Abstract
Objectives:
To describe disease management patterns and associated outcomes in patients with type 2 diabetes initiating a second-line glucose-lowering therapy in routine clinical practice in South Africa.
Design:
Non-interventional observational study.
Setting:
General and specialist private practices.
Subjects
Patients with diabetes initiating second-line glucose-lowering therapy.
Outcome measures:
Variables collected at baseline and at 6-, 12- and 24-month follow-up visits included sociodemographics, first- and second-line glucose-lowering treatments and other medications, reasons for change in diabetes therapy, HbA1c target set by the attending clinician at the time of change, comorbidities and health-related quality of life (HRQoL).
Results:
Baseline data were collected for 519 patients (69% female). Mean age was 54.6 years and mean time since initial diagnosis was 7.5 years. Mean HbA1c at baseline was 9.0% and the most common second-line treatment approach was to combine metformin with a sulphonylurea. Median HbA1c and median fasting glucose measurements were marginally lower at 24 months than at baseline (8.0% vs. 8.4%, and 8.5 mmol/l vs. 8.8 mmol/l, respectively). Only approximately 5% of patients had had their diabetes medication changed at any time after the baseline visit.
Conclusions:
Management of type 2 diabetes mellitus in private practice in South Africa is suboptimal.
Introduction
Diabetes mellitus is one of the largest global health emergencies of the twenty-first century and among the top 10 causes of death worldwide.Citation1 In Africa the number of people with diabetes is approximately 19 million and, with an ageing population, economic development, increasing urbanisation, declining dietary standards and physical activity, that is expected to increase to more than 40 million in 2045.Citation1,Citation2
South Africa has one of the highest prevalence rates of diabetes in sub-Saharan Africa. The International Diabetes Federation estimates that there are approximately 4.6 million South African adults with diabetes, about half of whom remain undiagnosed.Citation1,Citation2
Poorly managed diabetes leads to serious complications, disability, poor quality of life and early death. It is a leading cause of cardiovascular disease, blindness, kidney failure and lower limb amputation.Citation1 In South Africa, diabetes is the second leading cause of death and in 2019 almost 90 000 South Africans died from diabetes-related causes.Citation1,Citation3
The most effective way to prevent or reduce diabetes-related morbidity and mortality is meticulous control of glycaemia, blood pressure and dyslipidaemia, and regular examinations to facilitate timeous intervention for complications.Citation2 However, glycaemic control remains poor worldwide.Citation4,Citation5 Studies from Europe, Australia, China and the USA report that approximately 40% of people receiving treatment for diabetes fail to achieve the recommended target of HbA1c < 7%.Citation6–11 In Africa, India and the Middle East control rates are reportedly even lower, with 60% to > 80% not achieving target HbA1c.Citation12–22 In South Africa, even in tertiary centre specialist clinics, the proportion of people with diabetes whose blood glucose is well controlled is alarmingly low. At best, only approximately 1 out of every 4 patients with type 2 diabetes achieves HbA1c < 7%.Citation23–34
However, currently, there are few or no data on usual prescribing practices for patients with diabetes in South Africa.
DISCOVER is a worldwide study to investigate management patterns for type 2 diabetes in everyday clinical practice across different regions and countries. The objectives are to describe clinical evolution in patients with type 2 diabetes who are starting a second-line glucose-lowering therapy (defined as adding a glucose-lowering agent or switching between therapies) after failure of first-line oral treatment with a monotherapy, dual therapy or triple therapy; determinants of treatment patterns at baseline and thereafter; and the associations between treatment patterns and a broad range of outcomes, including glycaemic control, changes in bodyweight, blood pressure (BP) and lipid profile, hypoglycaemic episodes, incidence of diabetes-related complications, patient-reported outcomes and healthcare resource utilisation.Citation35 Here we report the baseline characteristics and 24-month outcomes for the South African cohort of DISCOVER.
Study design
The design of the DISCOVER study has been described in detail elsewhere.Citation35 It is a multinational, prospective, 3-year, observational, longitudinal, non-interventional study involving 15 992 patients in 38 countries across six continents. Eligible patients are adults (age ≥ 18 years) with type 2 diabetes initiating a second-line glucose-lowering treatment (add-on or switching) after a first-line oral treatment. Eligible patients were invited to participate by their doctor (primary care physicians, diabetologists, endocrinologists, cardiologists and other specialists). Exclusion criteria include type 1 diabetes, pregnancy, initiation of dual therapy after having previously received two different lines of monotherapy, first-line treatment with insulin or another injectable agent, other illness or condition that would compromise three-year follow up.
Data were collected at baseline (initiation of second-line therapy) using a standardised electronic case report form and transferred to a central database via a web-based data capture system. Some data were collected retrospectively from medical records. Variables collected included sociodemographics, first- and second-line glucose-lowering treatments and other medications, reasons for change in diabetes therapy, HbA1c target set by the attending clinician at the time of change, and comorbidities. Health-related quality of life (HRQoL) was assessed using the Short-Form version 2 (SF-36v2) Health Survey. The SF-36 consists of 36 items comprising 8 domains that measure the extent to which physical and/or mental health problems affect physical, emotional and social aspects of quality of life. Each domain yields a percentage ranging from 0 (worst possible health) to 100 (best possible health), such that higher scores indicate better HRQoL.Citation36
In accordance with the observational nature of the study, methods to measure HbA1c and glucose were those routinely used by the individual practices and diagnosis of complications and comorbidities was dependent on the attending clinician. There was no external independent adjudication of events.
Results
Baseline data
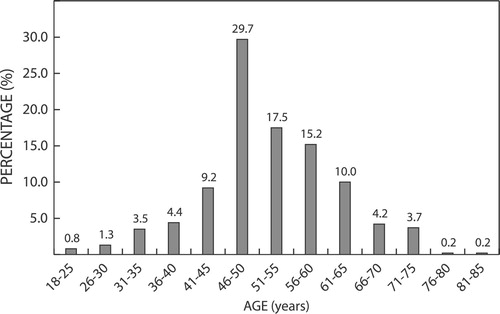
Baseline data for the South African cohort were collected from 519 patients enrolled by a general practitioner/family doctor (72.2%), endocrinologist/diabetologist (11.1%), specialist physician (internist) (5.6%) or cardiologist (5.6%). The majority of enrolled patients were being treated in the private healthcare sector (94%). Data collection was variable and the majority of patients had incomplete data. Demographic and other baseline characteristics are shown in and and .
Figure 2: First-line and second-line diabetes medications at baseline visit. Met: metformin; SU: sulphonylurea; DPP4i: dipeptidyl peptidase-4 inhibitor; TZD: thiazolidinedione. *Patients on insulin may also have received oral therapy.
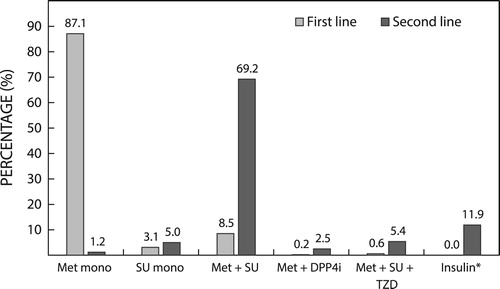
Figure 3: Most common co-prescribed medications at baseline and at 24-month follow-up. ACEi: angiotensin converting enzyme inhibitor; BB: beta blocker; CCB: calcium channel blocker.
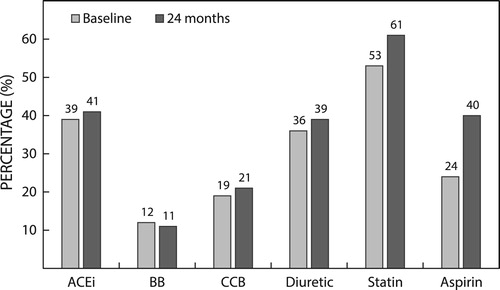
Table 1: Baseline demographics and vital data
Table 2: Metabolic parameters at baseline and during follow-up
The mean time since initial diagnosis of diabetes mellitus was 7.5 (±6.0) years. Mean baseline HbA1c was 9.0% ± 2.1, with a median value of 8.4% (interquartile range, IQR 7.5% to 10.1%). Macrovascular and microvascular pathologies were reported in 24% and 10.8%, respectively, and nonvascular pathologies were reported in 24%. In total, 67% of patients had a prior diagnosis of hypertension and 51% had a prior diagnosis of hyperlipidaemia. Previous episodes of major and minor hypoglycaemic events were reported in 1% and 6% of patients, respectively.
Overall, including monotherapy or combination therapy, 96% of patients were receiving metformin as first-line therapy and approximately 11% were receiving a sulphonylurea (SU). The most common second-line approach was to combine metformin with an SU ().
The most common reasons given for changing first-line glucose-lowering therapy were lack of efficacy (96%), weight gain or other side effect (2%), patient convenience (2%) and affordability (2%). At initiation of a new therapy, 67% of patients had an HbA1c target set, of whom 43% were specifically aware of their HbA1c target.
Mean scores (out of a total of 100) across the subdomains of SF-36 ranged from 44 to 53.
24-month data
At 24 months, data were available for 466 (88%) patients. Eleven patients had died, including 4 from cardiovascular causes, 2 from infection, 1 from kidney disease and 4 unspecified.
In the remaining patients, body mass index, waist circumference, and systolic and diastolic blood pressures remained largely unchanged from the values recorded at baseline. From baseline to 24 months, incident hypertension and hyperlipidaemia were diagnosed in a further 135 (26%) and 83 (16%) patients, respectively.
Mean HbA1c, mean fasting glucose and random glucose measurements were lower at 24 months than at baseline (8.3% vs. 9%, 8.7 mmol/l vs. 9.0, and 10 mmol/l vs. 16 mmol/l, respectively) ().
Despite failing to meet treatment targets, at each of the 6-, 12- and 24-month follow-up visits only approximately 5% of patients had had their diabetes medication changed at any time after the baseline visit. At the 24-month follow-up, the most common treatment discontinuations were a sulphonylurea (0.9%), sulphonylurea plus thiazolidinedione (0.9%) or basal or premix insulin (0.6%), whereas the most common treatment addition was basal insulin (2.8%). The most common reasons given for changing treatment regimen were efficacy (70%), poor adherence (9%), weight issues (9%), hypoglycaemia or side effects (9%), affordability (4%) and physician preference (4%).
A single major hypoglycaemic event was reported for only one patient at each of the follow-up visits. Minor hypoglycaemic events were reported for 13 patients (3.8%) at 6 months and 11 patients (2.5%) each at 1 and 2 years. There were few other incident diabetes complications. At the 24-month follow-up, recorded events included 51 patients with urinary tract infections, 18 with depression, 13 with peripheral neuropathy, 4 with chronic kidney disease, 4 with retinopathy and 1 with coronary artery disease.
Physical functioning and mental health summary scores on the SF-36v2 remained unchanged from baseline at 24 months (45.7 vs. 46.4 and 48.2 vs. 47.2, respectively).
Discussion
The South African DISCOVER population represents a diverse group of individuals from different ethnic groups and educational backgrounds being treated mainly in the private healthcare sector. Over two-thirds of the study population is female, which is disproportionately high in comparison with other South African studies of people being treated for diabetes and also in the DISCOVER population overall, in which 46% of the patients are female.Citation4,Citation24–27,Citation34
At baseline, the majority of patients had at least one comorbidity, predominantly hypertension and/or hyperlipidaemia, and those prevalences are similar to those reported previously.Citation24–27,Citation29,Citation30,Citation32,Citation34,Citation37 The prevalence of hypertension and hyperlipidaemia was consistent with the proportion of patients receiving any antihypertensive medication and statins, suggesting that pharmacotherapy is usually prescribed to manage these conditions when they are diagnosed.
Compared with previous published reports of South African patients, the baseline prevalence of macro- and microvascular complications in this DISCOVER population, with a mean time since diagnosis of 7.5 years, was low. However, since no specific tests were conducted to establish these findings, which were documented from history only, it is possible that the actual prevalence of complications may have been underestimated. In patients with a mean age of 63 years attending a state outpatient clinic (duration of diabetes not specified) the prevalence of coronary artery disease was 14.3%, nephropathy 11.7%, neuropathy 7.1% and retinopathy 6.3%.Citation24 In patients with a mean duration of diabetes of 12 years being treated at outpatient clinics in the State sector (mean age 58 years), and private sector (mean age 63 years) nephropathy was reported in 9.7% and 8.9%; neuropathy in 11.8% and 17.8%; retinopathy in 13.9% and 18.5%; and cardiovascular disease in 16% and 15.8%, respectively.Citation38
In a cross-sectional study of 50 institutional/private clinics, in patients with a mean time since diagnosis of 9 years and mean age 56 years, the prevalence of diabetic peripheral neuropathic pain was 30%, autonomic neuropathy 5%, nephropathy 11% and retinopathy 12%.Citation37 Furthermore, in the DISCOVER population, incident complications, including hypoglycaemia, were extremely uncommon during the two years’ follow-up. Data were relatively complete for these outcomes. Reasons for these discrepancies are unclear, except that the South African DISCOVER population was younger than that in the first study and had a shorter mean time since diagnosis in comparison with those in the second and third studies. Ethnicity may also play a role. It is notable that approximately 97% of the DISCOVER population self-reported being of either Black, Asian (Indian) or mixed ancestry and fewer than 3% were Caucasian (White). Pinchevsky reported that there are considerable differences between South African ethnic groups in the prevalence and incidence of diabetes-related complications ().Citation27 In his cohort, with a mean time since diagnosis of approximately 10–14 years, diabetes-related complications were considerably less common at baseline and after 4 years’ follow-up among the Black and mixed-ancestry groups (which together comprised 63% of the South African DISCOVER population).
Table 3: Prevalence of diabetes-related complications by ethnic group in patients attending the Charlotte Maxeke Johannesburg Academic Hospital in 2009 (data from Pinchevsky et al.)Citation27
In line with current South African diabetes management guidelines, metformin was the most commonly prescribed first-line oral therapy.Citation2 At baseline almost all of the patients were being treated with metformin (96%), whereas approximately 11% were receiving an SU, either as monotherapy or in combination with metformin. In almost all cases the reason for change to second-line therapy was poor glycaemic control. The most common second-line approach, which included 70% of patients, was to combine metformin with an SU. Basal insulin was prescribed to 12%. Although both of these approaches are supported by recent South African diabetes treatment guidelines, adding a sulphonylurea to metformin is the preferred approach and insulin is not recommended unless HbA1c target is not achievable with other agents.Citation2
In contrast to algorithmic sequential treatment, more recent guidelines recommend an individualised approach to diabetes therapy, with particular attention to comorbidities. Because they have been shown to reduce morbidity and/or mortality in these patients, glucagon-like peptide-1 receptor agonists (GLP1RA) and sodium-glucose co-transporter 2 inhibitors (SGLT2i) are recommended as early add-on therapies to metformin where there is established cardiovascular disease, high or very high cardiovascular risk and in patients with chronic kidney disease.Citation39,Citation40 A considerable proportion of DISCOVER patients had more than two cardiovascular risk factors and coronary artery disease was reported in almost 10%, suggesting that a not insignificant proportion might have been candidates for one of these add-on therapies. Although the SGLT2i class has only recently been introduced to South Africa and would not have been available during the course of the study, a GLP1RA had been prescribed to only one patient in the entire cohort. Nevertheless, these medications are unavailable in the public sector and in the private sector access to them is limited by cost.
During follow-up, the most common reason for changing therapy was lack of efficacy. However, although glucose control improved during follow up, mean HbA1c remained above the usual target value of 7% and diabetes therapy was changed in only 5% of patients. It is worrying that only 43% of patients were aware of their HbA1c target and 75% of patients had missing data for glycaemic control, suggesting that clinicians are making treatment decisions without proper expectations or monitoring. Therapy is unlikely to be adjusted or to be appropriate if, at minimum, there is no monitoring of glycaemic control.
Considering the long duration of type 2 diabetes (> 7 years), mean HbA1c at which second-line treatment was started and prevailing HbA1c levels over the following 24 months, it appears that diabetes management in South African patients is, at present, suboptimal.
The SF-36 HRQoL summary scores for both physical and mental health in the South African DISCOVER patient population are low. Although there are no published normative data for SF-36 in a South African population, these scores are lower than normative scores reported for various general populations in other countries.Citation36 However, they are similar to those reported for South African adults 12 months after discharge from a surgical intensive care unit.Citation41 These patients perceived their emotional and physical health to negatively affect their social interactions with family and friends, they perceived themselves to be more nervous and depressed than calm and happy, and they regarded themselves as generally sicker than other people they knew.Citation41
Conclusions
The South African cohort in the multinational DISCOVER study has revealed that diabetes management in private practice in South Africa is suboptimal. While poor glycaemic control among people with diabetes has been consistently recorded across the world, DISCOVER suggests that South Africans are neither being sufficiently monitored nor having their medication adjusted appropriately or timeously. Despite a high level of comorbidities, many patients are still managed exclusively at the general practitioner level, when referral to a specialist physician or diabetologist would be appropriate. It is unsurprising that HRQoL among this patient group remains low.
Results from the 3-year follow-up in DISCOVER will be available later this year.
Disclosure statement
The authors report the following disclosures: DW is a medical writer and was paid by AstraZeneca to write the report. AH is an employee of AstraZeneca, South Africa. AK and AA declare no conflicts of interest that are relevant to their participation in the DISCOVER study or this report.
Additional information
Funding
References
- International Diabetes Federation. IDF diabetes atlas. 9th ed. Brussels: International Diabetes Federation; 2019 [cited 2020 Feb 14]. Available from: http://www.diabetesatlas.org
- The Society for Endocrinology, Metabolism and Diabetes of South Africa Type 2 Diabetes Guidelines Expert Committee. Epidemiology of type 2 diabetes. In 2017 SEMDSA guideline for the management of type 2 diabetes guideline committee. JEMDSA. 2017;21(1)(Supplement 1):S1–S196.
- Statistics South Africa. Statistical Release P0309.3. Mortality and causes of death in South Africa: Findings from death notification 2017. [cited 2020 Oct 13]. http://www.statssa.gov.za/publications/P03093/P030932017.pdf.
- Gomes MB, Rathmann W, Charbonnel B, et al. Treatment of type 2 diabetes mellitus worldwide: baseline patient characteristics in the global DISCOVER study. Diab Res Clin Practi. 2019;151:20–32. http://doi.org/10.1016/j.diabres.2019.03.024
- Pinchevsky Y, Butkow N, Chirwa T, et al. Glycaemic, blood pressure and cholesterol control in 25 629 diabetics. Cardiovasc J Afr. 2015;26:188–192. http://doi.org/10.5830/CVJA-2015-050
- Stone MA, Charpentier G, Doggen K, et al. Quality of care of people with type 2 diabetes in eight European countries: findings from the guideline adherence to enhance care (GUIDANCE) study. Diabetes Care. 2013;36:2628–2638. http://doi.org/10.2337/dc12-1759
- de Pablos-Velasco P, Parhofer KG, Bradley C, et al. Current level of glycaemic control and its associated factors in patients with type 2 diabetes across Europe: data from the PANORAMA study. Clin Endocrinol (Oxf). 2014;80:47–56. http://doi.org/10.1111/cen.12119
- Brož J, Janíčková Žďárská D, Urbanová J, et al. Current level of glycemic control and clinical inertia in subjects using insulin for the treatment of type 1 and type 2 diabetes in the Czech Republic and the Slovak Republic: results of a multinational, multicenter, observational survey (DIAINFORM). Diabetes Ther. 2018;9:1897–1906. https://doi.org/10.1007/s13300-018-0485-2
- Kemp TM, Barr ELM, Zimmet PZ, et al. Glucose, lipid, and blood pressure control in Australian adults With type 2 diabetes: the 1999–2000 AusDiab. Diab Care. 2005;28(6):1490–1492. http://doi.org/10.2337/diacare.28.6.1490
- Bi Y, Zhu D, Cheng J, et al. The status of glycemic control: a cross-sectional study of outpatients with type 2 diabetes mellitus across primary, secondary, and tertiary hospitals in the Jiangsu province of China. Clin Ther. 2010;32:973–983. http://doi.org/10.1016/j.clinthera.2010.05.002
- Ong KL, Cheung BM, Wong LY, et al. Prevalence, treatment, and control of diagnosed diabetes in the U.S. national health and nutrition examination Survey 1999–2004. Ann Epidemiol. 2008;18:222–229. http://doi.org/10.1016/j.annepidem.2007.10.007
- Omar SM, Musa IR, Osman OE, et al. Assessment of glycemic control in type 2 diabetes in the Eastern Sudan. BMC Res Notes. 2018;11:373. https://doi.org/10.1186/s13104-018-3480-9
- Fekadu G, Bula K, Bayisa G, et al. Challenges and factors associated with poor glycemic control among type 2 diabetes mellitus patients at Nekemte Referral Hospital, Western Ethiopia. J Multidiscip Healthc. 2019;12:963–974. http://doi.org/10.2147/JMDH.S232691
- Camara A, Baldé NM, Sobngwi-Tambekou J, et al. Poor glycemic control in type 2 diabetes in the South of the Sahara: the issue of limited access to an HbA1c test. Diabetes Res Clin Pract. 2015;108:187–192. http://doi.org/10.1016/j.diabres.2014.08.025
- Musenge EM, Michelo C, Mudenda B, et al. Glycaemic control and associated self-management behaviours in diabetic outpatients: a hospital based observation study in Lusaka, Zambia. J Diabetes Res. 2016. https://doi.org/10.1155/2016/7934654
- Kamuhabwa A, Charles E. Predictors of poor glycemic control in type 2 diabetic patients attending public hospitals in Dar es Salaam. Drug Health Patient Saf. 2014;6:155–165. http://doi.org/10.2147/DHPS.S68786
- BeLue R, Ndiaye K, NDao F, et al. Glycemic control in a clinic-based sample of diabetics in M’Bour Senegal. Health Educ Behav. 2016;43:112S–116S. http://doi.org/10.1177/1090198115606919
- Kibirige D, Atuhe D, Sebunya R, et al. Suboptimal glycaemic and blood pressure control and screening for diabetic complications in adult ambulatory diabetic patients in Uganda: a retrospective study from a developing country. J Diabetes Metab Disord. 2014;13:40. http://www.jdmdonline.com/content/13/1/40.
- Ngwogu K, Mba IE, Ngwogu AC. Glycaemic control amongst diabetic mellitus patients in Umuahia Metroppolis, Abia State, Nigeria. Int J Basic Appl Innov Res. 2012;1(3):98–104.
- Afroz A, Ali L, Karim N, et al. Glycaemic control for people with type 2 diabetes mellitus in Bangladesh – An urgent need for optimization of management plan. Nature Sci Rep. 2019;9:10248. https://doi.org/10.1038/s41598-019-46766-9
- Al Rasheedi AA. Glycaemic control among patients with type 2 diabetes mellitus in countries of Arabic Gulf. Int J Health Sci Quassim Univ. 2015;9(3):345–350.
- Qaddoumi M, Al-Khamis Y, Channanath A, et al. The status of metabolic control in patients with type 2 diabetes attending Dasman Diabetes Institute, Kuwait. Front Endocrinol. 2019;10:412. https://doi.org/10.3389/fendo.2019.00412
- Ngassa Piotie P, Van Zyl DG, Rheeder P. Diabetic nephropathy in a tertiary care clinic in South Africa: a cross-sectional study. JEMDSA. 2015;20(1):67–73.
- Pinchevsky Y, Butkow W, Raal FJ, et al. The implementation of guidelines in a South African population with type 2 diabetes. JEMDSA. 2013;18(3):154–158.
- Pinchevsky Y, Butkow N, Chirwa T, et al. Treatment gaps found in the management of type 2 diabetes at a community health centre in Johannesburg, South Africa. J Diab Res. 2017;2017:9536025. https://doi.org/10.1155/2017/9536025
- Pinchevsky J, Shukla V, Butkow N, et al. The achievement of glycaemic, blood pressure and LDL cholesterol targets in patients with type 2 diabetes attending a South African tertiary hospital outpatient clinic. J Endocrin Metab Diab S Afr. 2015;20(2):81–86. http://doi.org/10.1080/16089677.2015.1056468
- Pinchevsky J, Shukla V, Butkow N, et al. Multi-ethnic differences in HbA1c, blood pressure, and low-density-lipid cholesterol control among South Africans living with type 2 diabetes, after a 4-year follow-up. Int J Gen Med. 2016;9:419–426. http://doi.org/10.2147/IJGM.S119965
- Erasmus RT, Blanco EB, Okesina AB, et al. Assessment of glycaemic control in stable type 2 black South African diabetics attending a peri-urban clinic. Postgrad Med J. 1999;75:603–606.
- Pillay S, Aldous C, Mahomed F. Diabetic patients served at a regional level hospital: what is their clinical picture? JEMDSA. 2015;20(1):60–66.
- Rotchford AP, Rotchford KM. Diabetes in rural South Africa: an assessment of care and complications. S Afr Med J. 2002;92(7):536–541.
- Steyn K, Levitt D, Patel M, et al. Hypertension and diabetes: poor care for patients at community health centres. S Afr Med J. 2008;l98(8):618–622.
- Klisiewicz AM, Raal F. Sub-optimal management of type 2 diabetes mellitus: a local audit. JEMDSA. 2009;14(1):13–16.
- Distiller LA, Brown MA, Joffe BI, et al. Striving for the impossible dream: a community-based multi-practice collaborative model of diabetes management. Diabet Med. 2010;27:197–202. http://doi.org/10.1111/j.1464-5491.2009.02907.x
- Amod A, Riback W. Diabetes guidelines and clinical practice: is there a gap? The South African cohort of the international diabetes management practices study. JEMDSA. 2012;17(2):85–90.
- Ji L, Bonnet F, Charbonnel B, et al. Towards an improved global understanding of treatment and outcomes in people with type 2 diabetes: rationale and methods of the DISCOVER observational study program. J Diabetes Complications. 2017;31(7):1188–1196. http://doi.org/10.1016/j.jdiacomp.2017.03.011
- McDowell I. Measuring health: a guide to rating scales and questionnaires. New York: Oxford University Press; 2006.
- Jacovides A, Bogoshi M, Distiller LA, et al. An epidemiological study to assess the prevalence of diabetic peripheral neuropathic pain among adults with diabetes attending private and institutional outpatient clinics in South Africa. J Int Med Res. Published online 2 June 2014. http://doi.org/10.1177/0300060514525759
- Pinchevsky Y, Raal F, Butkow N, et al. Quality of care delivered to type 2 diabetes mellitus patients in public and private sector facilities in Johannesburg, South Africa. Int J Gen Med. 2018;11:383–390. http://doi.org/10.2147/IJGM.S165545
- Consentino F, Grant PJ, Aboyans V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD The Task Force for diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and the European Association for the Study of Diabetes (EASD). 2019. http://doi.org/10.1093/eurheartj/ehz486
- American Diabetes Association. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes – 2019. Diabetes Care. 2019;42(Suppl. 1):S90–S102. https://doi.org/10.2337/dc19-S009
- Karachi F, Hanekom S, Faure M. Health-related quality of life of patients 12 months following surgical intensive care discharge. SA J Physiother. 2011;67(1):28–34.