Abstract
Ovarian neoplasms are rare tumours of steroid cell origin. These tumours present clinically due to the associated excess production of either androgenic or oestrogenic gonadal steroid hormones. The clinical picture is dictated by the specific hormone(s) produced and influenced by the age of the patient. The case is a 59-year-old woman who presented with a five-year history suggestive of androgen excess. She underwent a hysterectomy and right-sided oophorectomy at age 28 years for dysfunctional uterine bleeding. Virilisation was confirmed on clinical examination and the testosterone excess biochemically localised to the ovaries. A left-sided oophorectomy was performed. The clinical picture and testosterone excess persisted after surgery. Follow-up radiological investigations identified adnexal material that on resection proved to be remnant ovarian tissue. Histopathology confirmed the presence of a steroid cell tumour within the remnant tissue. The biochemical androgen excess resolved and the clinical features improved dramatically.
Case presentation
A 59-year-old Caucasian woman presented with complaints of excessive hair growth, a deepening of the voice and temporal balding. She had first noted the symptoms five years previously, with an initial concern of new onset facial hair growth. The hirsutism was progressive, followed by the onset of male-pattern baldness and subsequent deepening of the voice. The voice changes prompted endocrine consultation. There was no other significant prior medical history apart from well-controlled asthma that never required oral corticosteroids. She denied use of any pharmacotherapy (topical and oral) or any over-the-counter supplements. Her weight remained unchanged over the course of the five years. Her menarche was at age 13 years with a regular menstrual cycle reported during adolescence. She conceived spontaneously and had two live children. At age 28 years, she underwent a total abdominal hysterectomy (TAH) and right salpingo-oophorectomy for dysfunctional uterine bleeding, which rendered her amenorrhoeic. She experienced symptoms compatible with perimenopause at 51 years and recalls hot flushes and vaginal dryness around the time that subsided spontaneously.
Physical examination revealed a hirsute woman, normotensive with a body mass index (BMI) of 30 kg/m2 (weight: 77 kg; height: 1.60 m) indicating obesity class I with central accentuation. No other clinical features of insulin resistance were present. Terminal hair growth involved the chin, upper lip, face, breasts, thighs, back and abdomen. Severe hirsutism was confirmed with a modified Ferriman–Gallwey score of ≥ 15. She had no acne or abnormal skin pigmentation. Features of virilisation in the form of severe hirsutism, profound male-pattern baldness and clitoromegaly (1.5 × 2.5 centimetres) were present. Abdominal examination revealed no palpable masses. The rest of the systemic examination was unremarkable with no features suggestive of cortisol or mineralocorticoid excess.
Baseline biochemical results are tabulated in . Biochemical testing documented elevated serum luteinising hormone (LH) and follicle stimulating hormone (FSH) levels along with low circulating oestradiol in keeping with the patient’s postmenopausal state. The serum total testosterone was markedly high at 10.8 nmol/l (normal female range: 0.5–2.6 nmol/l). The weaker androgens, dehydroepiandrosterone sulphate (DHEAS) and androstenedione, respectively predominantly from adrenal and ovarian origin, were both within the normal laboratory reference ranges. A random, unstimulated 17α- hydroxyprogesterone (17OHP) level was slightly elevated at 4.3 nmol/l (female reference range 0.6–2.2). Pathological cortisol excess was excluded based on an 8:00 am serum cortisol level that suppressed following administration of low dose (1 mg) exogenous dexamethasone to less than 50 nmol/l (32 nmol/l).
Table 1: Baseline biochemical assessment
The clinical and baseline biochemical assessment confirmed excess androgen production and an ovarian origin was considered to be most likely. The differential diagnosis included ovarian hyperthecosis, a virilising ovarian tumour, and also late-onset congenital adrenal hyperplasia (CAH). Last-mentioned aetiology was thought unlikely to result in virilisation and was excluded with the documentation of an unstimulated 17α-OHP level below 5.1 nmol/l, the level above which ACTH stimulation is suggested.Citation1
Pelvic ultrasound (US) was requested with due cognisance that a virilising ovarian tumour may be too small to visualise. US confirmed an absent uterus, in keeping with the reported hysterectomy. The right-sided ovary was not seen, as expected with the history of prior surgical removal. The left ovary appeared anatomically normal. Despite the normal ultrasonography, a possible ovarian tumoral source of androgen was still considered and the patient was referred for a left salpingo-oophorectomy, before further imaging was performed. Histopathology demonstrated no tumour and the ovarian histology was reported as being within normal limits. A benign papillary cyst adenofibroma in the Fallopian tube was deemed an incidental finding.
No clinical improvement followed the surgical removal of the left ovary. Biochemistry following the surgical intervention is tabulated in . The virilisation worsened and was accompanied by the documentation of persistently elevated serum testosterone levels. Anti-androgenic therapy was initiated and resulted in some clinical improvement with a less frequent need to shave and a lowering of serum testosterone levels to 4.6 nmol/l. Upon discontinuation of anti-androgen therapy, the testosterone level increased again to a value more than three times the upper female normal range, i.e. to 8.3 nmol/l. The persistently elevated serum testosterone level despite an apparent complete surgical removal of the uterus, fallopian tubes and both ovaries prompted further investigation to identify the persistent source of testosterone. A repeat normal DHEAS (2.8 nmol/,) and a normal ACTH-stimulated 17α-OHP again excluded the likelihood of an adrenal source. A normal β-human chorionic gonadotropin (HCG) of < 2 IU/L and a normal α-fetoprotein level (8.0 ug/l) eliminated a rare androgen secreting tumour associated with these tumour markers, such as hepatoblastomas.
Table 2: Biochemical assessment following left oophorectomy
The pelvic US was repeated. A small (20 × 17 millimetre), solid mass of uncertain significance, located posterior and cranial to the right cervical stump, was noted. Peristalsis was absent, but US-doppler demonstrated some arterial flow in the centre of the mass. The cervix was visualised, the uterus was noted to be absent and yet again neither ovary could be identified. A pelvic magnetic resonance imaging (MRI) study was performed to further delineate the right adnexal mass. The MRI reported only varicosities in the parametrium bilaterally and no mass lesions.
The patient underwent laparoscopic surgery almost two years after initial presentation. Tissue in the right adnexa, macroscopically identified to be ovarian tissue, was noted in keeping with the most recent pelvic US findings and was laparoscopically resected. The remainder of the right infundibulopelvic ligament was mobilised proximally and resected as well. No macroscopic ovarian remnant was identified here. The cervical stump was found to be embedded in an adhesive complex that involved the urinary bladder and ureters. The anatomy in this area was restored and the cervical stump excised. No other masses or abnormalities were noted. One month after the laparoscopic surgery, the clinical features of hyperandrogenism improved significantly with biochemical resolution of the testosterone excess (testosterone level 0.6 nmol/l).
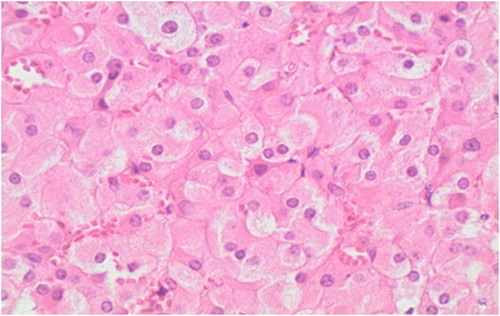
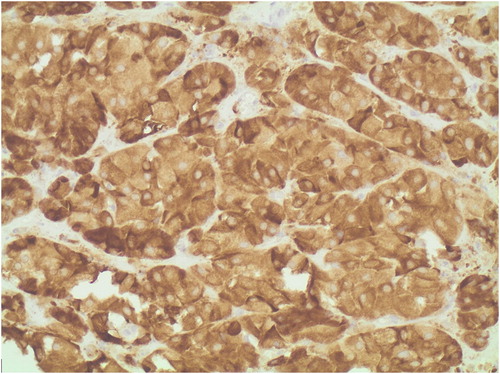
On histopathology, the excised adnexal tissue was confirmed to be ovarian in nature, with a 9 mm tumour identified adjacent to the hilum. The tumour comprised polygonal cells with central monomorphic nuclei and eosinophilic cytoplasm. Many cells contained lipofuscin pigment. No Reinke’s crystalloids, nuclear clustering or fibrinoid necrosis of vessel walls to suggest a Leydig cell tumour were present. Immunohistochemical staining was positive for both melan-A and inhibin. The diagnosis of a steroid cell tumour not otherwise specified (NOS) was therefore made (). No ovarian tissue was present in the right infundibulopelvic ligament or cervical stump.
Discussion
The case presented with new-onset hirsutism after menopause and demonstrated ongoing hirsutism and virilisation despite surgical removal of both ovaries. Ultrasound detected tissue of uncertain significance in the right adnexa that was surgically removed. Histopathology confirmed residual ovarian tissue and identified a steroid cell tumour (NOS) within. This case illustrates the importance of evaluating new-onset hirsutism after menopause and highlights the importance and utility of clinical and biochemical surveillance after surgical resection of hormone-producing lesions. The importance is demonstrated in this case where persistent ovarian androgen production occurred despite bilateral oophorectomies.
Hirsutism is defined as male-pattern terminal hair growth in women. It is a common finding in certain ethnic groups and is not necessarily pathological. Hirsutism is quantified by the Ferriman–Gallwey score in premenopausal women and a score of ≥ 8 are consistent with a diagnosis of hirsutism. A score greater than 15 is regarded as severe hirsutism as evident in our case with a calculated score of 16.Citation2 The Ferriman–Gallwey score has been criticised for its inability to take all androgenic skin areas into account and for lacking validation in certain ethnic groups as well as in postmenopausal women.Citation3 The Ferriman–Gallwey score, however, remains a valuable clinical tool as it provides us with a quantitative, standardised assessment of the extent of male-pattern hair growth. Hirsutism presenting after menopause should always prompt a meticulous history aimed at differentiating new-onset hirsutism from ongoing hirsutism. Accompanying signs of virilisation are always pathological and concerning and necessitate further investigations to exclude or confirm the presence of an androgen-secreting tumour.Citation3
Our patient presented with new-onset hirsutism after menopause. The hirsutism was accompanied by virilisation (voice deepening, male-pattern balding and clitoromegaly) and an investigation of the source of androgen production was appropriately undertaken.
Androgen-secreting tumours arise from the adrenal gland or ovary. Biochemically, serum testosterone levels in excess of 4 mmol/l in females generally support the suspicion of a tumorous aetiology.Citation4,Citation5 Virilising ovarian tumours are exceedingly scarce and represent about 10% of ovarian neoplasms.Citation4 The term ‘virilising tumour of the ovary’ includes Leydig cell tumour, Sertoli cell tumour, ovarian thecomas and steroid cell tumour.Citation4,Citation6
A steroid cell tumour not otherwise specified (NOS) was diagnosed in our case. These tumours originate from ovarian tissue and from ectopic adrenal glands.Citation4,Citation6 Steroid cell tumours account for less than 0.1% of ovarian neoplasms. The subtype ovarian steroid cell tumour NOS is the most common variant and represents approximately 60% of all steroid cell tumours.Citation6
Ovarian hyperthecosis, where testosterone production is attributed to persistent, functional theca cells, must also be considered in the differential diagnosis of postmenopausal females with new-onset hirsutism. The androgen production is thought to be unmasked in the postmenopausal state through the loss of granulosa cell mediated aromatisation of testosterone to oestradiol.Citation7 This process is reinforced by increased levels of LH and insulin that reduces hepatic sex-hormone binding globulin (SHBG) production. Analogous to polycystic ovarian syndrome (PCOS), the decreased SHBG leads to increases in bioavailable, unbound testosterone with enhanced androgenic effects.Citation7 Mildly increased ovarian 17OHP levels, similar to the level initially noted in our patient (4.3 nmol/l, female reference range 0.6–2.2), can occur in PCOS and are attributed to intrinsic theca cell overactivity.Citation8
The differentiation between ovarian hyperthecosis and virilising ovarian tumour is often challenging, with significant overlap between the clinical picture and biochemical findings of these conditions.Citation7 It has been proposed that ovarian hyperthecosis, unlike primary ovarian tumour, is gonadotrophin dependent, with higher levels of FSH and LH seen in hyperthecosis compared with corresponding levels in women with virilising ovarian tumour. Absolute FSH cut-off values have been proposed in the literature to support the diagnosis of virilising ovarian tumour. An FSH value ≤ 35 IU/l has a sensitivity of 90% and specificity of 92%, whereas a cut-off FSH value of < 22.3 IU/l has a sensitivity and specificity of 77% and 87% to diagnose virilising ovarian tumour.Citation9 Our patient had a baseline FSH level of 72.4 IU/l. She would therefore have been erroneously categorised as ovarian hyperthecosis based on these FSH cut-off criteria. The bilateral oophorectomy and the absence of readily demonstrable ovarian tissue on imaging, along with the fact that the hirsutism onset occurred three years after the onset of menopause, made a diagnosis of ovarian hyperthecosis unlikely.
Steroid cell tumour NOS are usually benign and often too small to be detected with radiological imaging, including MRI.Citation7,Citation9 The fact that the pelvic US revealed the abnormality in our patient highlights the fact that radiological investigation remains operator dependent and underpins the importance of skilled ultrasonographers in settings with limited access to MRI. Steroid cell tumour NOS presents at any age, with 43 years the mean age at presentation.Citation10,Citation11 In the majority of patients (75%), these tumours are functional and present with a clinical picture of hormonal excess.Citation11 The most common presentation is with virilisation (56–77%) due to testosterone overproduction and as noted in our case. The clinical presentation of androgen excess is modified by age and masked in postmenopausal women who seldom seek medical attention due to associated menstrual irregularities. Oestradiol secretion occurs in 6–23% of patients with these tumours and hyperoestrogenemia can result in irregular menstruation or postmenopausal bleeding.Citation11,Citation12 Cushing’s syndrome, due to ectopic ACTH or CRH production, is described in up to 10% of patients.Citation11 Our patient did not develop any overt clinical features of Cushing’s syndrome and cortisol excess was also excluded biochemically.
Histopathology remains the gold standard for the diagnosis of steroid cell tumours NOS. Characteristically these tumours are benign, well circumscribed and non-calcified with a lobulated appearance. The tumours are also typically solid with a yellow or orange section surface because of intracytoplasmic lipids. On microscopic examination, the tumour cells typically have a nested arrangement but can be organised into columns or cords resembling adrenal zona glomerulosa and zona fasciculata. Cytologically, cells are polygonal or round with distinctive cell borders, central nuclei and prominent nucleoli. The cytoplasm varies from eosinophilic to clear and vacuolated. The absence of cytoplasmic Reinke’s crystals helps differentiate this tumour from the Leydig cell neoplasm. Immunohistochemical staining helps to distinguish steroid cell tumours from other stromal cell tumours as shown in the index case (see and ). Based on the limited data available, the recommended management of steroid cell tumour NOS is primarily surgical.
Conclusion
This case emphasizes the importance of having a high index of suspicion for an occult testosterone-secreting ovarian tumour in a virilised postmenopausal woman without an obvious ovarian mass on radiologic studies. Prompt surgical management can lead to complete resolution of symptoms and normalisation of testosterone levels as illustrated in our case.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Candice Sher-Lockitz http://orcid.org/0000-0002-6657-7801
Annelize Barnard http://orcid.org/0000-0001-6450-8354
Viju Thomas http://orcid.org/0000-0002-9125-8079
Magda Conradie http://orcid.org/0000-0003-3092-4098
References
- Escobar-Morreale HF, Sanchon R, San Millan JL. A prospective study of the prevalence of nonclassical congenital adrenal hyperplasia among women presenting with hyperandrogenic symptoms and signs. J Clin Endocrinol Metab. 2008;93:527–533. https://doi.org/10.1210/jc.2007-2053
- Ferriman D, Gallwey JD. Clinical assessment of body hair growth in women. J Clin Endocrinol Metab. 1961;21:1440–1447. https://doi.org/10.1210/jcem-21-11-1440
- Martin KA, Chang RJ, Ehrmann DA, et al. Evaluation and treatment of hirsutism in premenopausal women: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008;93:1105–1120. https://doi.org/10.1210/jc.2007-2437
- Brojeni NR, Salehian B. Androgen secreting ovarian tumors. MOJ Womens Health. 2017;5(6):00145.
- O’Driscoll JB, Mamtora H, Higginson J, et al. A prospective study of the prevalence of clear-cut endocrine disorders and polycystic ovaries in 350 patients presenting with hirsutism or androgenic alopecia. Clin Endocrinol (Oxf). 1994;41:231–236. https://doi.org/10.1111/j.1365-2265.1994.tb02535.x
- Rothman MS, Wierman ME. How should postmenopausal androgen excess be evaluated? Clin. Endocrinol. (Oxf). 2011 Aug;75(2):160–164. https://doi.org/10.1111/j.1365-2265.2011.04040.x
- Wang PH, Chao HT, Lee RC, et al. Steroid cell tumors of the ovary: clinical, ultrasonic, and MRI diagnosis—a case report. Eur J Radiol. 1998 Feb 1;26(3):269–273. https://doi.org/10.1016/S0720-048X(96)01133-3
- Ibanez L, Hall JE, Potau N, et al. Ovarian 17-hydroxyprogesterone hyperresponsiveness to gonadotropin-releasing hormone (GnRH) agonist challenge in women with polycystic ovary syndrome is not mediated by luteinizing hormone hypersecretion: evidence from GnRH agonist and human chorionic gonadotropin stimulation testing. J Clin Endocrinol Metab. 1996 Nov 1;81(11):4103–4107.
- Yance VR, Marcondes JA, Rocha MP, et al. Discriminating between virilizing ovary tumors and ovary hyperthecosis in postmenopausal women: clinical data, hormonal profiles and image studies. Eur J Endocrinol. 2017 Jul 1;177(1):93–102. https://doi.org/10.1530/EJE-17-0111
- Sood N, Desai K, Chindris AM, et al. Symptomatic ovarian steroid cell tumor not otherwise specified in a post-menopausal woman. Rare Tumors. 2016 Jun 28;8(2):69–72. https://doi.org/10.4081/rt.2016.6200
- Hayes MC, Scully RE. Ovarian steroid cell tumors (not otherwise specified). A clinicopathological analysis of 63 cases. Am J Surg Pathol. 1987 Nov;11(11):835–845. https://doi.org/10.1097/00000478-198711000-00002
- Mamoojee Y, Ganguri M, Taylor N, et al. Clinical case Seminar: postmenopausal androgen excess–challenges in diagnostic work-up and management of ovarian thecosis. Clin. Endocrinol. (Oxf). 2018 Jan;88(1):13–20. https://doi.org/10.1111/cen.13492