Abstract
Background:
Diabetic neuropathy is one of the most common microvascular complications associated with diabetes mellitus. Diabetic peripheral neuropathy (DPN) has been linked to hyperglycaemia and long duration of uncontrolled type 2 diabetes mellitus (T2DM) as measured by glycosylated haemoglobin (HbA1c). To our knowledge the estimated duration between diagnosis and developing DPN and the level of HbA1c have not yet been investigated in Sudanese patients with type 2 DM. Therefore, this study aims to investigate the relationship between the duration of diabetes and HbA1c with nerve conduction velocity (NCV) in patients with type 2 DM.
Methods:
This cross-sectional study recruited 63 male and female patients with T2DM who attended the diabetic outpatient clinic of Academy Charity Teaching Hospital (ACTH) and Alzaytouna Private Hospital for Nerve Conduction Velocity (NCV) and electromyography (EMG) tests. Nerve conduction was done by using ADInstruments PowerLab series 26. SPSS was used to analyse the data and p-value < 0.05 was considered significant.
Results:
The mean duration of DM was 14.7 (± SD 9.24) years and the mean age of participants was 57.71 (± SD 12.2) years. The most common symptom was numbness (50%). Pearson's correlation test revealed a significant negative correlation between HbA1c and nerve conduction velocity (r = 0.4, p < 0.05) and negative significant correlation between the duration and the amplitude (r = 0.35, p < 0.05).
Conclusion:
There is a slowing of nerve conduction velocity in type 2 diabetic patients, which is accelerated by the poor glycaemic control (HbA1c). These findings support the need for tight glycaemic control to avoid drastic neuropathic complications of diabetes.
Background
Diabetes mellitus (DM) is a metabolic disorder which is characterized by an increased risk of microvascular and macrovascular complicationsCitation1 It is characterized by insulin resistance (IR) and relative insulin deficiency. Microangiopathy lesion in type 2 DM has become a common condition among patients and of major concern to diabetologists. It is featured by several biochemical and structural changes in the microvasculature that lead to extracellular matrix protein synthesis and thickening of the capillary basement membrane. These changes are developed as consequences of increased glycation end products, oxidative stress, low grade inflammation and neovascularization of the vasa vasorum, which are directly related to micro- and macrovascular complications. Therefore, patients are prone to long-term damage and failure of various organ systems leading to retinopathy, neuropathy, nephropathy, coronary artery disease and stroke. The risk is directly proportional to both the duration and magnitude of hyperglycaemia. On the other hand, there are some individuals who may have a genetic susceptibility to develop such complications besides other risk factors such as B12 deficiency, excessive alcohol, infections and certain types of cancers such as lymphoma.Citation2 The prevalence of diabetic neuropathy is about 1.91% of all diabetic patients worldwide.Citation3. In Northern Africa, including Morocco, Algeria, Tunisia, Libya, Egypt, Sudan, South Sudan and Western Sahara, diabetic neuropathy was found to range from 21.9% to 60%.Citation4 In 2017, Awadalla estimated the prevalence of diabetic polyneuropathy in Sudan, which was around 68.2%.Citation5 Poor glucose control is one of the risk factors that are strongly related to the progression of diabetic neuropathy in patients with type 2 DM.Citation6 Early screening for symptoms and signs of diabetic neuropathy is important, since it creates a chance to detect the neuropathy at its earliest asymptomatic stages and thus prevent further progression.Citation7 The NCS enables sensory and motor abnormalities associated with neuropathy to be diagnosed even if the dysfunction is subclinical.Citation8 It is important to understand that the nature of diabetic polyneuropathy is controversial as it might be demyelinating and/or axonal. Therefore, some studies have been done to determine whether diabetic polyneuropathy is electrophysiologically axonal, demyelinating, or both. Diabetic neuropathy patients can either have demyelinating disease with or without symptoms of polyneuropathy, or axonal loss that is responsible for most of the symptoms.Citation9 Various preventive methods, such as blood glucose control, improvement of lipid and blood pressure indexes, and the avoidance of cigarette smoking and excess alcohol consumption, are useful for the prevention of DPN or other delayed complications.Citation10 There is increasing concern regarding diabetic neuropathy complications as these are considered a leading cause of disability due to foot ulceration and amputation, gait disturbances and injuries secondary to falls. Such complications severely lower the quality of life of patients and increases health costs associated with diabetes.Citation11 The aim of this study was to explore the relationship between the duration, glycaemic control and the DPN in T2DM patients who underwent nerve conduction measurement using a nerve conduction test.
Material and methods
This study was a descriptive cross-sectional facility-based study. The study included all diabetic patients. Both diagnosed male and female patients with T2DM were recruited from the diabetic outpatient clinic in the Academy Teaching Hospital (ACTH) and Alzaytouna Private Hospital. The Research Ethical Committee institute of the University of Medical Sciences and Technology (UMST) approved the present study and verbal consent was taken from all participants following full disclosure of the procedure and assured confidentiality.
Inclusion criteria
Age: 18–80 years old.
Diagnosed with type 2 DM.
Exclusion criteria
Alcoholics, hypertensives, smokers, pregnant females.
Diabetic subjects having acute diabetic complications (DKA or DSF).
Patients with a history of myopathy, neuromuscular diseases or inherited neuropathy.
Sixty-three patients were involved in this study selected by simple random sampling technique. In the outpatient clinics of both ACTH and the clinic of Alzaytouna Private Hospital, a questionnaire including socio-demographic data was filled in by patients under the supervision of investigators. The nerve conduction study (NCS) was conducted by a trained technician using the ADInstruments’ PowerLab device (ADInstruments – Australia, Bella Vista, NSW, Australia) provided by the hospital's skills lab in the Physiology Department. Important precautions and procedures were taken prior to the NCS. The patient was asked to remove any metal accessories and was asked if he/she had any implanted metal devices, e.g. a pacemaker. A measuring tape was used to measure the distance from the elbow to the wrist in a position of full extension of the left forearm. The patients were fully informed that they would feel a small electric shock with stimulation in this procedure. The electrodes were then placed on the patient's left palm to stimulate the left median nerve at the wrist and record muscle activity from a muscle on the thumb, the abductor pollicis brevis. Before placing the stimulating bar, a reading was taken and a flat line indicated correct positioning of electrodes and absence of artefacts. Gel was then applied to the two silver pads of the stimulating bar, the stimulating bar was placed over the patient's median nerve at the wrist and this was followed by a shock felt by the patient, and multiple readings were taken. This was repeated until a good wave was seen on the screen. Another set of readings were taken from the median nerve on the elbow by following the same steps but by placing the stimulating bar on the cubital fossa opposite to the elbow. Wrist and elbow latencies were recorded, and the velocity and amplitude of the left median nerve were calculated by the application.
Statistical analysis
Statistical analysis was done by using the Statistical Package for the Social Sciences software (SPSS version 22; IBM Corp, Armonk, NY, USA). Pearson's correlation coefficient was used to find the strength and direction of correlation between nerve conduction parameters, duration and HbA1c. The statistical significance of the test was set at a p-value of < 0.05.
Results
The mean age of type 2 DM patients was (mean ± SD) 57.71 ± 12.2 years. The mean duration of type 2 DM, in years, was (mean ± SD) 14.67 ± 9.24 years ().
Table 1: Basic characteristics of patients by age and duration of DM among type 2 DM (n = 63)
The percentage of females in the study population was 55.6% and the percentage of males was 44.4% ().
Table 2: Distribution of participants by gender (n = 63)
The commonest symptom among participants was numbness (50%), followed by burning pain (26.4%), muscle weakness (15.3%) and stabbing pain (8.3%) ().
Table 3: Frequencies and percentages of symptoms in the study group (n = 63)
HbA1c showed a mean of (mean ± SD) 7.61 ± 1.76 with a minimum of 6 and a maximum of 13 (). The nerve conduction velocity (NCV) showed a mean of (mean ± SD) 51.5 ± 12.1 m/s and a minimum of 26.7 m/s and a maximum of 82.4 m/s. The amplitude showed a mean of (mean ± SD) 7.6 ± 4.04 mV with a minimum of 0.66 mV and a maximum of 20.30 mV.
Table 4: HbA1c, amplitude and nerve conduction velocity of study participants
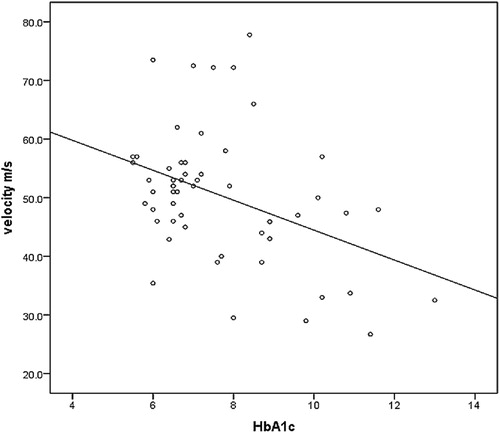
shows a significant negative correlation between HbA1c and nerve conduction velocity (r = −0.4, p = 0.002).
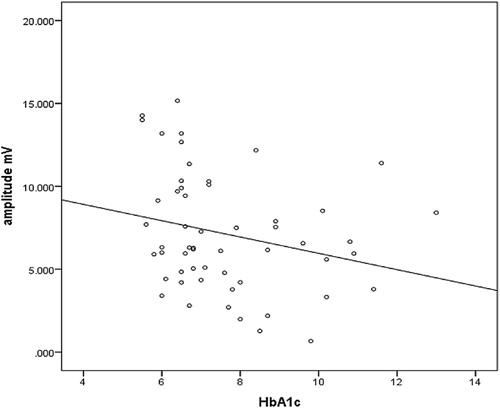
shows an insignificant negative correlation between HbA1c and amplitude (r = −0.2, p = 0.4).
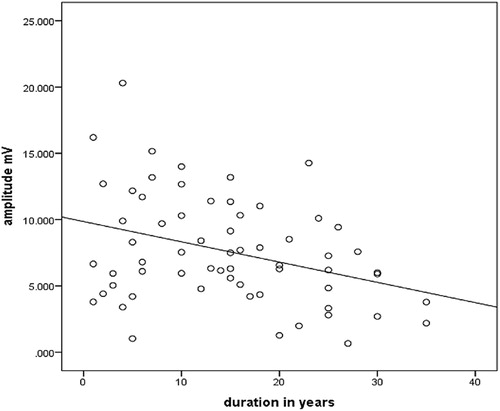
There was a significant negative correlation between amplitude and duration in years of Type2 DM (r = −0.3, p = 0.005) ().
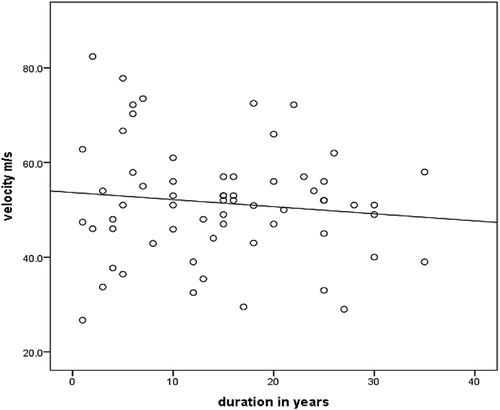
There was no significant correlation between nerve conduction velocity and duration of type 2 DM (r = 0.2, p = 0.07) ().
Discussion
The aim of this study was to explore the relationship between NC with duration and HA1c in diabetic patients. There is a consensus among diabetologists that non-invasive and simple tools like NCS can be clinically used in practice for asymptomatic stages of the disease in favour of early detection of DPN, which will result in fewer diabetic complications such as diabetic foot ulcer and amputation. In Sudan, poor glycaemic control and the high diabetic complication costs can be reduced by screening diabetics for neuropathy using regular HbA1c levels and nerve conduction studies in the subclinical stage. According to the results, the commonest presenting symptoms of neuropathy in T2DM patients in our study were numbness and burning pain, numbness being the most common (50%), which corroborates a study done by Qureshi MS et al. in 2017 in Islamabad, Pakistan.Citation12
The prevalence of diabetic neuropathy in this study was 49.21%, which falls within the ranges reported by other studies (21.9% to 60%).Citation4 On the other hand, this result was slightly higher than the results of the study conducted in the Middle East and North Africa (MENA) region in 2018.Citation13
The estimation of NCV and the HbA1c levels in diabetics is helpful in identifying the risk category for diabetic neuropathy, which is considered one of the main causes for morbidity among diabetes mellitus patients.
In this study HbA1c has a significant negative correlation with nerve sensory NCV, which is similar to a number of studies like those conducted in India in 2019Citation14, the UK in 2016Citation15, China in 2018Citation16, another study in India in 2016Citation17 and in 2017 study carried out in Sudan.Citation5
The pathophysiological mechanism behind the microvascular neuropathy in diabetic patients is thought to be caused by activation of aldose reductase enzyme. Therefore, aldose reductase activation can reduce nitric oxide (NO) synthesis, leading to vasoconstriction, prolonged hypoxia and ischaemia, damage to the nerves and thus a reduction in NCV. Low NO in diabetic patient is also can be caused by the metabolic disturbance hyperglycaemia.Citation18 On the other hand, uncontrolled hyperglycaemia results in increased formation of sorbitol leading to its accumulation, which is also neurotoxic, and this in turn decreases nerve conduction velocity, and therefore a reduction in sorbitol can be beneficial to increase NCV.Citation19
Glycosylated haemoglobin has an insignificant correlation with amplitude in this study; this is contradicted by the Magnus Peterson et al. study, which revealed an association between sural nerve, amplitude and HbA1c levels, but the sural nerve was used rather than the median nerve in that study.Citation20
Decreased amplitude was more pronounced than decreased conduction velocity, and this justifies that axonal degeneration is an earlier and more prominent effect of hyperglycaemia than demyelination.Citation20 This was contrary to the study by Yun-Ru Lai et al., which found that those with higher HbA1c levels showed lower amplitudes and reduced nerve conduction velocity in tested nerves, and higher HbA1c even lowers nerve conduction velocity in the sural nerveCitation21
According to our results there is no signification correlation between NCV and duration of DM. On the other hand, a cross-sectional study done in 2017 in India has concluded that increasing duration of diabetes was a significant risk factor for abnormal nerve conduction velocity, but it agrees that age has no statistical significance in terms of velocity.Citation22 In another Indian study conducted in 2018, the result was that there was a significant decrease in the NCV among the diabetic population aged five years or more compared with those with duration of less than five years, concluding the that risk of complications of neuropathy increases with increasing duration and severity of hyperglycaemia.Citation23
In this study the amplitude has significant negative correlations with duration of DM; this result is similar to a study that was conducted by de Souza et al. (Citation2015), which concluded that nerve conduction changes in DPN is correlating with clinical features and long-term glycaemic control.Citation24
This finding was opposed by Kim et al., in a 2018 study, which concluded that there was a non-significant change in amplitude among the diabetic populations with a longer duration,Citation25 but, in general, the commonest abnormality in diabetics is the reduction of amplitude of motor or sensory nerve action potential because of axonopathy, caused by the different mechanisms explained previously.
In conclusion, the results of this study indicate that the median nerves are affected in type 2 diabetic mellitus patients, with a significant drop in sensory conduction velocity in patients with poor glycaemic control (HbA1c) and a reduction of amplitude with longer duration of diabetes. Therefore, nerve conduction is impaired in patients with long duration of DM and poor glycaemic control. Hence, NCS should be considered as a screening tool with regular HbA1c tests for such patients to prevent the progression of neuropathy together with dietary control, medications and lifestyle modification.
Ethical permission
Ethical permission was sought from the research technical and ethical committee at the Faculty of Medicine, UMST, and the Academy Teaching Hospital administration. Patients’ privacy and confidentiality were maintained. Verbal informed consent was taken from the patients, or their legal representatives in case of disability, to fill out the questionnaire, view their records and to perform the nerve conduction study. The subjects’ right of withdrawal was preserved. All data obtained from the patients are kept confidential with limited access by the research team only.
Acknowledgement
We would like to acknowledge the efforts of all participants, Dr Asmaa, Dr Mounkeila Noma, Dr Eltaher Obeid, Dr Raid and Dr Ahmed Karar for their support and permission to conduct this research in their clinics, and for helping us throughout this research.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Zaccardi F, Webb DR, Yates T, et al. Pathophysiology of type 1 and type 2 diabetes mellitus: a 90-year perspective. Postgrad Med J. 2016;92(1084):63–9.
- Chawla A, Chawla R, Jaggi S. Microvasular and macrovascular complications in diabetes mellitus: distinct or continuum? Indian J Endocrinol Metab. 2016;20(4):546.
- Bhutani J, Bhutani S. Worldwide burden of diabetes. Indian J Endocrinol Metab. 2014;18(6):868.
- Bos M, Agyemang C. Prevalence and complications of diabetes mellitus in Northern Africa, a systematic review. BMC Public Health. 2013;13(1):387.
- Awadalla H, Noor SK, Elmadhoun WM, et al. Diabetes complications in Sudanese individuals with type 2 diabetes: overlooked problems in sub-Saharan Africa? Diab Metab Syndr Clin Res Rev. 2017;11:S1047–51.
- Feldman EL, Callaghan BC, Pop-Busui R, et al. Diabetic neuropathy. Nat Rev Dis Primers. 2019;5(1):41.
- Pop-Busui R, Boulton AJ, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136–54.
- Picon AP, Ortega NR, Watari R, et al. Classification of the severity of diabetic neuropathy: a new approach taking uncertainties into account using fuzzy logic. Clinics. 2012;67(2):151–6.
- Valls-Canals J, Povedano M, Montero J, et al. Diabetic polyneuropathy. axonal or demyelinating? Electromyogr Clin Neurophysiol. 2002;42(1):3–6.
- Boulton AJ, Vinik AI, Arezzo JC, et al. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005;28(4):956–62.
- Juster-Switlyk K, Smith AG. Updates in diabetic peripheral neuropathy. F1000Res. 2016;5:738.
- Qureshi MS, Iqbal M, Zahoor S, et al. Ambulatory screening of diabetic neuropathy and predictors of its severity in outpatient settings. J Endocrinol Invest. 2017;40(4):425–30.
- Garoushi S, Johnson MI, Tashani OA. Point prevalence of painful diabetic neuropathy in the Middle East and North Africa region: a systematic review with meta-analysis. Libyan J Med Sci. 2018 Jul 1;2(3):85.
- Masood A, Rizvi SA, Siddiqi SS, et al. A study on effects of HbA1c levels on nerve conduction velocity in type 2 diabetes mellitus patients. Int J Cur Res Rev. 2019;11(03):6–8.
- Themistocleous AC, Ramirez JD, Shillo PR, et al. The Pain in Neuropathy Study (PiNS): a cross-sectional observational study determining the somatosensory phenotype of painful and painless diabetic neuropathy. Pain. 2016;157(5):1132–45.
- Su JB, Zhao LH, Zhang XL, et al. Hba1c variability and diabetic peripheral neuropathy in type 2 diabetic patients. Cardiovasc Diabetol. 2018;17(1):47.
- Sunandini R, Somani SG. Median nerve conduction velocity as a tool to detect subclinical neuropathy in type II diabetes mellitus. Int J Physiol. 2016;4(1):103–9.
- Stevens MJ, Dananberg J, Feldman EL, et al. The linked roles of nitric oxide, aldose reductase and, (Na+, K+)-ATPase in the slowing of nerve conduction in the streptozotocin diabetic rat. J Clin Invest. 1994;94(2):853–9.
- Greene DA, Arezzo JC, Brown MB, Zenarestat Study Group. Effect of aldose reductase inhibition on nerve conduction and morphometry in diabetic neuropathy. Neurology. 1999;53(3):580.
- Peterson M, Pingel R, Lagali N, et al. Association between HbA1c and peripheral neuropathy in a 10-year follow-up study of people with normal glucose tolerance, impaired glucose tolerance and type 2 diabetes. Diabetic Med. 2017;34(12):1756–64.
- Lai YR, Chiu WC, Huang CC, et al. Hba1c variability is strongly associated with the severity of peripheral neuropathy in patients with type 2 diabetes. Front Neurosci. 2019;13:90.
- Shah A, Shah D. To study clinical profile of peripheral neuropathy in type 2 diabetes mellitus using nerve conduction velocity. J Integr Health Sci. 2017;5(2):69.
- Chethan K, Sowjanya T. A study on the effect of duration of type 2 diabetes mellitus on nerve conduction velocity. Natl J Physiol Pharm Pharmacol. 2018;8(12):1622–4.
- de Souza RJ, de Souza A, Nagvekar MD. Nerve conduction studies in diabetics presymptomatic and symptomatic for diabetic polyneuropathy. Journal of Diabetes and its Complications. 2015 Aug 1;29(6):811–7.
- Kim SE, Park KM, Park J, et al. Vascular factors and neuropathy in lower limb of diabetic patients. J Clin Neurosci. 2019;59:130–5.