ABSTRACT
Objective
To investigate and compare the effects of basic preconditioning regimens Bu/Cy, Cy/TBI and Flu/Bu for the treatment of patients in allogeneic hematopoietic stem cell transplantation.
Methods
It comprised exploring the published literature in the databases of PubMed, EMBASE, Cochrane Library, and Web of Science, using suitable keywords pertaining to various basic pretreatments Bu/Cy, Cy/TBI, and Flu/Bu, prior to allogeneic hematopoietic stem cell transplantation, and then extracting the searched outcome indicators of Overall Survival (OS) and survival (herein represented as OS and survival). Further, the results were estimated with meta-analysis using R, where the incidence of GVHD was reported in odds ratio (OR) with its 95% confidence interval (95%CI).
Results and Discussion
A total of 14 papers were included in this study, including 1436 cases were treated with Bu/Cy, 1816 cases with Cy/TBI, and 549 cases with Flu/Bu in the preconditioning regimen. After OS was the outcome pooled, compared with Flu/Bu in the preconditioning group, the results (Cy/TBI HR = 1.12 (95% Cl:1.04,1.61), Bu/Cy HR = 1.24 (95% Cl. 1.13,2.06)) showed that Flu/Bu preconditioning regimen significantly improved the overall survival rate of allogeneic HSCT patients. With the incidence of GVHD as the outcome summary, compared with Flu/Bu in the pretreatment group, the results (Cy/TBI HR = 1.24 (95% Cl:1.12, 1.82), Bu/Cy HR = 1.14 (95% Cl. 1.03, 2.12)) indicated that Flu/Bu in the pretreatment regimen group also significantly reduced the incidence of GVHD after allogeneic HSCT.
Conclusion
Patients who received the basal preconditioning regimen Flu/Bu before allogeneic hematopoietic stem cell transplantation had the lowest hazard ratio for overall survival (OS) development. This indicates that the use of the basal preconditioning regimen Flu/Bu for the treatment of patients was the most effective, although the quality of the studies included needs to be confirmed by high-quality randomized controlled trials.
1. Introduction
The only known cure for relapsing hematological diseases is allogeneic hematopoietic stem cell transplantation (allo-HSCT), and it has been observed to be the best form of prevention in such diseases, especially for patients in first remission [Citation1]. All-HSCT is part of the preconditioning regime in which it is able to kill the cancer cells and provide a stem cell niche for new stem cells in the bone marrow of the host [Citation2]. Myeloablative Conditioning Regimen (MAC) is used in Allo-HSCT to allow disease control and to provide adequate immunosuppression that may prevent graft rejection [Citation3]. E. Donnall Thomas et al. showed that Cy/TBI was the first successful HSCT in the MAC regimen [Citation4]. However, the difficulty of using TBI uniformly across multiple centers, and the high transplant-related mortality (TRM) associated with the early regimen led to associated complications, including childhood growth delay, interstitial pneumonitis, and secondary malignancies. Therefore, it is necessary to develop the non-radiation-based combination chemotherapy regimens [Citation5].
In the early 1980s, Bu/Cy combination therapy was introduced as an alternative to Cy/TBI, and the goal of the new preconditioning regimen was to reduce toxicity, improve prognosis, and provide an alternative for patients who were not suitable for TBI [Citation5]. Clinically, the interaction between preconditioning regimen Bu/Cy may lead to increased hepatotoxicity, and include shortcomings such as delayed immune reestablishment, high viral reactivity, and the need for transfusion for symptomatic treatment. Therefore, it is urgent to find less toxic immunosuppressants to replace cyclophosphamide to improve transplantation outcomes [Citation6]. Fludarabine (Flu), a potent immunosuppressive purine analogue with significant antitumor and immunosuppressive activity, has been selected to replace cyclophosphamide in a pulp stripping pretreatment regimen [Citation7,Citation8]. However, there is a lack of clinical randomized controlled trials evaluating the efficacy of Bu/Cy, Cy/TBI, and Flu/Bu in myeloablative preconditioning regimen before allogeneic hematopoietic stem cell transplantation. Moreover, most of these trials are from a single center, and the volume of each trial is small, and the clinical research results are also controversial.
Through an evidence-based approach, this study summarized existing databases and conducted a meta-analysis to evaluate the efficacy of the three basic preconditioning regimens and rank the advantages and disadvantages. This paper aims to summarize the current evidence to find the best basic preconditioning regimen before allogenic hematopoietic stem cell transplantation and provide data support.
2. Methods
2.1. Search strategy
The relevant literatures on the comparison of prognosis of prognosis of basic pretreatment for allogeneic hematopoietic stem cell transplantation published from self-built English databases such as PubMed, EMBASE, Cochrane Library was searched from inception to March 2023. The search terms included ‘allogeneic hematopoietic stem cell transplantation OR allo-HSCT’ AND ‘Busulfan plus Fludarabine’ OR ‘Busulfan plus Cyclophosphamide’ OR ‘Body irradiation plus Cyclophosphamide’ OR ‘TBI plus Cyclophosphamide’ OR ‘Fludarabine’ OR ‘Busulfan’ OR ‘Cyclophosphamide’ OR ‘TBI,’ the MeSH subject headings were queried and both subject and free words were included in the search. Relevant literature references were also manually searched as a supplement to ensure the comprehensiveness of the data obtained.
2.2. Inclusion criteria
The inclusion criteria of the literature were as follows: at first, the study subjects were patients who need to undergo myeloablative pretreatment for allogeneic hematopoietic stem cell transplantation, age between 18 and 45 years old, with disease remission in CR1 phase; Secondly, the basic pretreatment regimen consisted of Bu/Cy (i.e. Bu), Cy/TBI (TBI = 10 Gy or 15Gy), and Flu/Bu (i.v. Bu), which involved the comparison of two or more basic pretreatment regimen, with consistency of the preconditioning dose and cycle; Thirdly, the outcome index was the overall survival rate (Overall Survival Rate; OS rate) of patients receiving different basic preconditioning regimens. The outcome indicators were Overall Survival Rate (OS rate) of patients who received allogeneic HSCT with different basal preconditioning regimens, in which OS was the duration of follow-up until death from any cause after the patients received the treatment, and the literature included in this study was the OS at 3 years of post-transplantation follow-up; the incidence of Graft-versus-Host Disease (GVHD); the incidence of GVHD; the incidence of GVHD; and the incidence of GVHD. Graft-versus-host disease (GVHD); Fourthly, hazard ratio (HR) and its 95% confidence interval (95% CI) were directly or indirectly available in the article; Fifthly, the type of study was a randomized controlled trial; Sixthly, studies with data from the same institution were only included if they were the most recent study or had the highest quality score.
2.3. Exclusion criteria
Exclusion criteria for literature articles were as follows: first, the ones not comparing the above schemes of preprocessing or those with incomplete data to be extracted; second, the sample size was too small for each group, less than 20, which will be excluded from interventions. Third, the type of literature that excludes duplication of published literature, review, system review, meta-analysis, etc., includes case reports, animal experiments, and so on.
2.4. Literature screening and data extraction
This literature was screened independently by two authors, and the retrieved literature was screened strictly according to the inclusion and exclusion criteria, and any disagreement was resolved through discussion or negotiation with a third party. Data extraction included: At first, general information of the included studies: first author, publication time; Secondly, information of the study subjects: number of patients, disease stage, specific protocol of basic pretreatment; Thirdly, outcome indicators were OS; incidence of GVHD; Fourthly, key information of risk of bias evaluation.
2.5. Risk of bias assessment of included studies
The included studies for risk of bias using the Cochrane Handbook's RCT Risk of Bias tool, in which 2 researchers independently evaluated the literature and discussed decisions in cases of disagreement. The population scale included the following factors: randomization process, deviation from expected intervention, selection of reported outcomes, and overall bias.
2.6. Statistical analysis
The meta-analysis was conducted using Stata 14.0 software and R 4.0 software. The overall survival (OS) was assessed using heart rate (HR) and its 95% confidence interval (CI), whereas the incidence of graft-versus-host disease (GVHD) was quantified using odds ratio (OR) and its 95% CI as the indicator of effect. The hazard ratio (HR) for overall survival (OS) was directly obtained in trials reporting summary statistics. In the absence of aggregated statistics, data were extracted from the Kaplan-Meier curves provided in the article using Digitizer software according to the method proposed by Liu et al. and HR was calculated at the same time. Statistical analyses were performed using the HR of OS as the main indicator of efficacy. The HR OS was used as the main indicator index for statistical analysis. Statistical analysis of the data used the HR of OS as the main index of efficacy. The Meta-analysis was performed in R Studio using R3.6.3 software. The I2 test was used to assess the heterogeneity of the study results, and if the I2 was <50%, it suggested that there was no significant heterogeneity among the studies. The node splitting method was used to evaluate the inconsistency. If P > 0.05, it suggested that the difference was not statistically significant, and the results of direct and indirect comparisons were consistent. The test level α = 0.05.
3. Results
3.1. Results of literature search
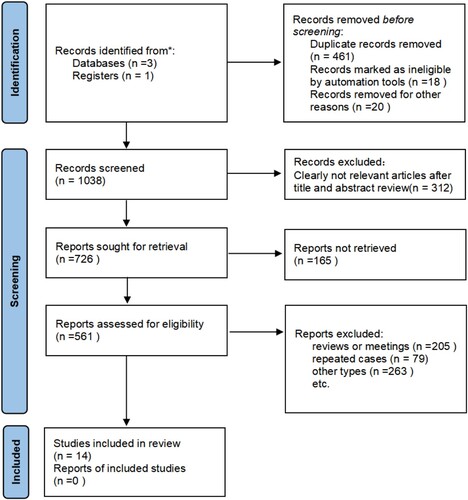
According to the pre-established search strategy and data collection methods, various databases were searched, with 1,235 documents. After using EndNote 22 management software to de-focus, 1,038 relevant documents were obtained. According to the inclusion and exclusion criteria, 312 literatures that did not meet the criteria were excluded by reading the titles and abstracts, and 726 documents that might meet the criteria were initially included. The full-text screening of these documents was carried out to exclude 23 retrospective studies, 205 reviews, 79 repetitive reports, and 263 non-randomized controlled trials, and 14 documents that met the criteria were obtained. The process of screening was shown in .
3.2. Basic characteristics of the included literature
14 randomized controlled trials were included in this study, including 6 comparisons of preconditioning regimen Bu/Cy versus Cy/TBI, 1 comparison of Flu/Bu versus Cy/TBI, and 7 comparisons of Flu/Bu versus Bu/Cy. The basic characteristics of the studies included were shown in .
Table 1. Basic characteristics of the included literature.
3.3. Methodological qualitative evaluation of included studies
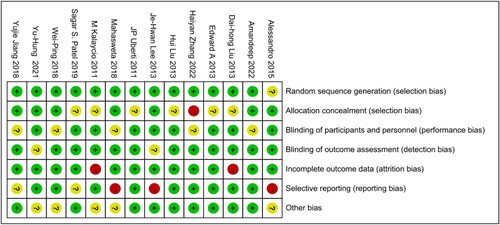
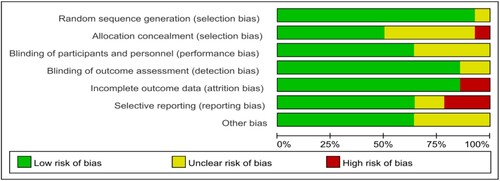
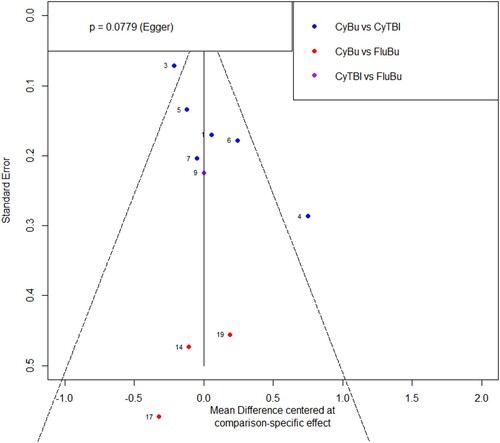
According to the quality evaluation requirements, the quality evaluation of the 14 included literatures was conducted, including the randomization method, the concealment of the middle pairing, the implementation of the blind method, completeness of the outcome data, selective reporting, conflict of interest and other risks of bias. In terms of risks of bias, the risk of bias was assessed for each study included, and the results were shown in and .
3.4. Net meta-analysis results
3.4.1. Network relationship diagram
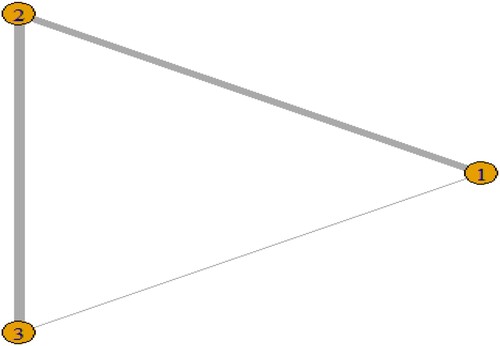
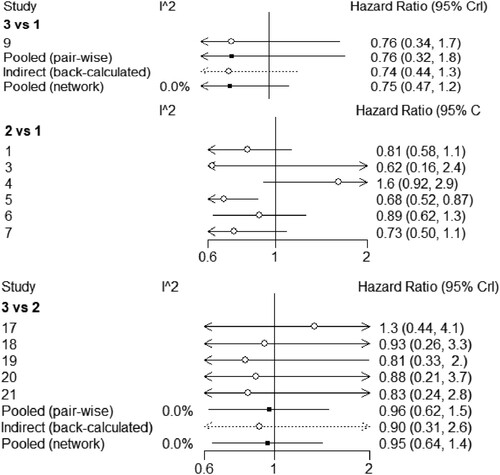
14 studies reported survival rates, including 1436 cases in the pre-treatment regimen Bu/Cy group, 1816 cases in the Cy/TBI group, and 549 cases in the Flu/Bu group. There were 6 studies in the pre-treatment regimen Bu/Cy group and Cy/TBI group, and 7 studies in the Cy/TBI group and Flu/Bu group, and 1 study in the Bu/Cy group and Flu/Bu group. Bu group studies were 1. The size of the dots in the graph represents the number of people in the group, and the thickness of the inter-arms between the dots represents the number of direct study comparisons. The network relationship diagrams 1–4 were created as follows .
3.4.2. Model fit goodness-of-fit test
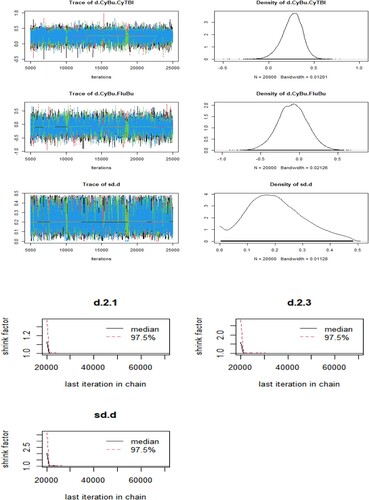
In R software, this article set model parameters likelihood = binom, link = cloglog, linear model = random, and select the number of pre-iterations as 5000 times and the number of iterations as 50000 times for iterative operation. After the iterative operation was completed, the trajectory middle and density plots and Brooks-Gelman-Rubin plots were plotted separately. As shown in , the plots suggested that the model convergence degree was satisfactory.
3.4.3. Analysis based on total inclusion studies
3.4.3.1. League tables of HR after aggregation with OS as the outcome
Exclusion criteria for literature articles were as follows: first, the ones not comparing the above schemes of preprocessing or those with incomplete data to be extracted; second, the sample size was too small for each group, less than 20, which will be excluded from interventions. Third, the type of literature that excludes duplication of published literature, review, system review, meta-analysis, etc., includes case reports, animal experiments, and so on. Compared with the HR column values obtained by behavioral comparison (), it was found that Cy/TBI HR = 1.12 (95% Cl:1.04,1.61), Bu /Cy HR = 1.24 (95% Cl:1.13,2.06), compared with the pretreatment group.
Table 2. OS as ending three pretreatment options league table.
3.4.3.2. League table of OR after aggregation of GVHD incidence as outcome
Survival analysis of the three preconditioning regimens was performed, and 9 studies met the inclusion criteria. The 95% confidence intervals of HR values of each group of pretreatment regimens were compared, and a league table of the OR after pooling of the three pretreatment regimens with the incidence of GVHD as the endpoint, with the values in the behavioral comparisons to derive columns of the ORs compared with the pretreatment group (), the results were as follows: Flu/Bu with Cy/TBI HR = 1.24 (95% Cl:1.12, 1.82), Bu/Cy HR = 1.14 (95% Cl:1.03, 2.12).
Table 3. League table of three pretreatment options with GVHD incidence as an outcome.
3.4.3.3. Relative effect ranking with OS as the outcome
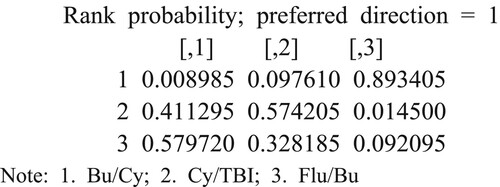
From the individual ranking results, As can be seen from the individual ranking results, the probability of the basic pretreatment scheme Flu/Bu ranking first was 0.5797, and the probability of the third was 0.0920, while the probability of the basic pretreatment scheme Bu/Cy ranking first place was 0.00089, and the probability of ranking in the third place was 0.8934. The results showed that the basic pretreatment scheme Flu/Bu has the best effect, and the Bu/Cy effect was the worst. Cy has the worst effect, as shown in :
3.4.4. Analysis based on research related to AML disease
3.4.4.1. League tables of HR after aggregation with OS as the outcome
Survival analysis was performed for 3 preconditioning regimens with a total of 9 studies that met the inclusion criteria, respectively comparing the 95% confidence intervals of the HR values of each preconditioning regimen, and the league table of HR after pooling of the three pretreatment regimens with OS as the endpoints. Compared with the HR column values obtained by behavioral comparison (), it was found that Cy/TBI HR = 1.18(95%Cl:1.11,1.42), Bu/Cy HR = 1.32 (95%Cl:1.22,1.96), compared with the pretreatment group.
Table 4. OS as ending three pretreatment options league table.
3.4.4.2. League table of OR after aggregation of GVHD incidence as outcome
Survival analysis of the three preconditioning regimens was performed, and 9 studies met the inclusion criteria. The 95% confidence intervals of HR values of each group of pretreatment regimens was compared, and a league table of the OR after pooling of the three pretreatment regimens with the incidence of GVHD as the endpoint, with the values in the behavioral comparisons to derive columns of the ORs compared with the pretreatment group (), the results were as follows: Flu/Bu with Cy/TBI, Cy/TBI HR = 1.15 (95%Cl:1.08, 1.72), Bu/Cy HR = 1.22 (95%Cl:1.08, 2.02).
Table 5. League table of three pretreatment options with GVHD incidence as an outcome.
3.4.4.3. Relative effect ranking with OS as the outcome
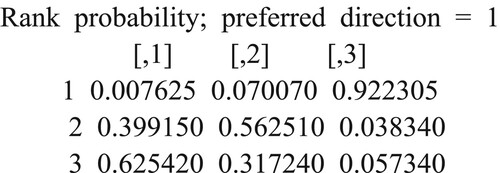
The probability of the basic pretreatment scheme Flu/Bu being the first ranked is 0.6254, while on the other hand, the probability of it occupying the third position was 0.0573. For the basic pretreatment scheme Bu/Cy, the probability of being placed first is 0.0076, and the probability of being placed in the third place is 0.9223. The probability of the basic pretreatment scheme Flu/Bu being the first ranked is 0.6254, while the probability of it occupying the third position was 0.0573 .
3.4.5. Heterogeneity test
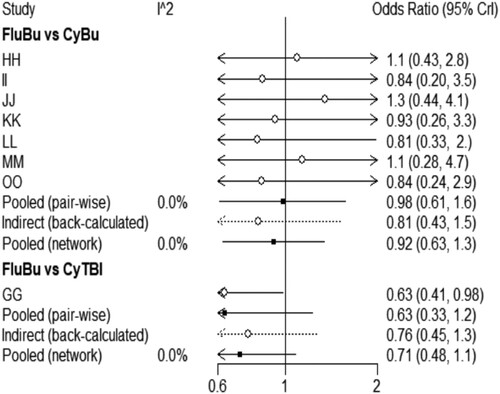
As can be seen from , the I2 between the direct comparison of the base pretreatment scheme Cy/Bu and Flu/Bu was 0%, indicating that there was no heterogeneity between them. The I2 between the results of the reticulation comparison between the two was 0%, indicating that they met the assumption of homogeneity between them. The comparison between the basic preprocessing schemes Cy/TBI and Flu/Bu is the same.
3.4.6. Consistency test
A nodal analysis method was used to test the consistency of comparison results. It can be seen from the nodal analysis chart () that the P-value of indirect comparison results and mesh comparison results of intervention of Cy/Bu and Flu/Bu, Cy/TBI and Flu/Bu were all greater than 0.05, and there was no statistically significant difference, i.e. the consistency was better.
4. Discussion
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is the recommended method of treatment for leukemia patients who are at an intermediate or high risk. The practice of transplantation has experienced substantial transformations because of advancements in human leukocyte antigen (HLA) mapping [Citation23], infection prevention and treatment [Citation24], prevention and management of graft versus-host disease (GVHD) [Citation25], and efficient management of organ toxicity. Myeloablative preconditioning (MAC) is the most used regimen to maximize disease control and provide adequate immunosuppression to prevent GVHD [Citation26]. However, MAC regimens are prone to higher non-relapse mortality (NRM) due to drug-related toxicity and graft-versus-host disease (GVHD) [Citation27,Citation28]. There are also differences in NRM among different MAC regiments, so it is urgent to find the optimal basal preconditioning regimen for allogeneic HSCT to improve prognosis. The three most used clinical MAC regimens Cy/TBI, Bu/Cy and Flu/Bu have never been compared in a randomized manner. It is difficult to consider the survival benefit in the pretreatment scheme. However, some observational studies have made two comparisons of these protocols, with conflicting results. Therefore, by aggregating the 14 relevant studies included, by comparing patients with consistent clinical characteristics, and by performing a net meta-analysis of the included studies, this study showed that the use of the basic pretreatment regimen Flu/Bu was the most effective. But the effect still needs to be evaluated by high-quality randomized controlled trials.
Fludarabine is widely used as a pretreatment regimen for chemotherapy and allo-HSCT in acute leukemia (especially refractory leukemia). Fludarabine interacts synergistically with leucovorin by inhibiting DNA ligases and DNA initiating enzymes as well as preventing DNA polymerization and impairing alkylator-induced damage repair [Citation29–31]. In addition, fludarabine does not rely on liver storage for detoxification, and there is a non-overlapping organ toxicity between fludarabine and leucovorin. There is increasing evidence that fludarabine combined with leucovorin as a purging or non-purging pretreatment regimen can strike a good balance between anti-tumor efficacy and reduced toxicity [Citation32,Citation33].
For OS, there was a statistically significant difference in pretreatment groups between the combination Cy/TB and Flu/Bu (HR = 1.12; 95% CI: 1.04–1.61) compared to Bu/Cy vs. the Flu/Bu combination (HR = 1.24; 95%) CI: Results from the SUCRA sorting showed that Flu/Bu under the pretreatment group yielded the highest chance of the best benefit in improving OS. There was an improvement in GVHD incidence in terms of Cy/TBI HR = 1.24 (95% Cl: 1.12, 1.82), and Bu/Cy HR = 1.14 (95% Cl) This study observed that improved survival outcomes with Flu/Bu were associated with lower NRM, most notably due to the lower incidence of aGVHD. In the past, flu has been shown to decrease aGVHD in a mouse model of aljsonc bone marrow transplantation [Citation34]. It has been noted that patients in the Flu/Bu group had a shorter duration of neutropenia and a shorter median time to neutrophil and platelet implantation than those in the Bu/Cy group. The need for haematocrit transfusion (especially platelets) was much lower in the Flu/Bu group than in the Bu/Cy group [Citation35,Citation36], suggesting that the BuFlu regimen is associated with milder myelotoxicity and a better safety profile than the BuCy regimen.
To better understand how Flu affects different T cell subsets and reduces aGVHD, it is necessary to perform an immunoreconstructive analysis of Flu used in preconditioning. GVHD is a complex pathological process mediated by allogenic reactive donor T cells recognizing different HLA antigens and involves tissue-specific immune cells and inflammatory cytokines [Citation37]. GVHD is a complex pathological process mediated by the recognition of different HLA antigens by alloreactive donor T cells, involving tissue-specific immune cells and inflammatory cytokines. This regulatory effect can stimulate the release of inflammatory factors, which play a key role in GVHD. Chae et al. reported that the BuFlu regimen had a lower incidence and severity of acute and chronic GVHD than the BuCy regimen [Citation9]. The results of the study showed that the pretreatment regimen of fludarabine instead of cyclophosphamide was less severe in terms of nausea and vomiting reactions and liver and cardiotoxicity, which was like previous reports in the literature [Citation13].
Despite the best efforts to control confounding factors in this study, some practical questions remain unanswered. Current improvements in clinical practice, including new microbial agents, antibodies that alert the immune response, GVHD prevention protocols, donor selection based on allele typing, and stem cell sources, have improved outcomes for non-related transplants. In addition, the number of Peripheral Blood Stem Cells (PBSC) transplants has increased dramatically, and it is unclear whether the results observed in bone marrow transplant recipients are also true in PBSC transplant recipients. Long-term quality of life indicators following the use of different MAC regimens in HSCT should also be studied in the future to better understand late effects.
5. Conclusion
This meta-analysis specifically focuses on Chinese and English literature, which may introduce language and publication bias. Also, the sample sizes in some studies are small due to limited available literature. Consequently, this study only examines the disease AML for further analysis. There may be unidentified or unregulated variables that can influence the pooled data. As preprocessing techniques have evolved, there have been variations in these protocols among studies, which can potentially affect the research outcomes. In this study, the results of a meta-analysis of several basic preconditioning protocols showed that the use of the basic pretreatment regimen Flu/Bu has the best clinical outcome in the late stage, we further selected disease AML for individual validation, and the results were the same. However, a high-quality, large sample randomized controlled trials was needed to rule out the possibility that other variables may have a greater impact on survival than the basic pretreatment regimen, and that there are still some adverse reactions to the Flu/Bu regimen. The Flu/Bu regimen still has some adverse effects, especially in terms of myelosuppression, as low granulocytes are prone to infection, and the clinic needs to pay close attention to the patient's condition during the pretreatment period and provide timely therapeutic interventions. Prospective and randomized trials of allogeneic hematopoietic stem cell transplantation in patients with hematological disorders remain challenging in clinical practice due to the disease and the treatment process's complexity.
Ethics approval statement
This is not a clinical trial; this study did not require the approval of an Ethics Committee because it is based entirely on previously published studies.
Patient consent statement
As it is based entirely on previously published studies, this study did not require the approval of patients.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Goldstone AH, Richards SM, Lazarus HM, et al. In adults with standard-risk acute lymphoblastic leukemia, the greatest benefit is achieved from a matched sibling allogeneic transplantation in first complete remission, and an autologous transplantation is less effective than conventional consolidation/maintenance chemotherapy in all patients: results of the International All Trial (MRC UKALL XII/ECOG E2993). Blood. 2008;111:1827–1833. doi:10.1182/blood-2007-10-116582
- Giebel S, Labopin M, Potter M, et al. Comparable results of autologous and allogeneic hematopoietic stem cell transplantation for adults with Philadelphia-positive acute lymphoblastic leukemia in first complete molecular remission: an analysis by the acute leukemia working party of the EBMT. Eur J Cancer. 2018;96:73–81. doi:10.1016/j.ejca.2018.03.018
- Tutschka PJ, Copelan EA, Klein JP. Bone marrow transplantation for leukemia following a new busulfan and cyclophosphamide regimen. Blood. 1987;70(5):1382–1388. doi:10.1182/blood.V70.5.1382.1382
- Thomas ED, Buckner CD, Banaji M, et al. One hundred patients with acute leukemia treated by chemotherapy, total body irradiation, and allogeneic marrow transplantation. Blood. 1977;49(4):511–533. doi:10.1182/blood.V49.4.511.511
- Bertz H, Potthoff K, Mertelsmann R, et al. Busulfan/cyclophosphamide in volunteer unrelated donor (VUD) BMT: excellent feasibility and low incidence of treatment-related toxicity. Bone Marrow Transplant. 1997;19:1169–1173. doi:10.1038/sj.bmt.1700823
- Slavin S, Nagler A, Naparstek E, et al. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood. 1998; 91(3):756–763.
- de Lima M, Couriel D, Thall PF, et al. Once-daily intravenous busulfan and fludarabine: clinical and pharmacokinetic results of a myeloablative, reduced-toxicity conditioning regimen for allogeneic stem cell transplantation in AML and MDS. Blood. 2004;104(3):857–864. doi:10.1182/blood-2004-02-0414
- Russell JA, Tran HT, Quinlan D, et al. Once-daily intravenous busulfan given with fludarabine as conditioning for allogeneic stem cell transplantation: study of pharmacokinetics and early clinical outcomes. Biol Blood Marrow Transplant. 2002;8(9):468–476. doi:10.1053/bbmt.2002.v8.pm12374451
- Uberti JP, Agovi MA, Tarima S, et al. Comparative analysis of BU and CY versus CY and TBI in full intensity unrelated marrow donor transplantation for AML, CML and myelodysplasia. Bone Marrow Transplant. 2011;46(1):34–43. doi:10.1038/bmt.2010.81
- Salhotra1 A, Hui S, Yang D, et al. Long-term outcomes of patients with acute myelogenous leukemia treated with myeloablative fractionated total body irradiation tbi-based conditioning with a tacrolimus and sirolimus-based graft-versus-host disease prophylaxis regimen: 6-year follow-up from a single center. Biol Blood Marrow Transplant. 2020;26(2):292–299. doi:10.1016/j.bbmt.2019.09.017
- Kalaycio M, Bolwell B, Rybicki L, et al. BU-vs TBI-based conditioning for adult patients with ALL. Bone Marrow Transplant. 2011;46(11):1413–1417. doi:10.1038/bmt.2010.314
- Copelan EA, Hamilton BK, Avalos B, et al. Better leukemia-free and overall survival in AML in first remission following cyclophosphamide in combination with busulfan compared with TBI. Blood. 2013;122(24):3863–3870. doi:10.1182/blood-2013-07-514448
- Eroglu C, Pala C, Kaynar L, et al. Comparison of total body irradiation plus cyclophosphamide with busulfan plus cyclophosphamide as conditioning regimens in patients with acute lymphoblastic leukemia undergoing allogeneic hematopoietic stem cell transplant. Leuk Lymphoma. 2013;54(11):2474–2479. doi:10.3109/10428194.2013.779691
- Wang YH, Ko BS, Liao XW, et al. Busulfan-containing conditioning regimens in allogeneic hematopoietic stem-cell transplantation for acute lymphoblastic leukemia: single tertiary center experience in Taiwan. Biol Blood Marrow Transplant. 2019;25(3):188–199. doi:10.1016/j.bbmt.2018.12.774
- Gooptu M, Kim H, Ho V, et al. A Comparison of the myeloablative conditioning regimen fludarabine/busulfan with cyclophosphamide/total body irradiation, for allogeneic stem-cell transplantation in the modern era: a cohort analysis. Biol Blood & Marrow Transplant. 2018;24:1733–1740. doi:10.1016/j.bbmt.2018.03.011
- Jiang Y, Fang X, Sui X, et al. Comparison of different conditioning regimens of haploidentical hematopoietic stem cell transplant in patients with acute myeloid leukemia. Exp Clin Transplant: Off J Middle East Soc Organ Transplant. 2018;16(6):736–744.
- Liu H, Zhai X, Song Z, et al. Busulfan plus fludarabine as a myeloablative conditioning regimen compared with busulfan plus cyclophosphamide for acute myeloid leukemia in first complete remission undergoing allogeneic hematopoietic stem cell transplantation: a prospective and multicenter study. J Hematol, Oncol. 2013;6(15):2–9.
- Lee JH, Joo YD, Kim H, et al. Randomized trial of myeloablative conditioning regimens: busulfan plus cyclophosphamide versus busulfan plus fludarabine. J Clin Oncol Off J Am Soc Clin Oncol. 2013;31(6):701–709. doi:10.1200/JCO.2011.40.2362
- Liu DH, Xu LP, Zhang XH, et al. Substitution of cyclophosphamide in the modified BuCy regimen with fludarabine is associated with increased incidence of severe pneumonia: a prospective, randomized study. Int J Hematol. 2013;98(6):708–715. doi:10.1007/s12185-013-1460-3
- Patel SS, Rybici L, Pohlman B, et al. Comparative effectiveness of busulfan/cyclophosphamide versus busulfan/fludarabine myeloablative conditioning for allogeneic hematopoietic cell transplantation in acute myeloid leukemia and myelodysplastic syndrome ScienceDirect. Hematol Oncol Stem Cell Ther. 2020;13(3):160–165. doi:10.1016/j.hemonc.2019.09.002
- Rambaldi A, Grassi A, Masciulli A, et al. Busulfan plus cyclophosphamide versus busulfan plus fludarabine as a preparative regimen for allogeneic haemopoietic stem-cell transplantation in patients with acute myeloid leukemia: an open-label, multicenter, randomized, phase 3 trial. Lancet Oncol. 2015;16(15):1525–1536. doi:10.1016/S1470-2045(15)00200-4
- Zhang W-P, Wang Z-W, Hu X-X, et al. Preconditioning with fludarabine, busulfan and cytarabine versus standard BuCy2 for patients with acute myeloid leukemia: a prospective, randomized phase II study. Bone Marrow Transplant. 2019;54(6):894–902. doi:10.1038/s41409-018-0356-5
- Flomenberg N, Baxter-Lowe LA, Confer D, et al. Impact of HLA class I and class II high-resolution matching on outcomes of unrelated donor bone marrow transplantation: HLA-C mismatching is associated with a strong adverse effect on transplantation outcome. Blood. 2004;104(7):1923–1930. doi:10.1182/blood-2004-03-0803
- Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic cell transplantation. N Engl J Med. 2010;363(22):2091–2101. doi:10.1056/NEJMoa1004383
- Tanaka Y, Kurosawa S, Tajima K, et al. Analysis of non-relapse mortality and causes of death over 15 years following allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2016;51:553–559. doi:10.1038/bmt.2015.330
- Holler E, Kolb HJ, Moller A, et al. Increased serum levels of tumor necrosis factor alpha precede major complications of bone marrow transplantation. Blood. 1990;75:1011–1016. doi:10.1182/blood.V75.4.1011.1011
- Kawamura K, Kako S, Mizuta S, et al. Comparison of conditioning with fludarabine/busulfan and fludarabine/melphalan in allogeneic transplantation recipients 50 years or older. Biol Blood Marrow Transplant. 2017;23:2079–2087. doi:10.1016/j.bbmt.2017.09.003
- Scott BL, Pasquini MC, Logan BR, et al. Myeloablative versus reduced-intensity hematopoietic cell transplantation for acute myeloid leukemia and myelodysplastic syndromes. J Clin Oncol. 2017;35:1154–1161. doi:10.1200/JCO.2016.70.7091
- Santos GW. Preparative regimens: chemotherapy versus chemoradiotherapy. A historical perspective. Ann N Y Acad Sci. 1995;770:1–7. doi:10.1111/j.1749-6632.1995.tb31039.x
- Oliansky DM, Camitta B, Gaynon P, et al. Role of cytotoxic therapy with hematopoietic stem cell transplantation in the treatment of pediatric acute lymphoblastic leukemia: update of the 2005 evidence based review. Biol Blood Marrow Transplant. 2012;18(4):505–522. doi:10.1016/j.bbmt.2011.12.585
- Santos GW, Tutschka PJ, Brookmeyer R, et al. Marrow transplantation for acute nonlymphocytic leukemia after treatment with busulfan and cyclophosphamide. N Engl J Med. 1983;309:1347–1353. doi:10.1056/NEJM198312013092202
- Santos GW, Tutschka PJ, Brookmeyer R, et al. Marrow transplantation for acute nonlymphocytic leukemia after treatment with busulfan and cyclophosphamide. N Engl J Med. 1983;309(22):1347–1353. doi:10.1056/NEJM198312013092202
- Tutschka PJ, Copelan EA, Klein JP. Bone marrow transplantation for leukemia following a new busulfan and cyclophosphamide regimen. Blood. 1987;70(5):1382–1388. doi:10.1182/blood.V70.5.1382.1382
- Bredeson C, LeRademacher J, Kato K, et al. Prospective cohort study comparing intravenous busulfan to total body irradiation in hematopoietic cell transplantation. Blood. 2013;122(24):3871–3878. doi:10.1182/blood-2013-08-519009
- Lee J-H, Joo Y-D, Kim H, et al. Randomized trial of myeloablative conditioning regimens: busulfan plus cyclophosphamide versus busulfan plus fludarabine. J Clin Oncol. 2013;31(6):701–709. doi:10.1200/JCO.2011.40.2362
- Andersson BS, de Lima M, Thall PF, et al. Once daily i.v. busulfan and fludarabine (i.v. Bu-Flu) compares favorably with i.v. busulfan and cyclophosphamide (i.v. BuCy2) as pretransplant conditioning therapy in AML/MDS. Biol Blood Marrow Transplant. 2008;14(6):672–684. doi:10.1016/j.bbmt.2008.03.009
- Or R, Weiss L, Teiman S. Fludarabine monophosphate reduces the incidence and severity of graft-versus-host disease in a murine model of bone marrow transplantation. Blood. 1997;90(1):461–471.