Abstract
Metastasis of a neuroendocrine tumour (NET) to the orbit is a rare occurrence, with published data limited to a few case reports. Orbital involvement usually presents with proptosis and decreased ocular mobility. Timing, in relation to the presentation of the primary tumour (PT), varies. The authors report a case of a 58-year-old female whose clinical presentation of orbital metastases preceded clinical suspicion of a midgut NET. Her presentation was with severe headaches and bilateral ptosis, histologically proven to be due to orbital muscle infiltration. The PT location was determined from the histopathological markers on orbital muscle biopsy, which suggested a gastrointestinal origin. Abdominal computed tomography (CT) was in keeping with a midgut NET with features characteristic of a desmoplastic reaction. Additional CT findings were widespread metastases, corroborated by nuclear imaging. The patient developed carcinoid syndrome and subsequently underwent surgical resection of the PT due to acute bowel obstruction. Her carcinoid symptoms and ocular findings improved on continued somatostatin receptor analogue therapy, but she sadly died within one month following surgery.
Introduction
Globally, there has been an increase in the prevalence of neuroendocrine tumours (NETs). This is attributed to increased clinical vigilance and improved diagnostic techniques.Citation1 The clinical presentation of NETs varies. Local experience of a multidisciplinary neuroendocrine team at a tertiary, public sector hospital (TH) in the Western Cape province, South Africa, is that patients present late, either with bowel obstruction or with carcinoid syndrome attributable to hepatic metastases with release of polypeptides, biogenic amines and prostaglandins. A further presentation is to surgery, with liver metastases, the diagnosis being made on immunohistochemistry and biopsy.
NETs derive from enterochromaffin cells of the gastrointestinal and bronchial tract. They are usually indolent tumours, with a wide five-year survival range documented (57–95% if localised; 38–67% for distant metastases).Citation1,Citation2 To predict the clinical behaviour, NETs are categorised based on their anatomical site of origin, degree of differentiation and proliferation index. The anatomical site of origin of the primary tumour (PT) can be foregut (lung, bronchus, stomach, proximal duodenum, pancreas), midgut (distal duodenum to ascending colon) or hindgut (transverse colon, descending colon, rectum), the commonest being midgut. According to the 2017 Wold Health Organization (WHO) statement, NETs are classified as either well differentiated or poorly differentiated based on their morphological growth pattern and proliferation indices, namely Ki-67 and mitotic rate (MR). Well-differentiated tumours are classified as Grade 1 (Ki-67 < 3% and MR < 2), Grade 2 (Ki-67 3–20% and MR 2–20) or Grade 3 (Ki-67 > 20% and MR > 20).Citation3 Poorly differentiated NETs, also referred to as neuroendocrine carcinomas, have a Ki-67 index > 20% and a MR exceeding 20/10 per high power field (HPF).Citation4
Midgut NETs mainly metastasise to the liver, lymph nodes and bone. The risk of metastases increases with the size of the PT, with tumours in excess of 1 cm having a greater risk.Citation5 Orbital metastasis from primary midgut origin NETs is rare, with around 70 cases described. A case of orbital metastasis in a female patient with bilateral ptosis that preceded diagnosis of the primary midgut NET is presented.
Case report
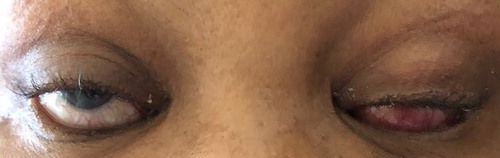
A 58-year-old female with hypertension and HIV (well controlled, with a suppressed viral load on antiretroviral therapy) presented to the TH ophthalmology clinic in late 2018. The patient was seeking care for severe, new onset (two to three months) headaches and dizziness. A cavernous sinus thrombosis was suspected based on a relative afferent pupillary defect, with decreased visual acuity and compatible clinical findings (periorbital oedema, chemosis, ptosis and ophthalmoplegia) as depicted in . Optic disc swelling was absent and pupils, although seemingly dilated, had normal responses to light.
Figure 1: Image depicting bilateral ptosis, periorbital oedema and chemosis at initial presentation.
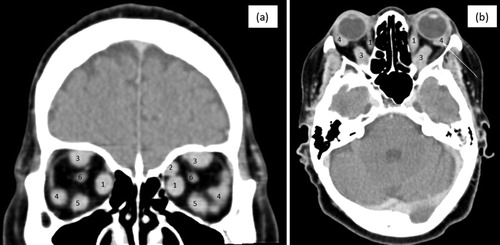
A contrasted computed tomography (CT) brain scan excluded cavernous sinus thrombosis, but noted bilateral enlargement of the extraocular muscles, as illustrated in (a and b). An inflammatory disorder was entertained and corticosteroids were initiated. Owing to a limited response to anti-inflammatory therapy, a right inferior orbital biopsy was performed and the histopathological evaluation showed a well-differentiated NET (Ki-67 3%) with strong immunoreactivity to CDX2, suggestive of the tumour being of mid-gut origin.
Figure 2: (a) and (b): Coronal and sagittal views of a contrasted brain CT illustrating enlargement and marked contrast enhancement of extraocular muscles (EOM) (inferior (5), medial (1) and superior rectus (3) bilaterally, left lateral rectus (4) and, to a lesser extent, right lateral rectus (4). The superior oblique (2) and optic nerve (6) appeared unaffected.)
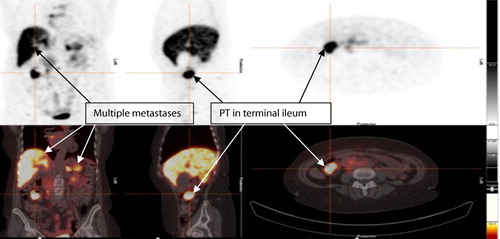
An abdominal CT () and DOTANOC PET () demonstrated multiple metastasis to the liver, pancreas, spleen, bone and right pleura. Asymptomatic intussusception of the distal ileum and characteristic features of a desmoplastic reaction, pathognomonic of NETs, was also present on CT.
Figure 3: Abdominal CT showing intussusception of the distal ileum (A) with no signs of small bowel obstruction and a desmoplastic reaction (B) with typical findings of linear opacities radiating outwards in a ‘spoke wheel’ or stellate pattern, with ‘in-drawing’ of the surrounding tissues.
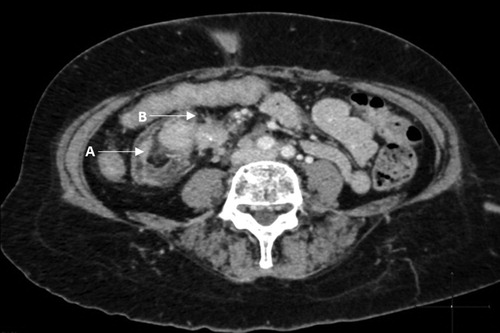
Figure 4: Ga 68 DOTANOC PET showing metastatic lesions and intense uptake of the primary lesion in the terminal ileum, with multiple metastases to liver, spleen and pancreas.
The patient subsequently presented with episodes of facial flushing and diarrhoea and carcinoid syndrome was biochemically confirmed with an elevated 5-hydroxyindoleacetic acid to creatinine ratio of 156 µmol/mmol, near twenty-fold higher than the upper laboratory range of 8.3 µmol/mmol. The significant tumour burden (primary and widespread metastasis) was biochemically confirmed with a chromogranin A level of 4090 ng/ml (normal range < 101.9 ng/ml). An echocardiogram demonstrated mild thickening of the tricuspid valve and moderate tricuspid regurgitation, as well as thickening and regurgitation of the pulmonary valve, features compatible with carcinoid heart disease.
Paraneoplastic myasthenia gravis was also considered in this case. It has been described in patients with NETs and may present with bilateral ptosis,Citation6,Citation7 but was excluded based on the absence of fatigability and undetectable acetylcholine receptor antibodies (< 0.5 nmol/l).
A suspicion of bowel obstruction and clinical deterioration with vomiting and abdominal pain prompted laparotomy. Somatostatin receptor agonist (SSRA) treatment was initiated perioperatively to protect the patient against a carcinoid crisis. At laparotomy a 35 mm polypoid mass in the ileocaecal valve was noted that obstructed the lumen and protruded into the caecum. The mass was resected by right hemicolectomy and primary anastomosis. Extensive intraperitoneal disease involving the proximal superior mesenteric artery and head of pancreas precluded lymph node resection. Histopathology of the polypoid mass confirmed a well-differentiated NET, grade 1 Ki-67 < 2%, with lympho-vascular and perineural invasion. TNM staging was determined as T4N1M1b.
The right-sided ptosis and diarrhoea improved with continued post-surgical SSRA therapy. The headaches also transiently disappeared, but returned within a month following her surgery. The patient’s condition acutely deteriorated and she sadly died one month after discharge.
Discussion
NETs are rare, but more frequently described owing to improved access to and quality of imaging, with only five previously reported cases presenting with unilateral ptosis due to metastasis from midgut NETs.Citation8–12 Our case is the first to present with bilateral ptosis with bilateral involvement of the extraocular muscles. The majority of orbital metastases are to the uveal tract, rather than the orbital space.Citation8 Uveal metastases are associated with NETs of bronchopulmonary origin, with orbital metastases mostly described in patients with NETs of gastrointestinal origin.Citation11 The age of presentation of orbital involvement attributed to NETs is wide (34–80 years with a mean of 61.4 years) and has a slight male preponderance. The most common presenting symptom under these circumstances is proptosis, followed by diplopia and decreased eye motility. The signs and symptoms of the PT in midgut NETs usually precedes orbital involvement and occurs in the presence of other metastasis. Initially, extraocular muscle involvement is unilateral, with the lateral and inferior rectus most commonly affected. Paraneoplastic myasthenia gravis presenting in NETs has been reported. The absence of clinical fatigability and negative acetylcholine receptor antibody in the index case, coupled with extensive infiltration of the periorbital muscles, made a neuromuscular junctional abnormality unlikely.Citation6,Citation13
Focal nodular enlargement of the periorbital muscles on CT/MRI, without invasion of the orbital fat or adjacent osseous structures, indicative of metastases to the extraocular muscle, has been proposed to be specific for metastatic midgut NETs. Das and co-authors propose that a tissue diagnosis and orbital biopsy is unnecessary in this setting.Citation13 The case presented was not known with a NET and the biopsy findings were invaluable in determining the underlying aetiology by allowing immunological staining and appropriately directed further investigations.
The subsequent abdominal CT in our patient was in keeping with radiological findings of a midgut NET typically showing a hypervascular, polypoid or plaque-like mass that is contrast avid. These tumours can also present as asymmetric or concentric bowel wall thickening with calcifications commonly present (in up to 70% of cases). Infiltration of the mesentery results in the pathognomonic radiological finding of a mass with soft tissue spokes radiating towards the small bowel, the so-called desmoplastic reaction. The infiltration can cause retraction with angulation and tethering of the bowel with obstruction.Citation14,Citation15
Guidelines on the management of small bowel NETs propose that, even in the presence of metastasis, morbidity and mortality can be improved by resecting the primary in selected patients.Citation16 Our patient had an intussusception with the PT as the lead point. The ideal aim of surgery is to do a complete oncological resection that includes regional lymphadenectomy. An oncological resection was not possible in the reported case due to distal metastasis. The proposed therapy for well- differentiated NETs with orbital metastasis is surgical debulking with adjuvant beam radiotherapy, peptide receptor radiotherapy or systemic medical therapy.Citation8 There is, however, currently no English literature stating the effect of PT resection on orbital metastases.
The National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program in 2017 showed that patients with metastases of small bowel NETs had a median overall survival of 5.83 years.Citation17 Mehta et al. described a five-year survival of 72% and 10-year survival of 38% for patients presenting with orbital metastasis of a NET, significantly better than other malignancies with orbital involvement.Citation18
Carcinoid syndrome, as evident in our patient, is present in the majority of small bowel NETs with metastases to the liver. The syndrome results from a surge in polypeptides and biogenic amines, especially serotonin and prostaglandins, into the systemic circulation due to a significant increase in tryptophan metabolism. This results in systemic symptoms of flushing, hypertension, diarrhoea and sweating, and causes valvular heart disease.Citation19 The liver typically inactivates these bioactive products produced by the PT, but with liver metastases the inactivation is bypassed allowing vast quantities of active product to enter the systemic circulation. It is postulated that these humeral substances are inactivated by the lungs, therefore so-called carcinoid heart disease typically affects the right side of the heart with fibrotic valvular changes.Citation20–22
SSRAs inhibit excessive production of these compounds and are indicated for symptomatic control in patients with carcinoid syndrome. The use of SSRAs is also recommended to limit tumour burden due to their established antiproliferative effect in well-differentiated NETs. This is evidenced by the improvement in orbital involvement in our patient.Citation23 SSRA therapy is locally available for perioperative use. Despite evidence for effectively addressing the debilitating symptoms of carcinoid syndrome, coupled with the well-documented mortality benefit globally, use of SSRAs for these indications for patients in the public health sector is, however, severely restricted, largely due to cost.
Conclusion
In the context of a known NET, orbital metastases should be high on the differential if ocular muscles are impaired. Classic CT findings may preclude the need for orbital biopsy in patients with known NETs. Histopathology is invaluable in cases presenting primarily with ocular disease. SSRAs addressed the carcinoid syndrome and improved the eye involvement in the index case. Local access to SSRAs remains limited.
Consent
Written consent from the next of kin has been obtained and ethical approval has been awarded.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97(4):934–959. doi: 10.1002/cncr.11105
- Couch DA, O’Halloran HS, Hainsworth KM, et al. Carcinoid metastasis to extraocular muscles: case reports and review of the literature. Orbit. 2003;19(4):263–269. doi: 10.1076/orbi.19.4.263.2656
- Klimstra DS, Modlin IR, Coppola D, et al. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas. 2010;39(6):707–712. doi: 10.1097/MPA.0b013e3181ec124e
- Kim JY, Hong SM. Recent updates on neuroendocrine tumors from the gastrointestinal and pancreatobiliary tracts. Arch Pathol Lab Med. 2016;140(5):437–448. doi: 10.5858/arpa.2015-0314-RA
- Khaw P, Ball D, Duchesne G. Carcinoid tumour of the orbital muscles: A rare occurrence. Australas Radiol. 2001;45(2):179–181. doi: 10.1046/j.1440-1673.2001.00899.x
- Shaygannejad V, Ghasemi M, Rajaee Z. Myasthenia gravis as a presenting feature in a patient with lung cancer: a case report. J Res Med Sci. 2011;16(2):229–232.
- Hermans MAW, Stelten BML, Haak HR, et al. Two patients with a neuroendocrine tumour of the small intestine and paraneoplastic myasthenia gravis. Endocrinol Diabetes Metab Case Reports. 2014;2014(May):140013. doi:10.1530/EDM-14-0013.
- Pusceddu S, Milione M, Ortolani S, et al. Orbital lesions, an exceedingly rare site of neuroendocrine tumor metastasis. J Cancer Metastasis Treat. 2016;2(8):341. doi: 10.20517/2394-4722.2016.41
- Mehta P, Malik S, Adesanya O, et al. Orbital carcinoid metastasis: diverse presentations and value of indium-octreotide imaging. Orbit. 2012;31(6):379–382. doi: 10.3109/01676830.2012.711890
- Fan JT, Buettner H, Bartley GB, et al. Clinical features and treatment of seven patients with carcinoid tumor metastatic to the eye and orbit. Am J Ophthalmol. 1995;119(2):211–218. doi: 10.1016/S0002-9394(14)73875-9
- Yang M, Loh A, Seah LL, et al. Neuroendocrine tumors of the orbit - clinicopathological findings in 3 more cases of this rare entity and review of the literature. Orbit. 2011;30(3):145–149. doi: 10.3109/01676830.2011.569629
- Turaka K, Mashayekhi A, Shields C, et al. A case series of neuroendocrine (carcinoid) tumor metastasis to the orbit. Oman J Ophthalmol. 2011;4:125–128. doi: 10.4103/0974-620X.91268
- Das S, Pineda G, Goff L, et al. The eye of the beholder: orbital metastases from midgut neuroendocrine tumors, a two institution experience. Cancer Imaging. 2018;18(1):1–7. doi: 10.1186/s40644-018-0181-5
- Horton KM, Kamel I, Hofmann L, et al. Carcinoid tumors of the small bowel: a multitechnique imaging approach. Am J Roentgenol. 2004;182(3):559–567. doi: 10.2214/ajr.182.3.1820559
- Ganeshan D, Bhosale P, Yang T, et al. Imaging features of carcinoid tumors of the gastrointestinal tract. Am J Roentgenol. 2013;201(4):773–786. doi: 10.2214/AJR.12.9758
- Capurso G, Rinzivillo M, Bettini R, et al. Systematic review of resection of primary midgut carcinoid tumour in patients with unresectable liver metastases. Br J Surg. 2012;99(11):1480–1486. doi: 10.1002/bjs.8842
- Dasari A, Shen C, Halperin D, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017;3(10):1335–1342. doi: 10.1001/jamaoncol.2017.0589
- Mehta JS, Abou-Rayyah Y, Rose GE. Orbital carcinoid metastases. Ophthalmology. 2006;113(3):466–472. doi: 10.1016/j.ophtha.2005.10.051
- Burke AP, Thomas RM, Elsayed AM, et al. Carcinoids of the jejunum and ileum. Cancer. 1997;79(6):1086–1093. doi: 10.1002/(SICI)1097-0142(19970315)79:6<1086::AID-CNCR5>3.0.CO;2-E
- Bhattacharyya S, Toumpanakis C, Burke M, et al. Features of carcinoid heart disease identified by 2-and 3-dimensional echocardiography and cardiac MRI. Circ Cardiovasc Imaging. 2010;3(1):103–111. doi: 10.1161/CIRCIMAGING.109.886846
- Veenendaal LM, Borel Rinkes IHM, Lips CJM, et al. Liver metastases of neuroendocrine tumours; early reduction of tumour load to improve life expectancy. World J Surg Oncol. 2006;4:1–6. doi: 10.1186/1477-7819-4-35
- Hendrix TR, Atkinson M, Clifton JA, et al. The effect of 5-hydroxytryptamine on intestinal motor function in man. Am J Med. 1957;23(6):886–893. doi: 10.1016/0002-9343(57)90298-X
- Modlin IM, Pavel M, Kidd M, et al. Review article: somatostatin analogues in the treatment of gastroenteropancreatic neuroendocrine (carcinoid) tumours. Aliment Pharmacol Ther. 2010;31(2):169–188.
