Abstract
Objectives:
A study was undertaken to assess the feasibility and safety of the Tshwane Insulin Project (TIP) intervention, describe patients’ and healthcare professionals’ experiences with the intervention, and determine preliminary treatment effects on glycaemic control.
Design:
This was a single-group feasibility study.
Setting:
The study was carried out in the City of Tshwane, South Africa.
Subjects:
People with type 2 diabetes on maximum oral drugs with suboptimal glycaemic control (HbA1c: 9–12%), and healthcare professionals who were involved in the implementation of the TIP intervention were included.
Outcome measures:
Implementation outcome measures included satisfaction, acceptability, appropriateness and safety; and efficacy by assessing change in HbA1c levels.
Results:
Healthcare professionals and patients were satisfied with the intervention. Healthcare professionals agreed that the intervention was acceptable and appropriate. No symptomatic or severe hypoglycaemic events were reported. Improved glycaemic control was recorded with 2.2% lowering of HbA1c values (95% CI, 1.6–2.8%).
Conclusions:
The TIP intervention was feasible and can be implemented with minor amendments. Most participants recommended scaling up the intervention. Lessons learned from this study include: (1) high rates of insulin refusal should be anticipated, and insulin resistance amongst people with type 2 diabetes in primary care should be addressed; and (2) the challenges of initiating and titrating insulin in primary care can be addressed through task sharing and by involving allied healthcare workers.
Introduction and background
Insulin is an evidence-based treatment for achieving glycaemic control in people with diabetes. Despite being effective and in use for more than 100 years,Citation1 managing people with insulin-requiring type 2 diabetes remains a global challenge. In South Africa, particularly in the Tshwane District, glycaemic control remains suboptimal in people with diabetes, with fewer than 30% of patients at the primary care level recording an acceptable HbA1c.Citation2 The primary care patients who have already been switched to insulin have the worst glycaemic control.Citation2
Numerous barriers to insulin therapy, including initiation and titration, especially in patients with type 2 diabetes have been reported globally.Citation3–5 Current diabetes management guidelines recommend the early initiation of insulin therapy,Citation6,Citation7 which is often delayed or not commenced at all,Citation8 leading to poor clinical outcomes and complications for people living with diabetes. In South Africa, insulin titration in patients with type 2 diabetes is vastly inadequate.Citation9 Titration of insulin is inconsistent and may occur only once a month during clinic visits. There is a gap between research evidence, which demonstrates the effectiveness of insulin in people with diabetes, and the actual use of insulin therapy in the real world including in primary health care, especially in resource-constrained settings.
In primary health care settings, task shifting has been identified as a sustainable solution to insulin management.Citation10 Task shifting involves allocating different roles to nurses and allied healthcare workers, including community health workers. In South Africa, nurse practitioners form the backbone of the primary healthcare system. Nurses see most patients with chronic conditions in primary care,Citation11 and routinely manage patients with type 2 diabetes who are on oral glucose-lowering drugs. According to the South African diabetes management guidelines, doctors are responsible for guiding the initiation of insulin and prescribing insulin, but often visit the clinic only once a week.Citation11 Nurses are thus ideally placed to lead the initiation of insulin in primary care. Nurse-led insulin initiation has been suggested as one of the most successful methods to address initiation inertia, and primary care nurse practitioners should be allowed to assist or lead insulin initiation.Citation12 In Australia, nurse-led insulin initiation was associated with increased insulin initiation rates and better glycaemic control.Citation10,Citation13 In rural areas in South Africa, nurse-led diabetes care was associated with improved glycaemic control, demonstrating that with adequate training and support, nurses can play a prominent role in diabetes management.Citation14
Besides health system barriers, patients may delay the initiation of insulin because they are afraid or anxious.Citation15 Feelings of anxiety may be exacerbated by the volume of information that people with diabetes must absorb in a short space of time. In South Africa, overburdened and overworked primary healthcare professionals are expected to dedicate enough time at every visit to educate and empower people living with diabetes.Citation16 However, individual ad-hoc education is limited due to time constraints.Citation17 Community health workers, or lay personnel serving as a link between healthcare professionals and the community, are ideally placed to support diabetes self-management and patient education. In South Africa, community health workers who are adequately trained and empowered could play a role in preventing and treating diabetes.Citation18
Information and communication technologies (ICT) also have the potential to improve diabetes care, including insulin therapy. Healthcare providers are using telehealth to deliver health care remotely using various telecommunication tools, including telephones and smartphones, with or without a video connection.Citation19 Telehealth enables long-distance care and saves patients’ time and money by eliminating the need to travel long distances to access healthcare services.Citation20 Telehealth may also improve clinical outcomes in type 2 diabetes, improving glycaemic control and quality of care.Citation7 Telehealth has not been widely tested or used for insulin management in primary care despite the availability of digital health tools and mobile technology.
The Tshwane Insulin Project (TIP) intervention was developed to facilitate initiating and titrating insulin in people with type 2 diabetes in South Africa.Citation21 The TIP intervention is a nurse-driven and home-based telehealth intervention designed to improve insulin management in primary health care. This intervention was developed according to the framework recommended by the Medical Research Council (MRC) for designing and evaluating complex interventions.Citation22,Citation23 According to this framework, the fourth developmental phase includes a pilot study of the TIP intervention to assess feasibility and acceptability to healthcare providers and participants.Citation24 According to the MRC framework, a feasibility trial provides important insights on the appropriateness of procedures, the recruitment process, participant retention and how acceptable the participants find the intervention.Citation23 Evaluating acceptability during the initial development of the intervention may identify aspects that need to be modified before a definitive trial.Citation25 The MRC framework recommends using a mixture of qualitative and quantitative methods to assess the feasibility of complex interventions.Citation23 Here, we report on the pilot study of the TIP intervention, to inform any refinements ahead of a larger scale study. This pilot study assessed the feasibility and safety of the TIP intervention, and described patients’ and healthcare providers’ experiences with the intervention. We also determined preliminary treatment effects on glycaemic control.
The pilot study was registered with the National Health Research Ethics Committee (NHREC) and the South African National Clinical Trials Register (SANCTR) (SANCTR_5234; 11/02/2019). Reporting of this feasibility study is presented according to the guidelines of Consolidated Standards of Reporting Trials (CONSORT).Citation26
Methods
Study design
In this single-group feasibility study, we used a mixed-methods approach to assess the feasibility, safety and efficacy of the TIP intervention.
Recruitment of participants
The study population comprised healthcare providers and people with type 2 diabetes being treated at selected primary care clinics in the Tshwane District. The Tshwane District is situated in the northern part of Gauteng province in South Africa. The population is urban and consists of mixed socioeconomic groups living in traditional and informal dwellings. People who attend these primary care clinics are often from middle to low socioeconomic groups and cannot afford medical insurance. The primary care clinics were selected conveniently after the facility managers expressed interest in the study, because the intervention could only be implemented if clinic staff supported and participated in the intervention.
Healthcare providers
Healthcare providers included nurse practitioners, medical officers and community health workers who were working at various primary care clinics. The participating healthcare providers attended a workshop on Integrated Diabetes Management in Primary Health Care. They also received training on the study procedures including sessions on the evidence and rationale for insulin therapy, patient counselling, initiation and titration of basal insulin and the use of the mobile app. The community health workers were trained on what to do during home visits.
People living with type 2 diabetes
People with type 2 diabetes qualified for the study if they were insulin-naive, aged between 18 and 70 years, with suboptimal glycaemic control (most recent HbA1c between 9 and 12%), on maximum tolerated doses of two oral glucose-lowering agents for at least three months and compliant, and willing to commence insulin therapy and to self-monitor blood glucose. The participants were required to sign an informed consent document. Exclusion criteria were poor kidney function (eGFR ≤ 30); BMI ≥ 40; other chronic conditions e.g. heart failure; liver disease; history of non-compliance; any previous episodes of hypoglycaemia; consume more than two alcoholic drinks on any day of the week; or unable to secure two meals a day. During the first contact, patients and healthcare providers discussed the participant’s glycaemic control and the treatment options available including insulin therapy and the benefits and challenges of using insulin.
Procedure
Between August 2019 and March 2020, clinical staff (nurses and doctors) at participating primary care clinics, assisted by TIP field researchers, identified potential participants who met the inclusion criteria. Some participants were identified through a review of their medical records, while others were informed about the study when they attended their routine clinic visit. Participants who were identified through their medical records were contacted telephonically and invited either to visit the clinic or to avail themselves of a home visit.
Each potential participant was screened by a nurse practitioner assisted by field researchers. During the screening visit, participants gave written informed consent, and a blood sample was taken and sent to the National Health Laboratory Service for an HbA1c test to confirm eligibility (HbA1c between 9% and 12%). If the HbA1c test results met the eligibility criteria, participants attended the clinic, were enrolled in the study and were initiated on basal insulin. The initiation of basal insulin (Protophane Humulin N [NPH]) was nurse-led, assisted remotely by a doctor via the mobile app. All enrolled participants were initiated on basal insulin at 10 units in the evening at bedtime (but not after 10pm) as recommended by the current South African diabetes management guidelines.Citation27 Patients who had HbA1c < 9% were excluded from the study and continued to receive their usual diabetes care at their local clinic.
Enrolled participants received information on insulin management. Participants attended an education session to explain the use of a blood glucose meter and how to use a blood glucose monitoring record book (diary). Patients were requested to perform finger-prick glucose monitoring twice a day, including one fasting reading before breakfast and another reading two hours after a meal, and to record the readings in the diary.
Intervention
The intervention consisted of using the TIP model to initiate and titrate basal insulin for qualifying type 2 diabetes participants at primary care clinics. TIP field researchers were assigned to primary care clinics, where they supported healthcare providers to identify, educate and follow up participants.
Healthcare providers planned their follow-up according to a visit schedule, which comprised 5 clinic visits (screening visit, enrolment (initiation) visit, week 6, week 10 and week 14) and 14 weekly home visits, of which one was an optional pre-insulin initiation preparatory visit and 13 were post-insulin initiation follow-up visits. At each visit, healthcare providers were encouraged to use a diabetes education booklet to educate the participants on a specific topic: ‘Starting and using insulin’, ‘Food and eating’, ‘Controlling diabetes’, ‘Testing blood sugar’, ‘Hyper- and Hypoglycaemia’ and ‘Emotional wellbeing’.
After the initiation of insulin, follow-up visits presented nine opportunities to titrate insulin, three during clinic visits and six during home visits. Insulin was titrated according to fasting morning blood glucose readings, using the average of the previous two fasting morning blood glucose readings. Healthcare providers received a simplified insulin titration algorithm, which was conservative to minimise the risk of hypoglycaemia (see ). A similar insulin titration algorithm was used in a previous study.Citation28 The titration of insulin was telephonically or electronically directed by a physician (physician-directed titration), and implemented by nurses at clinics and by community health workers at home. Healthcare providers were instructed to refer to the hospital participants who needed more than 20 units of basal insulin to achieve glycaemic control. In March 2020, we amended the follow-up procedure to accommodate the COVID-19 pandemic lockdown restrictions and to ensure the safety of participants and staff. These included implementing telephonic follow-ups instead of home visits. The initial study protocol was also amended to allow for visits with working participants close to their place of work and home visits by TIP field researchers in the absence of community health workers.
Table 1: Simplified insulin titration algorithm used to inform titration in the Tshwane Insulin Project intervention
At baseline, demographic data, clinical history (complications, and medications), smoking status, clinical examination (body mass index, blood pressure), and co-morbidity data were collected. HbA1c was tested at baseline and at the end of the study (14 weeks). For the purpose of this study, HbA1c target was 7.5% (between 7% and 8%) as recommended by the latest American College of Physicians (ACP) guidelines.Citation29
Primary outcome measures
The first primary outcome measured was the feasibility of the intervention. To measure feasibility, we focused on two areas out of the eight outlined by Bowen et al.,Citation30 namely implementation (the extent, likelihood and manner in which the proposed intervention could be implemented) and acceptability (the reaction of the intended individual recipients to the intervention). Implementation outcome measures included satisfaction, acceptability and appropriateness. We recorded the recruitment and completion rates, and documented the number of primary care clinics involved, number of insulin initiations performed, number of insulin titrations done (clinic and home), number of visits conducted at the clinic and at home, and number of referrals to a higher level of care. After the trial, healthcare providers and participants completed a questionnaire concerning their views and experiences of the TIP intervention. The questionnaire was adapted from tools developed by Weiner et al.Citation31 Satisfaction was assessed with two statements for both healthcare providers and participants: (1) how satisfied were you with the TIP intervention with a 5-graded scale from ‘extremely dissatisfied’ to ‘extremely satisfied’; (2) would you recommend the TIP intervention to be implemented on a larger scale with a 5-graded scale from ‘definitely would not’ to ‘definitely would’. Acceptability and appropriateness were evaluated with four statements each for the healthcare providers with a 5-graded scale from ‘completely disagree’ to ‘completely agree’. The experiences of the participants were evaluated with seven statements with a 3-graded scale from ‘disagree’ to ‘agree’.
The second primary outcome was determining the safety of the intervention. We assessed safety using the following indicators: frequency of hypoglycaemia (< 4 mmol/l); frequency of symptomatic hypoglycaemia; frequency of severe hypoglycaemia (hypoglycaemia resulting in loss of consciousness or requiring third-party assistance); and frequency of hyperglycaemia (> 20 mmol/l) from home and clinic readings.
Secondary outcome measures
As a secondary outcome measure, we assessed the efficacy of the TIP intervention, using change in HbA1c after 14 weeks. We also recorded the proportion of participants who achieved an HbA1c ≤ 7.5% at 14 weeks, and the proportion of participants who transitioned to prandial insulin injection to assess the preliminary treatment effects of the intervention.
Sample size
As this was a pilot study, we did not perform a formal sample-size calculation.Citation32 We aimed to initiate 30 people with type 2 diabetes on basal insulin and follow up for 14 weeks, similar to previous feasibility studies.Citation33,Citation34
Statistical analysis
Data were analysed using STATA version 15 (StataCorp, College Station, TX, USA). The feasibility outcomes were reported descriptively and narratively. Descriptive data are presented with a number, or a median and interquartile range due to the small sample size. The 3-graded and 5-graded scales in the questionnaires for acceptability, appropriateness and experience were dichotomised. We described the frequency of categorical outcomes using raw counts (proportions). The paired t-test was used to compare participants’ HbA1c before and after the intervention.
Ethical consideration
The study was approved by the Research Ethics Committee of the Faculty of Health Sciences of the University of Pretoria, South Africa (Ethics Reference No.: 156/2019) and the Tshwane Research Council (No: GP_201810_049). The procedure is described in the methods section. The study was performed in accordance with the Declaration of Helsinki, and all methods were performed in accordance with the relevant guidelines and regulations. All participants were informed about the study and signed informed consent forms.
Results
Participant recruitment
Ten primary care clinics were involved in the study, but only participants from six facilities were initiated on insulin during the study period. At least two nurse practitioners, two community health workers and one doctor were trained at each clinic to be part of the intervention. Patients were recruited in two waves. The first recruitment wave was from August to mid-November 2019, at which time recruitment was paused due to December being holiday season in South Africa. Patients do not often visit health facilities for routine care and only skeleton staff are available at clinics. The second recruitment wave started from mid-January 2020 to March 2020 when recruitment was halted due to the COVID-19 pandemic and a nationwide lockdown announcement in South Africa on March 23, 2020.
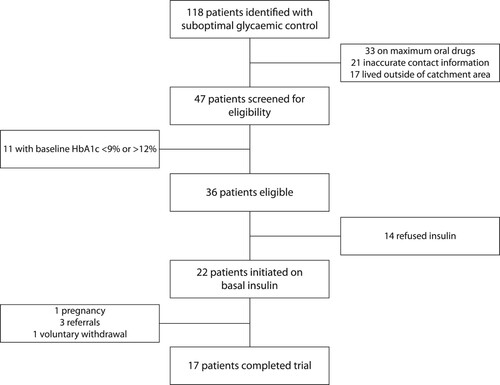
During the two recruitment waves, we identified 118 patients with suboptimal glycaemic control, meaning that their most recent HbA1c was between 9% and 12% (see ). We excluded 71 participants; 33 were not on maximum oral drugs, 21 could not be contacted because of inaccurate information in their records and 17 lived outside the clinic catchment area, making home visits impossible. Of the remaining 47 participants, we excluded 11 participants whose baseline HbA1c was outside the desired range and 36 eligible participants remained. Fourteen participants declined insulin and 22, rather than the 30 envisaged participants, were initiated on basal insulin. Participants refused insulin because they believed that insulin was a ‘death sentence’, they held on to their negative beliefs of insulin despite the counselling that was provided, they had fear of injections and needles or because of the insulin myths that are often prevalent in the communities. We believed that the study provided sufficient data to assess the objectives of the pilot study.
Figure 1: Flow chart showing the participant recruitment and reasons for exclusion during the pilot study.
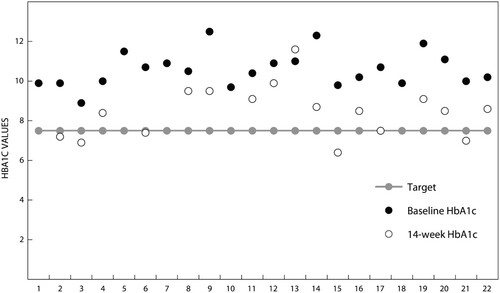
The baseline demographic and clinical characteristics of the participants are summarised in . Of the trial participants, 17 were women, 15 completed secondary school and 12 were married. Median age was 52.5 years (Q1–Q3 47–55).
Table 2: Baseline demographics and clinical characteristics of type 2 diabetes participants enrolled in the TIP intervention (n = 22)
Of the 22 enrolled participants, 17 (77%) completed the pilot study. Of those who did not complete the pilot, one became pregnant and was referred to a specialised obstetric unit at an academic hospital for follow-up; three were referred to hospital for prandial insulin to be added to their treatment and one chose to withdraw from the study voluntarily.
Follow-up after insulin initiation
After insulin initiation, participants were followed up during 3 routine monthly clinic visits and 13 weekly home visits. For 22 participants, we expected 66 clinic visits. Ten visits could not be done because five participants did not complete the trial and two clinic visits were missed due to travel. In total, participants completed 54 clinic visits (96%).
We expected 286 home visits. Forty-one were not done because participants did not complete the trial and 53 home visits were missed. Reasons for missing home visits included patients not available, at work, not responding to phone calls or travel. We recorded 192 home visits (78%), of which 119 were done at home, 16 at the workplace and 57 were done telephonically due to COVID-19 lockdown restrictions. Of the 119 visits conducted at the homes of participants, 42 (35%) were conducted by TIP field staff without community health workers. Community health workers became unavailable due to prior training commitments or for COVID-19-related reasons, e.g. accessibility due to nationwide lockdown restrictions.
App-enabled insulin titration
Physician-directed insulin titration using a mobile app was performed when indicated during home or clinic visits. We recorded 62 insulin titrations, 22 happened at clinics while 40 occurred at the participants’ homes. However, the healthcare providers experienced some challenges when titrating insulin, for example poor mobile network coverage, the prescribing doctor not being available, or patients not recording blood glucose values accurately.
Primary outcome: assessing feasibility
Twelve healthcare professionals completed the questionnaire after the trial. The remaining health professionals did not respond to phone calls or emails, or had relocated. Twelve of the 17 (71%) participants who completed the study answered the questionnaire. The remaining participants could not be reached or had relocated.
Satisfaction
Both healthcare professionals and participants were satisfied with the TIP intervention. Most recipients (71%) indicated that they were extremely satisfied, while the remaining 29% were satisfied. In addition, most recipients indicated that they would recommend implementing the TIP intervention on a larger scale, with 25% saying that they probably would and 75% saying that they definitely would.
Acceptability and appropriateness
Most healthcare professionals supported nurse-led initiation, directed remotely by a doctor. Participants also felt that the use of the app for prescribing and titrating insulin was appealing (). Healthcare professionals also believed that the TIP intervention supported diabetes management guidelines and was aligned with local primary care guidelines on initiation and titration of insulin. All the healthcare professionals thought that the TIP intervention was replicable to other South African primary care clinics.
Table 3: Acceptability and appropriateness of the TIP intervention according to participating healthcare professionals (n = 12)
Most participants believed that home visits by the community health workers were convenient, and that the home visits helped them to transition to insulin (). Most participants indicated that injecting insulin was not as painful (n = 9) and using the glucometer was not difficult (n = 11). Over half of the participants (n = 7) agreed that learning to self-inject was not difficult, but recording sugar levels in the diary was not easy to do. Most participants found the diabetes education booklet to be useful.
Table 4: Experiences of the participants regarding the feasibility of the TIP intervention (n = 12)
Safety of the TIP intervention
During the trial, the researchers recorded 10 occurrences of hypoglycaemia and 14 occurrences of hyperglycaemia from the glucometer readings. No symptomatic or severe hypoglycaemia was recorded.
Secondary outcome: efficacy
Six out of 17 (35.3%) participants achieved glycaemic control (HbA1c ≤ 7.5%) after 14 weeks (). Three out of 22 participants (13.6%) needed prandial insulin and were referred to a hospital for further care. A paired t-test was run on a sample of 17 participants who completed the study to determine whether there was a difference between the mean HbA1c at the baseline and after completing the intervention. Participants’ glycaemic control at the end of the intervention (8.5 ± 1.3%) had improved when compared with the start of the study (10.6 ± 0.9%), a reduction of 2.2% (95% CI 1.6–2.8).
Discussion
Our findings suggest the nurse-driven and home-based telehealth intervention can be implemented in a similar manner to the pilot after minor amendments. Both healthcare professionals and participants were extremely satisfied with the intervention and would recommend the TIP to be implemented on a larger scale. A large proportion of the participants completed the trial.
In this study, healthcare professionals agreed that the TIP intervention was acceptable and appropriate according to all the assessment measures.Citation31 Nurse-driven insulin initiation has been recommended as a potential solution to overcome barriers for insulin initiation, titration and intensification during diabetes management,Citation12 and has been effective in AustraliaCitation13 and in Ohio, USA.Citation35 Healthcare professionals agreed that the TIP intervention seems to fit and is aligned with diabetes management and local primary care guidelines, which bodes well for its replication to other primary care clinics in South Africa.
For people with type 2 diabetes, healthcare workers provide education that supports multiple aspects of diabetes management, including injecting insulin and using a blood glucose metre. The TIP intervention addressed many barriers that delay insulin intensification such as lack of healthcare provider resources, and assistance and education for patients regarding effective titration.Citation12,Citation36 Through the TIP intervention, patients had access to a diabetes education booklet and home visits. Participants felt that home visits specifically provided by the community health workers were convenient and helped them to transition smoothly to insulin. The pilot study revealed that participants struggled to record their sugar levels in the diary. In future, the TIP intervention should include additional education material and training on how to record sugar levels.
While piloting the TIP intervention, we did not record any symptomatic or severe hypoglycaemic events, suggesting that the TIP intervention was safe. Furthermore, hypoglycaemic and hyperglycaemic occurrences were rare. The rarity of adverse events could be due to the use of a conservative insulin titration algorithm. Glucose meters were used to document hypoglycaemic or hyperglycaemic occurrences, rather than self-reporting. Relatively few patients diagnosed with type 2 diabetes are aware of diurnal and nocturnal episodes of hypoglycaemia.Citation37 Preventing hypoglycaemia in newly initiated type 2 diabetes patients is critical to avoid discontinuation of insulin therapy, because patients who experienced hypoglycaemic events within six months of basal insulin initiation are more likely to discontinue therapy.Citation38 Fear of hypoglycaemia is an important reason for discontinuing insulin therapy amongst people living with type 2 diabetes in developing countries.Citation39
In this pilot study, the TIP intervention improved glycaemic control. Over the 14-week intervention, the mean HbA1c dropped from 10.6% to 8.5%, indicating a 2.2% from baseline to Week 14. These levels are somewhat similar to changes observed in a similar length study (12 weeks)Citation40 and other studies of much longer duration (approximately 24 months).Citation41 Turner et al.Citation42 reported a small change from 9.5% to 9.0% (−0.52%) following the implementation of 12-week telehealth support for patients with type 2 diabetes using insulin treatment. The large improvement in glycaemic control observed in our study may be due to including patients with high baseline HbA1c levels (between 9 and 12%) or it could be due to the intensive weekly follow-up home visits. Polonsky et al.Citation43 found that proactive follow-up was critical for successful insulin initiation, adding that proactive contact is important to encourage and guide patients before problems arise.
Our findings support the generalisability of our pilot study methods, and the implementation of the TIP intervention on a larger scale. First, the TIP intervention is based on a strong theoretical and pragmatic framework. The TIP intervention overcomes several known barriers to starting insulin treatment. For example, in the TIP intervention, primary care nurses are responsible for managing patients with type 2 diabetes and community health workers can spend more time on educating and empowering patients. Second, the pilot study was conducted in a real-world setting. The TIP intervention showed promising results despite the many challenges related to the South African environment such as an overburdened health system and limited healthcare providers, patients with limited knowledge and numeracy skills, and difficulties in implementing telehealth interventions (inconsistent mobile network coverage). Another strength of the TIP intervention is the app-enabled titration assisted by a nurse or a community health worker. In South Africa, services at primary care clinics are nurse-driven with doctors doing sessional work (usually 4–8 hours per week),Citation16 therefore it is not practical for insulin initiation to be led by doctors. In addition, the TIP intervention uses a mobile app called Vula that was not specifically developed for this study and is available free of charge.
We experienced a number of unanticipated challenges during the pilot study, including the follow-up of participants who were working and those who resided outside of the clinic catchment area. We amended the protocol to address the follow-up of working participants, but had to exclude patients who lived outside the catchment area of the clinic. Better coordination and communication within the community health worker programme should allow patients who live beyond the catchment area to be followed up by community health worker teams closer to their homes. Lastly, the TIP intervention will be enhanced if we can address technical challenges encountered during app-enabled titration and when trying to contact prescribing doctors.
This pilot study had several limitations. We recruited clinics based on the interest expressed by facility managers. Although this was done for practical reasons, there may be potential bias. Consequently, our convenience sample of clinics may not be representative of the wider population of primary care facilities. We struggled to identify eligible patients because of poor medical records and lack of patient registries. We could have identified more participants if the medical records were electronic. We encountered many patients who declined due to insulin resistance, which was expected as preliminary studies suggest that insulin resistance is prevalent among people living with diabetes in the Tshwane district.Citation44 Despite positive results, the study design and the small sample size do not allow us to draw any conclusions regarding treatment effects. Further evaluation of the TIP intervention with a larger sample size and a control group should focus on effectiveness.
Conclusions
The main conclusions of this pilot study are that: (a) nurse-led insulin initiation assisted remotely by a prescribing doctor is feasible and acceptable to primary healthcare professionals; (b) app-enabled physician-directed insulin titration assisted by a nurse or a community health worker is feasible; (c) home visits by community health workers provide additional opportunities for patient education and insulin titration; and, finally, (d) the TIP intervention holds promise for achieving glycaemic control.
Lessons learned from this study can be summarised as follows: (1) high rates of insulin refusal should be anticipated, and insulin resistance amongst people with type 2 diabetes in primary care should be addressed; (2) with the necessary support, patients with type 2 diabetes can be initiated safely on insulin in primary care and reach their glycaemic goals; and (3) the challenges of initiating and titrating insulin in primary care can be addressed through task sharing and the involvement of allied healthcare workers. Given that many patients on insulin-based therapy fail to reach glycaemic goals,Citation45,Citation46 and that even more type 2 diabetes patients who need insulin are not initiated, innovative telehealth interventions that are integrated within the health system present a solution even in resource-constrained settings.
Authors’ contributions
PNP designed the study, developed the study tools, performed data analysis, wrote the first draft and was responsible for collating all co-authors’ comments, preparing the final version and submitting the manuscript to the journal. PW contributed to writing of the results and discussion sections in the draft and contributed to interpretation of the results. JWM developed the study tools and edited the draft. EMW designed the study and developed the study tools. PR designed the study, developed the study tools, performed statistics and edited the draft. All authors read and approved the final manuscript.
Acknowledgements
The authors thank the people living with diabetes and healthcare providers who participated in the study, as well as the health facility managers and the health authorities from the City of Tshwane Metropolitan Municipality, Tshwane district, Gauteng province and the South African National Department of Health for supporting the Tshwane Insulin Project (TIP); and the TIP fieldwork team: Ms Gotabeng Mohlala, Ms Amanda Segale, Ms Ntokozo Zulu, Ms Betty Manyapetsa and Mr Charles Koenaite. Dr Cheryl Tosh is thanked for editing the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bliss M. Rewriting medical history: Charles Best and the Banting and Best myth. J Hist Med Allied Sci. 1993;48:253–274.
- Webb EM, Rheeder P, Van Zyl DG. Diabetes care and complications in primary care in the Tshwane district of South Africa. Prim Care Diabetes. 2015;9:147–154. https://doi.org/10.1016/j.pcd.2014.05.002.
- Haque M, Navsa M, Emerson SH, et al. Barriers to initiating insulin therapy in patients with type 2 diabetes mellitus in public-sector primary health care centres in Cape Town. J Endocrinol Metab Diabetes S Afr. 2005;10:94–99. https://doi.org/10.1080/22201009.2005.10872127.
- Ng CJ, Lai PS, Lee YK, et al. Barriers and facilitators to starting insulin in patients with type 2 diabetes: a systematic review. Int J Clin Pract. 2015;69:1050–1070. https://doi.org/10.1111/ijcp.12691.
- Kunt T, Snoek FJ. Barriers to insulin initiation and intensification and how to overcome them. Int J Clin Pract. 2009;63:6–10. https://doi.org/10.1111/j.1742-1241.2009.02176.x.
- The Society for Endocrinology, Metabolism and Diabetes of South Africa Type 2 Diabetes Guidelines Expert Committee. The 2017 SEMDSA Guideline for the Management of Type 2 Diabetes Guideline Committee. JEMDSA. 2017;22(Supplement 1):S1–S196.
- Diabetes Canada Clinical Practice Guidelines Expert Committee. Diabetes Canada 2018 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada. Can J Diabetes. 2018;42(Suppl 1):S1–S325.
- Mayet L, Naidoo SS. An evaluation of insulin therapy initiation among patients with type 2 diabetes attending a public health facility in South Africa. S Afr Fam Pract (2004). 2012;54:525–530. https://doi.org/10.1080/20786204.2012.10874287.
- Kalweit KL, Van Zyl DG, Rheeder P. Titrating insulin in patients with type 2 diabetes using a structured self-monitoring blood glucose regimen. S Afr Med J. 2018;108:654–659. https://doi.org/10.7196/SAMJ.2018.v108i8.12801.
- Furler JS, Blackberry ID, Manski-Nankervis JA, et al. Stepping up: A nurse-led model of care for insulin initiation for people with type 2 diabetes. Fam Pract. 2014;31:349–356. https://doi.org/10.1093/fampra/cmt085.
- Mash B, Fairall L, Adejayan O, et al. A morbidity survey of South African primary care. PloS ONE. 2012;7:e32358. https://doi.org/10.1371/journal.pone.0032358.
- Russell-Jones D, Pouwer F, Khunti K. Identification of barriers to insulin therapy and approaches to overcoming them. Diabetes Obesity Metab. 2018;20:488–496. https://doi.org/10.1111/dom.13132.
- Furler J, O’Neal D, Speight J, et al. Supporting insulin initiation in type 2 diabetes in primary care: results of the Stepping Up pragmatic cluster randomised controlled clinical trial. Br Med J. 2017;356; https://doi.org/10.1136/bmj.j783.
- Price C, Shandu D, Dedicoat M, et al. Long-term glycaemic outcome of structured nurse-led diabetes care in rural Africa. QJM: Mon J Assoc Physicians. 2011;104:571–574. https://doi.org/10.1093/qjmed/hcr005.
- Benroubi M. Fear, guilt feelings and misconceptions: barriers to effective insulin treatment in type 2 diabetes. Diabetes Res Clin Pract. 2011;93(Supplement 1):S97–S99. https://doi.org/10.1016/S0168-8227(11)70021-3.
- Webb EM, Rheeder P, Wolvaardt JE. The ability of primary healthcare clinics to provide quality diabetes care: An audit. Afr J Prim Health Care Fam Med. 2019;11:e1–e6. https://doi.org/10.4102/phcfm.v11i1.2094.
- Dube L, Van den Broucke S, Dhoore W, et al. An audit of diabetes self-management education programs in South Africa. J Public Health Res. 2015;4(3):581. https://doi.org/10.4081/jphr.2015.581.
- Hughes GD, Puoane T, Bradley H. Ability to manage diabetes—community health workers’ knowledge, attitudes and beliefs. J Endocrinol Metab Diabetes S Afr. 2006;11:10–14. https://doi.org/10.1080/22201009.2006.10872134.
- Dorsey ER, Topol EJ. State of telehealth. N Engl J Med. 2016;375:1400. https://doi.org/10.1056/NEJMra1601705.
- Al-Badri M, Hamdy O. Diabetes clinic reinvented: will technology change the future of diabetes care? Ther Adv Endocrinol Metab. 2021;12:2042018821995368. https://doi.org/10.1177/2042018821995368.
- Ngassa Piotie P, Wood P, Webb EM, et al. Designing an integrated, nurse-driven and home-based digital intervention to improve insulin management in under-resourced settings. Ther Adv Endocrinol Metab. 2021;12:20420188211054688, https://doi.org/10.1177/20420188211054688.
- Craig P, Dieppe P, Macintyre S, et al. Developing and evaluating complex interventions: the new Medical Research Council guidance. Int J Nurs Stud. 2013;50:587–592. https://doi.org/10.1016/j.ijnurstu.2012.09.010.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: new guidance. Swindon: Medical Research Council; 2008. https://aka.mrc.ukri.org/complexinterventionsguidance [Accessed 11 May 2022].
- Campbell M, Fitzpatrick R, Haines A, et al. Framework for design and evaluation of complex interventions to improve health. Br Med J. 2000;321:7262. https://doi.org/10.1136/bmj.321.7262.694.
- Saracutu M, Edwards DJ, Davies H, et al. Protocol for a feasibility and acceptability study using a brief ACT-based intervention for people from southwest Wales who live with persistent pain. BMJ Open. 2018;8:e021866. https://doi.org/10.1136/bmjopen-2018-021866.
- Eldridge SM, Chan CL, Campbell MJ, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. Br Med J. 2016;355:i5239. https://doi.org/10.1136/bmj.i5239.
- Republic of South Africa. Essential Drugs Programme. Primary Healthcare Standard Treatment Guidelines and Essential Medicines List. 6th ed Pretoria: National Department of Health; 2018.
- Blackberry ID, Furler JS, Ginnivan LE, et al. An exploratory trial of insulin initiation and titration among patients with type 2 diabetes in the primary care setting with retrospective continuous glucose monitoring as an adjunct: INITIATION study protocol. BMC Fam Pract. 2014;15:82. https://doi.org/10.1186/1471-2296-15-82.
- Qaseem A, Wilt TJ, Kansagara D, et al. Hemoglobin A1c targets for glycemic control with pharmacologic therapy for nonpregnant adults with type 2 diabetes mellitus: a guidance statement update from the American College of physicians. Ann Intern Med. 2018;168:569–576. https://doi.org/10.7326/m17-0939.
- Bowen DJ, Kreuter M, Spring B, et al. How we design feasibility studies. Am J Prev Med. 2009;36:452–457. https://doi.org/10.1016/j.amepre.2009.02.002.
- Weiner BJ, Lewis CC, Stanick C, et al. Psychometric assessment of three newly developed implementation outcome measures. Implement Sci. 2017;12:108, https://doi.org/10.1186/s13012-017-0635-3.
- Thabane L, Ma J, Chu R, et al. A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol. 2010;10:1. https://doi.org/10.1186/1471-2288-10-1.
- Billingham SAM, Whitehead AL, Julious SA. An audit of sample sizes for pilot and feasibility trials being undertaken in the United Kingdom registered in the United Kingdom clinical Research network database. BMC Med Res Methodol. 2013;13:104–109. https://doi.org/10.1186/1471-2288-13-104.
- Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract. 2004;10(2):307–312. https://doi.org/10.1111/j..2002.384.doc.x.
- Litaker D, Mion L, Kippes C, et al. Physician - nurse practitioner teams in chronic disease management: the impact on costs, clinical effectiveness, and patients’ perception of care. J Interprof Care. 2003;17:223–237. https://doi.org/10.1080/1356182031000122852.
- Khunti K, Millar-Jones D. Clinical inertia to insulin initiation and intensification in the UK: A focused literature review. Prim Care Diabetes. 2017;11:3–12. https://doi.org/10.1016/j.pcd.2016.09.003.
- Pistrosch F, Ganz X, Bornstein SR, et al. Risk of and risk factors for hypoglycemia and associated arrhythmias in patients with type 2 diabetes and cardiovascular disease: a cohort study under real-world conditions. Acta Diabetol. 2015;52:889–895. https://doi.org/10.1007/s00592-015-0727-y.
- Dalal MR, Kazemi M, Ye F, et al. Hypoglycemia after initiation of basal insulin in patients with type 2 diabetes in the United States: implications for treatment discontinuation and healthcare costs and utilization. Adv Ther. 2017;34:2083–2092. https://doi.org/10.1007/s12325-017-0592-x.
- Chan JCN, Gagliardino JJ, Ilkova H, et al. One in seven insulin-treated patients in developing countries reported poor persistence with insulin therapy: real world evidence from the cross-sectional International diabetes management practices study (IDMPS). Adv Ther. 2021;38:3281–3298. https://doi.org/10.1007/s12325-021-01736-4.
- Quinn CC, Clough SS, Minor JM, et al. Welldoc™ mobile diabetes management randomized controlled trial: change in clinical and behavioral outcomes and patient and physician satisfaction. Diabetes Technol Ther. 2008;10:160–168. https://doi.org/10.1080/10.1089/dia.2008.0283.
- Jabbar A, Abdallah K, Hassoun A, et al. Patterns and trends in insulin initiation and intensification among patients with type 2 diabetes mellitus in the Middle East and north Africa region. Diabetes Res Clin Pract. 2019;149:18–26. https://doi.org/10.1016/j.diabres.2019.01.017.
- Turner J, Larsen M, Tarassenko L, et al. Implementation of telehealth support for patients with type 2 diabetes using insulin treatment: an exploratory study. J Innov Health Inform. 2009;17:47–53. https://doi.org/10.14236/jhi.v17i1.714.
- Polonsky WH, Arsenault J, Fisher L, et al. Initiating insulin: how to help people with type 2 diabetes start and continue insulin successfully. Int J Clin Pract. 2017;71(8):e12973. https://doi.org/10.1111/ijcp.12973.
- Ngassa Piotie P, Wood P, Webb EM, et al. Willingness of people with type 2 diabetes to start insulin therapy: evidence from the South African Tshwane Insulin Project (TIP). Diabetes Res Clin Pract. 2020;168:108366. https://doi.org/10.1016/j.diabres.2020.108366.
- Dalal MR, Grabner M, Bonine N, et al. Are patients on basal insulin attaining glycemic targets? characteristics and goal achievement of patients with type 2 diabetes mellitus treated with basal insulin and physician-perceived barriers to achieving glycemic targets. Diabetes Res Clin Pract. 2016;121:17–26. https://doi.org/10.1016/j.diabres.2016.08.004.
- Mauricio D, Meneghini L, Seufert J, et al. Glycaemic control and hypoglycaemia burden in patients with type 2 diabetes initiating basal insulin in E urope and the USA. Diabetes Obesity Metab. 2017;19:1155–1164. https://doi.org/10.1111/dom.12927.