1007/s12185-011-0919-3
- Bazarbachi AH, Al Hamed R, Labopin M, et al. Allogeneic stem-cell transplantation with sequential conditioning in adult patients with refractory or relapsed acute lymphoblastic leukemia: a report from the EBMT Acute Leukemia Working Party. Bone Marrow Transplant. 2020;55(3):595–602. doi:10.1038/s41409-019-0702-2
- Koh K, Ogawa C, Okamoto Y, et al. Phase 1 study of clofarabine in pediatric patients with relapsed/refractory acute lymphoblastic leukemia in Japan. Int J Hematol. 2016;104:245–255. doi:10.1007/s12185-016-2004-4
- Hijiya N, Gaynon P, Barry E, et al. A multi-center phase I study of clofarabine, etoposide and cyclophosphamide in combination in pediatric patients with refractory or relapsed acute leukemia. Leukemia. 2009;23:2259–2264. doi:10.1038/leu.2009.185
- Saito T, Hatta Y, Hayakawa F, et al. Combination of clofarabine, etoposide, and cyclophosphamide in adult relapsed/refractory acute lymphoblastic leukemia: a phase 1/2 dose-escalation study by the Japan Adult Leukemia Study Group. Int J Hematol. 2021;113(3):395–403. doi:10.1007/s12185-020-03032-3
- Tachibana T, Kanda J, Ishizaki T, et al. Outcomes and prognostic factors for patients with relapsed or refractory acute lymphoblastic leukemia who underwent allogeneic hematopoietic cell transplantation: a KSGCT multicenter analysis. Biol Blood Marrow Transplant. 2020;26(5):998–1004. doi:10.1016/j.bbmt.2020.01.007
- Tachibana T, Kanda J, Ishizaki T, et al. Pre-conditioning intervention in patients with relapsed or refractory acute lymphoblastic leukemia who underwent allogeneic hematopoietic cell transplantation: a KSGCT multicenter retrospective analysis. Ann Hematol. 2021;100(11):2763–2771. doi:10.1007/s00277-021-04607-8
- Kobayashi S, Kanda Y, Konuma T, et al. Outcomes of third allogeneic hematopoietic stem cell transplantation in relapsed/refractory acute leukemia after a second transplantation. Bone Marrow Transplant. 2022;57(1):43–50. doi:10.1038/s41409-021-01485-6
- Yaniv I, Krauss AC, Beohou E, et al. Second hematopoietic stem cell transplantation for post-transplantation relapsed acute leukemia in children: a retrospective EBMT-PDWP study. Biol Blood Marrow Transplant. 2018;24(8):1629–1642. doi:10.1016/j.bbmt.2018.03.002
- Ferra Coll C, Morgades de la Fe M, Prieto García L, et al. Prognosis of patients with acute lymphoblastic leukaemia relapsing after allogeneic stem cell transplantation. Eur J Haematol. 2023;110(6):659–668.
