Abstract
Background:
Despite highly active antiretroviral therapy (HAART) leading to a decline in human immunodeficiency virus (HIV)-induced morbidity and mortality, in recent years HAART has been implicated in abnormal lipid profiles, diabetes mellitus (DM) and predisposition of patients to cardiovascular disease (CVD).
Objectives:
In this comparative study, the side effects of HAART as well as other lifestyle factors such as diet, exercise, alcohol and/or smoking were assessed, as well as family history of diabetes between HIV-infected and HIV-uninfected patients of African ancestry with DM.
Methods:
The study population consisted of 80 Black African diabetic patients (18–65 years old) stratified by HIV status (HIV-infected n = 40; HIV-uninfected n = 40). Anthropometric measurements (weight, height and BMI) and blood pressure (BP), as well as biochemical tests for glucose, cholesterol, high-density lipoproteins (HDL), low-density lipoproteins (LDL) and triglycerides were performed.
Results:
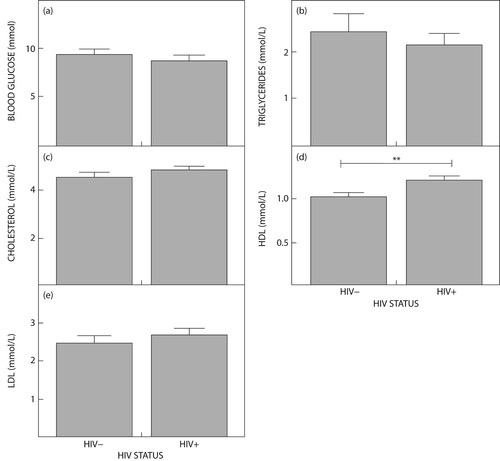
The median BMI indicated overweight in the HIV-infected compared with the HIV-uninfected, which was congruent with obesity. Systolic BP was higher in the HIV-infected compared with the HIV-uninfected groups, at 138.15 and 134.75 mmHg (p = 0.1651), respectively. Glucose was high in both groups, confirming diabetes (p = 0.3900). Cholesterol was high (4.85 mmol/l) in the HIV-infected group while HDL was lower (0.95 mmol/l) in the HIV-uninfected group. Triglycerides were elevated in the HIV-uninfected (1.90 mmol/l) compared with the HIV-infected (1.61 mmol/l) (p = 0.7500) group.
Conclusion:
Despite HAART being documented as a contributor to DM and abnormal lipid profiles in the HIV-infected group, lifestyle factors such as diet also affect obesity phenotype in the uninfected group. Thus, irrespective of DM and/or HIV status, a lack of exercise, behavioural and lifestyle risk factors exacerbate abnormal lipid profiles. Notably, a family history of DM showed a strong susceptibility to its development.
Introduction
Despite the devastation and disruption of the coronavirus disease 2019 (COVID-19) in the period 2019–2022, the world is still grappling with human immunodeficiency virus (HIV) infection/acquired immune deficiency disorder syndrome (AIDS), a serious public health challenge. In 2020, 37.7 million people worldwide were HIV-infected.Citation1 South Africa (SA) is considered the epicentre of the HIV pandemic with an overall prevalence of 13.7% (8.2 million) in 2021.Citation2 Moreover, 20% of all people living with HIV are from SA.Citation3 The World Health Organization has listed the Sustainable Development Goals 2030 (SDGs), which includes 10 SDGs specific to the response to AIDS that address advancing the right to health, gender equality, human rights, employment and social protection.Citation1 South Africa has the largest antiretroviral therapy (ART) rollout programme in the world.Citation4 Despite ART increasing the life expectancy of HIV-infected patients,Citation5,Citation6 it has been associated with predisposition to diabetes mellitus (DM) and other metabolic disorders. The former threat leads to insulin resistance and abnormal lipid profiles that increase the risk of cardiovascular disease (CVD) development.Citation7
The latest IDF Diabetes Atlas reports that the global prevalence of diabetes is 536.6 million cases. It projects that by 2045, 783.2 million adults or 1 in 10 adults will be living with DM.Citation8 This would be an escalation of 46%, which is more than twice the predicted population growth (20%) over the same time frame. The reality is that DM is projected to be the leading cause of death.Citation9,Citation10 Moreover, DM is associated with a higher risk of serious and life-threatening complications, such as heart attack, stroke, kidney failure, blindness and lower-limb amputation that eventuate in reduced quality of life and higher healthcare costs.Citation11
The prevalence of adult DM in Africa is 19.8 million.Citation12 Diabetes is a metabolic disorder, characterised by an impairment of insulin production/action, which results in abnormal metabolism of carbohydrates, leading to elevated levels of blood glucose.Citation13 The impaired activity of the hormone insulin is unable to regulate blood sugar levels. Diabetes is the second leading underlying cause of death in SA and the number one leading underlying cause of death for females.Citation14 The prevalence of DM in SA has tripled from 4.5% in 2010 to 12.7% in 2019. In 2019, diabetes affected approximately 4.58 million people between 20 and 79 years old.Citation15 Notably, another grave public health challenge in SA that leads to metabolic diseases such as diabetes is obesity, which progresses to dyslipidaemia. Dyslipidaemia is characterised by abnormal lipid components in plasma, which can lead to CVD. The varying levels of both high-density lipoprotein (HDL) and low-density lipoprotein (LDL) have a strong association with the incidence of CVD development. Decreased HDL indicates marked abnormalities while increased LDL results put individuals at cardiovascular risk.Citation16 High levels of triglycerides have also been associated with cardiovascular risk.
The triad of HIV infection, DM and obesity synergistically impact on one another. This relationship is further affected by ART, which dysregulates normal lipid profiles, insulin resistance and adipokine homeostasis, leading to body fat redistribution.Citation17 Antiretrovirals (ARVs) also dysregulate circulating adipocyte-secreted hormones, which contribute to metabolic disorders such as DM. This is exacerbated by protease inhibitors and nucleoside analogue inhibitor usage.Citation18 In light of the high prevalence of HIV infection, DM and obesity in SA, it is relevant to examine lipid profiles to assess the metabolic process and abnormalities that lead to DM in both HIV-infected and HIV-uninfected individuals. This profile, together with social factors, HIV parameters and drug interactions, may provide a meaningful risk profile of HIV-infected patients with DM.
Methods
Ethics
After institutional ethics approval (Reference number: BE686/18), this study was performed at a district hospital located in Chatsworth, a suburb in the eThekwini, KwaZulu-Natal area of SA.
Sampling
Study participants were selected through a systematic random sampling technique using daily clinic attendance as the sampling frame.
Study population
The study population consisted of 80 patients with DM stratified by HIV status into HIV-infected (n = 40) and HIV-uninfected (n = 40). Participants were recruited on clinic days if they met the inclusion criteria, which were age between 18–60 years, clinical diagnosis of DM and completion of written informed consent to participate in the study. All HIV-infected patients received ART, a standard of care in SA. Participants below the age of 18 years and above 60 years old, no informed consent and severely ill patients including those with Mycobacterium tuberculosis were excluded from the study.
Methods
Fasting blood glucose was done at the clinic site using an Accu-Check active blood glucose monitoring system (Roche, Indianapolis, IN, USA). Five millilitres of blood samples were collected from each participant by venepuncture after overnight fasting into collection tubes to procure biochemistry measurements of plasma glucose and serum lipids (total cholesterol, HDL cholesterol and total triglycerides). The blood samples were allowed to coagulate and spun at 3 000 rpm for 10 minutes. The serum samples were collected and stored at −80 degrees Celsius for the subsequent lipid profile assays. The Atellica™ CH Analyzer (Siemens Healthcare GmbH, Erlangen, Germany) was used for the cholesterol_2 (Chol_2), triglyceride (concentrated) (Trig) and direct HDL cholesterol (D-HDL) assay, which is an in vitro diagnostic test used in the quantitative determination of cholesterol, triglyceride and HDL cholesterol in human serum and plasma (lithium heparin). LDL-C was calculated using the Friedewald formula: LDL-C (mmol/l)= TC – HDL-C – TG/2.2. Presence of a derangement in any of the lipid profile parameters was defined as dyslipidaemia according to the SA National Health Laboratory Services reference range, therefore hypercholesterolemia (TC ≥ 6.2 mmol/l), hypertriglyceridemia (TG ≥ 2.25 mmol/l), low HDL (≤ 1.03 mmol/l), high LDL (≥ 4.11 mmol/l). Clinical demographics included weight, height, and systolic and diastolic blood pressure measurements. Body mass index (BMI) was calculated using the formulae BMI = weight (kg) /height (m2), and CD4 T-cell count and viral load was documented.
Oral hypoglycaemic agents included metformin, actrapid, protaphane, actraphane, or glimepiride and insulin therapies. The ARV treatment that was administered to the HIV-infected patients were either a single drug such as zidovudine also known as azidothymidine (AZT) or a combination of multiple drugs (tenofovir disoprovil fumarate [TDF, Viread], emtricitabine [FTC, Emtriva] and efavirenz [EFV]). The alternative drug combination administered to some of the patients was abacavar (ABC, Ziagen), lamivudine (3TC, Epivir) and efavirenz (EFV).
Statistical analysis
Data were analysed utilizing GraphPad Prism 5.00 for Windows (GraphPad Software, San Diego, CA, USA). The Kolmogorov–Smirnov normality test was used to check for parametric or non-parametric distribution. Non-parametric data are represented as median and interquartile range. Statistical significance across study groups was determined using a Mann–Whitney U-test. A p-value of < 0.05 was considered to be statistically significant.
Results
Of the 80 Black South African DM patients, 28 (35%) were male and 52 (65%) were female. Demographic, clinical and biochemical results are given in . The BMI of the HIV-infected participants was lower (median = 28.37 kg/m2; 95% CI 31.43–27.36) than that of the HIV-uninfected individuals (median BMI = 30.86 kg/m2; 95% CI 35.21–27.73). Both the HIV-infected and HIV-uninfected participants displayed high systolic blood pressure of 138.15 and 134.75 mmHg respectively, despite the diastolic blood pressure being within the normal range in both cohorts. Only 3 (7.5%) HIV-infected patients and 5 (12.5%) HIV-uninfected patients had high cholesterol levels, while the triglycerides were high in 15 (37.5%) and 12 (30%) HIV-uninfected and HIV-infected participants respectively. There was a statistically significant up-regulation of HDL in the HIV-infected group (median = 1.16 mmol/l; 95% CI 1.311–1.127) compared with the HIV-uninfected group (median = 0.95 mmol/l; 95% CI 2.887–2.034) (p = 0.0018) (see Table 1).
Table 1 : Clinical, demographic and biochemical data of the study population
All the HIV-infected patients received antiretroviral treatment over an average duration period of 108 months, whilst a low CD4 count of 74.52 cells/mm3 was noted in most patients. The majority of the study population consumed a diet rich in high fat and oil content, being higher in the HIV-infected compared with the HIV-uninfected (90% vs. 83%) groups respectively. Some 60% of HIV-infected and 65% of HIV-uninfected patients consumed a diet rich in red meat.
Only 15% of HIV-infected patients participated in some sort of exercise (cycling, weights, running, soccer and gymnasium) and these patients had low cholesterol, triglycerides and LDL. A few patients indulged in alcohol (10%) and smoked cigarettes (7.5%). Five patients from the HIV-uninfected group were involved in some sort of exercise (weights, gym, soccer, running or netball) and of these only one patient had high triglycerides.
Smoking was not a significant finding as below 7% of the participants from both cohorts smoked cigarettes. There were 67.5% of patients in the HIV-uninfected group and 50% of the patients in the HIV-infected group with a family history of diabetes (, ).
Table 2: Number of patients with abnormal demographics and lipid profiles
Although both cohorts in the study were diabetic, there were six (15%) HIV-infected patients and five (12.5%) HIV-uninfected patients with controlled glucose at below 5.5 mmol/l. Surprisingly, although 35 (87.5%) HIV-uninfected patients had high glucose levels, only 3 (7.5%) patients had abnormal cholesterol and LDL levels. Similarly, of the 34 (85%) HIV-infected patients who had high glucose levels, only 3 (7.5%) participants had increased cholesterol levels and 3 (7.5%) had abnormal LDL levels. Of note, the significant findings in both the HIV-infected and HIV-uninfected groups was the low HDL readings, at 12 (30%) and 25 (62.5%) respectively. The triglyceride level in 30% of the HIV-infected group was abnormal in contrast to 37.5% being out of normal range in the HIV-uninfected group. The BMI in both the HIV-infected and HIV-uninfected groups showed that 75% and 77.5% of the participants had overweight or obese phenotypes respectively.
Of the 40 HIV-positive patients enrolled in the study, 20 (11%) received nucleoside reverse transcriptase inhibitors, 68 (36%) received a regimen containing a non-nucleoside reverse transcriptase inhibitor and 64 (34%) received a protease inhibitor containing regimen. A statistically significant difference was noted in the parameters of the lipid panel (F = 106.13, p < 0.001) and by ARV regimen (F = 2.88, p = 0.03), although the interaction between these variables was not significant.
A Tukey post-hoc test revealed no significant difference in cholesterol, LDL and HDL among all the ARV treatments (i.e. ABC/3TC/EFV, FDC = TDF/FTC/EFV, FDC and AZT). The only significant increase was noted in triglyceride levels in patients treated with FDC compared with TDF/FTC/EFV (p = 0.01).
Discussion
The main finding of this study was elevated blood glucose levels in both HIV-infected and HIV-uninfected DM patients, with only a small percentage (15% and 12.5% respectively) having controlled glucose levels. The increased glucose levels are expected, as HIV infection is associated with abnormal/worsening glucose metabolism in diabetes.Citation19 Notably, there was also a family history possibly reflecting a genetic link of DM derived from either a mother, father or one of the siblings in our study population. It is noteworthy to mention that preceding 1990 DM was rare in Africa. However, Africa has experienced a surge in the burden of DM with a high prevalence of DM in urban-dwelling South Africans.Citation20 Of note too, the prevalence of type 2 diabetes in Africa (1–2%) is lower than for those ndividuals of African ancestry living in high-income countries (11–13%).Citation21
South Africa is considered a ‘fat’ nation, where 62.2% of women and 25.1% men are overweight or obese. A strong correlation between type 2 DM and overweight or obesity exists, with as high as 75% of DM patients being overweight or obese.Citation22 This pre-empts a body mass index of ≥ 30 kg/m2 as noted in our study, and correlates with increased metabolic disease risk and excess adipose tissue accumulation, which intensify insulin resistance.
It is surprising to report similar BMI in both HIV-infected and HIV-uninfected DM groups. However, weight was unacceptably high, with patients being either obese or overweight, hence this may have contributed to the abnormal glucose handling.Citation23 Previous studies have shown that the poor access to health care, extent of HIV infection, large abdominal circumference, obesity, inactive lifestyle and economic constraints led to poor glucose profiles in Africa.Citation24 Despite obesity being linked to wealth in Africa,Citation25 this discrepancy was obviated as our study population were of similar economic status.
Additionally, our results are disparate with regard to wasting in HIV infectionCitation26,Citation27 compared with obesity. This disparity may emanate from weight gain during ART.Citation28,Citation29 Nonetheless, weight gain underwrites hyperlipidaemia, hypertension, insulin resistance and cardiovascular disease development among HIV-infected patients.Citation30 The fact that both groups of patients had a diet rich in red meat and high fats and oils was a major contribution to their weight gain and exacerbates their poor lipid profiles. It is possible that a lack of access to wholesome food, poor health care and economic constraints may have underwritten hypercholesteraemia and high lipids in diabetic patients. Moreover, more than 80% of patients from both groups did not include any form of exercise in their routine daily activities except for walking to the closest area normally to access public transport.
Dyslipidaemia may be defined as high levels of lipids in circulation, and is associated with familial predisposition as well as lifestyle factors that predispose to CVD risk and development.Citation31 Our results of diabetic dyslipidaemia in the HIV-infected (77.5%) and HIV-uninfected (92.5%) are corroborated by the findings of Woyesa et al. (2021)Citation23 who reported diabetic dyslipidaemia (41.7% and 37.5%) in both HIV-infected and HIV-uninfected diabetic patients, respectively. In contrast, Hadigan et al.Citation32 noted higher hypercholesterolaemia and hypertriglyceridaemia in HIV-infected diabetic patients compared with HIV-uninfected diabetic patients.
Some 30% of the HIV-infected patients in our study showed markedly high triglycerides and 30% showed abnormally low HDL levels, while the HIV-uninfected patients showed high triglycerides (37.5%) and abnormally low (62.5%) HDL levels. Our findings justify the findings of Lu et al.Citation31 in that the high levels of triglycerides and low levels of HDL may be major contributors to CVD development.
Waters and HsueCitation33 demonstrate a low level of LDL in patients receiving new HIV drugs with more favourable effects on lipid levels. Diabetic complications include elevated levels of LDL-C and triglycerides, and low levels of HDL-C.Citation34 This was also confirmed in a study by Albrki et al.,Citation35 who observed higher lipoprotein abnormalities in diabetics compared with the non-diabetic patients.
There were no significant differences in the LDL levels of both the HIV-infected and HIV-uninfected groups, as only 7.5% of patients had increased LDL. The majority of the participants had low levels of LDL. These findings are contrary to the previous studies mentioned above but are corroborated by Ugwu et al.,Citation36 who found the lipid and lipoprotein profiles of the diabetics were lower than those of the control group. These findings are similar to our study in both the HIV-infected and HIV-uninfected groups. The findings in the current study could be due to the successful drug therapy that has been administered to both cohorts of diabetics. A recent study by Choudhry et al.,Citation37 who compared non-diabetic and diabetic patients using serum and saliva samples, found that the LDL levels were increased in both groups of patients and the HDL was lower in the non-diabetic patients when compared with the diabetic patients.Citation37
Cholesterol levels were within normal limits in both groups; however, 12.5% and 7.5% of HIV-uninfected and HIV-infected patients had high levels, respectively. Genetic variants in the LPL locus are associated with abnormal serum lipid concentrations, and predisposition to CVD development.Citation38 More specifically, genetic polymorphisms of the pro-protein convertase subtilisin/kexin type 9 (PCSK9) gene, apolipoprotein A1 (APOL-A1), ATP-binding cassette protein A1 (ABCA1), and lecithin: cholesterol acyltransferase (LCAT) predispose to abnormal LDL-C levels.Citation39–41
In contrast to other studies where smoking was a risk factor for DM development, we report minimal smoking across our study population (7.5%). This may reflect lifestyle factors but most probably emanates from economic constraints in our catchment area. The results of the study by Calvo-Sanchez et al.Citation42 showed that smoking, diabetes, hypertension, family history of CVD and hypercholesterolaemia were more common in both HIV-infected and HIV-uninfected patients with acute coronary syndrome (ACS) than those without ACS.
HIV infection itself elevates cholesterol and triglyceride levels, and this is further aggravated by ART.Citation43 It is important to note that the type of ART, lipodystrophy, co-infection with other viruses, metabolic state and interaction of glycaemic drugs with ART may influence the lipid profile of the diabetic HIV-infected patient. Protease inhibitors (PI) such as saquinavir may cause a fivefold increase in DM.Citation44 Similarly, certain nucleoside reverse transcriptase inhibitors (NRTIs) are associated with DM risk via mitochondrial dysfunction.Citation45 All HIV-infected patients in our study received ARVs, a standard of care in SA. ARVs are associated with increase in bodyweight, a ‘return to health’ phenomenon rather than from reduced energy intake related to viral suppression,Citation46 and may be accountable for the high weight of HIV-infected patients. More specifically, integrase inhibitors elevate body mass index compared with protease inhibitors as regards protection against weight gain.Citation46 Furthermore, interactions between DM medications and ART are known.
However, one limitation of this study is the small sample size. Nonetheless, all patients received a triple ARV regimen, therefore we could not evaluate the effect of different ARV drugs. Also, in SA, a single clinic glucose reading is a standard procedure in the diabetic clinic. A more universal approach is to perform an HbA1c reading.
Conclusion
Blood glucose levels were upregulated in both HIV-infected and HIV-uninfected DM patients and may reflect glucose dysregulation in patients receiving ARVs. This study demonstrates similar lipid profiles in diabetic HIV-infected and HIV-naive patients. The dyslipidaemia may be attributed to obesity but it may also have been exacerbated by ARV usage in the HIV-infected group. Notably, a lack of exercise and poor diet in both our study groups contributed to the obesity and resultant poor BMI, hence their hypercholesteraemia and dyslipidaemia profiles. It is urgent that intensive measures be taken to promote public awareness of DM risk factors such as obesity, ARVs and lifestyle in South Africa, as behavioural/lifestyle adaptation will ameliorate this susceptibility.
Future studies
Future studies should evaluate adipokine profiles in HIV-infected diabetic patients receiving HAART. Moreover, consideration of the exact type of new ARV drugs and lifestyle factors requires a greater depth of study to understand their contribution to abnormal lipid profiles.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- UNAIDS DJRF. Report. 2021.
- STATISTICS SOUTH AFRICA. Mid-year population estimates 2021. Pretoria: Stats SA; 2021. 1–39.
- Hansoti B, Stead D, Parrish A, et al. HIV testing in a South African emergency department: a missed opportunity. PloS ONE. 2018;13(3):e0193858. https://doi.org/10.1371/journal.pone.0193858
- Moosa A, Gengiah TN, Lewis L, et al. Long-term adherence to antiretroviral therapy in a South African adult patient cohort: a retrospective study. BMC Infect Dis. 2019;19(1):1–12.
- Ballocca F, Gili S, D’Ascenzo F, et al. HIV infection and primary prevention of cardiovascular disease. lights and shadows in the HAART era. Prog Cardiovasc Dis. 2016;58(5):565–576.
- Bhatta DN, Adhikari R, Karki S, et al. Life expectancy and disparities in survival among HIV-infected people receiving antiretroviral therapy: an observational cohort study in Kathmandu, Nepal. BMJ Global Health. 2019;4(3):e001319. https://doi.org/10.1136/bmjgh-2018-001319
- Palios J, Kadoglou NPE, Lampropoulos S. The pathophysiology of HIV-/HAART-related metabolic syndrome leading to cardiovascular disorders: the emerging role of adipokines. Exp Diabetes Res. 2012;2011:103063–103067.
- Ogurtsova K, Guariguata L, Barengo NC, et al. IDF diabetes atlas: global estimates of undiagnosed diabetes in adults for 2021. Diabetes Res Clin Pract. 2022;183:109118. https://doi.org/10.1016/j.diabres.2021.109118
- Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. https://doi.org/10.1016/j.diabres.2019.107843
- Ogurtsova K, da Rocha Fernandes JD, Huang Y, et al. IDF diabetes atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40–50. https://doi.org/10.1016/j.diabres.2017.03.024
- Razaq RA, Mahdi JA, Jawad R. Sciences A. Information About Diabetes Mellitus. 2020;28(3):243–252.
- Bailey SL, Ayles H, Beyers N, et al. Diabetes mellitus in Zambia and the Western Cape province of South Africa: prevalence, risk factors, diagnosis and management. Diabetes Res Clin Pract. 2016;118:1–11. https://doi.org/10.1016/j.diabres.2016.05.001
- Saad MI, Abdelkhalek TM, Saleh MM, et al. Insights into the molecular mechanisms of diabetes-induced endothelial dysfunction: focus on oxidative stress and endothelial progenitor cells. Endocrine. 2015;50(3):537–567. https://doi.org/10.1007/s12020-015-0709-4
- Maluleke RJPSSA. Mortality and causes of death in South Africa, 2016. Findings from Death Notification. 2018.
- Grundlingh N, Zewotir TT, Roberts DJ, et al. Population, nutrition. Assessment of Prevalence and Risk Factors of Diabetes and pre-Diabetes in South Africa. J Health Popul Nutr. 2022;41(1):1–12. https://doi.org/10.1186/s41043-022-00281-2.
- Opoku S, Gan Y, Fu W, et al. Prevalence and risk factors for dyslipidemia among adults in rural and urban China. Findings from the China National Stroke Screening and Prevention Project (CNSSPP). 2019;19(1):1–15.
- Lagathu C, Béréziat V, Gorwood J, et al. Metabolic complications affecting adipose tissue, lipid and glucose metabolism associated with HIV antiretroviral treatment. Expert Opin Drug Saf. 2019;18(9):829–840. https://doi.org/10.1080/14740338.2019.1644317
- Koethe JR, Lagathu C, Lake JE, et al. HIV and antiretroviral therapy-related fat alterations. Nature Reviews Disease Primers. 2020;6(1):1–20. https://doi.org/10.1038/s41572-019-0135-7
- Gebrie A, Tesfaye B, Gebru T, et al. Diabetes mellitus and its associated risk factors in patients with human immunodeficiency virus on anti-retroviral therapy at referral hospitals of Northwest Ethiopia. Diabetol Metab Syndr. 2020;12(1):1–8.
- Peer N, Steyn K, Lombard C, et al. Rising diabetes prevalence among urban-dwelling black South Africans. PLoS ONE. 2012;7(9):1–9. https://doi.org/10.1371/journal.pone.0043336
- Tishkoff SA, Williams SMJNRG. Genetic analysis of African populations: human evolution and complex disease. Nat Rev Genet. 2002;3(8):611–621. https://doi.org/10.1038/nrg865
- Finer NJM. Medical consequences of obesity. J Clin Endocrinol Metab. 2015;43(2):88–93.
- Woyesa S, Mamo A, Mekonnen Z, et al. Lipid and lipoprotein profile in HIV-infected and non-infected diabetic patients: a comparative cross-sectional study design, Southwest Ethiopia. HIV/AIDS-Research and Palliative Care. 2021;13:1119–26. https://doi.org/10.2147/HIV.S339539.
- Adeniyi OV, Yogeswaran P, Longo-Mbenza B, et al. Uncontrolled hypertension and its determinants in patients with concomitant type 2 diabetes mellitus (T2DM) in rural South Africa. PloS ONE. 2016;11(3):e0150033. https://doi.org/10.1371/journal.pone.0150033
- Arcaya MC, Arcaya AL, Subramanian S. Inequalities in health: definitions, concepts, and theories. Glob Health Action. 2015;8(1):27106.
- Wanke C, Silva M, Knox T, et al. Weight loss and wasting remain common complications in individuals infected with human immunodeficiency virus in the era of highly active antiretroviral therapy. Clin Infect Dis. 2000;31(3):803–805.
- Campa A, Zhifang Y, Lai S, et al. HIV-related wasting in HIV-infected drug users in the era of highly active antiretroviral therapy. Clin Infect Dis. 2005;41(8):1179–1185.
- Amorosa V, Synnestvedt M, Gross R, et al. A tale of 2 epidemics: the intersection between obesity and HIV infection in Philadelphia. JAIDS J Acquir Immune Defic Syndr. 2005;39(5):557–561.
- Hodgson LM, Ghattas H, Pritchitt H, et al. Wasting and obesity in HIV outpatients. Aids. 2001;15(17):2341–2342.
- Crum-Cianflone N, Roediger MP, Eberly L, et al. Increasing rates of obesity among HIV-infected persons during the HIV epidemic. PLoS ONE. 2010;5(4):e10106. https://doi.org/10.1371/journal.pone.0010106.
- Lu S, Bao M-Y, Miao S-M, et al. Prevalence of hypertension, diabetes, and dyslipidemia, and their additive effects on myocardial infarction and stroke: a cross-sectional study in Nanjing, China. Ann Transl Med. 2019;7(18):436. https://doi.org/10.21037/atm.2019.09.04.
- Hadigan C, Meigs JB, Corcoran C, et al. Metabolic abnormalities and cardiovascular disease risk factors in adults with human immunodeficiency virus infection and lipodystrophy. Clin Infect Dis. 2001;32(1):130–139.
- Waters DD, Hsue PY. Lipid abnormalities in persons living with HIV infection. Can J Cardiol. 2019;35(3):249–259. https://doi.org/10.1016/j.cjca.2018.11.005
- Haffner S. Management of dyslipidemia in adults with diabetes. Diabetes Care. 1998;21(1):160–178.
- Albrki W, Elzouki A, El-Mansoury A, et al. Lipid profiles in Libyan type II diabetics. J Sci Appls. 2007;1:18–23.
- Ugwu C, Ezeanyika L, Daikwo M, et al. Lipid profile of a population of diabetic patients attending Nigerian national petroleum corporation clinic, Abuja. African J Biochem Res. 2009;3(3):066–069.
- Choudhry AA, Sharma P, Mohapatra T, et al. Assessment of salivary lipid profile parameters in healthy and type 2 diabetes mellitus patients. J Clin Diagn Res. 2021;15(6):1–5.
- Zhang W-S, Zhang W-H, Liu Q-J, et al. Lipoprotein lipase gene hind III polymorphism was associated with hemorrhagic stroke. Int J Clin Exp Med. 2015;8(6):9575.
- Natarajan P, Young R, Stitziel NO, et al. Polygenic risk score identifies subgroup with higher burden of atherosclerosis and greater relative benefit from statin therapy in the primary prevention setting. Circulation. 2017;135(22):2091–2101.
- Sadananda SN, Foo JN, Toh MT, et al. Targeted next-generation sequencing to diagnose disorders of HDL cholesterol. J Lipid Res. 2015;56(10):1993–2001.
- Khera AV, Emdin CA, Drake I, et al. Genetic risk, adherence to a healthy lifestyle, and coronary disease. N Engl J Med. 2016;375(24):2349–2358.
- Calvo-Sanchez M, Perello R, Perez I, et al. Differences between HIV-infected and uninfected adults in the contributions of smoking, diabetes and hypertension to acute coronary syndrome: two parallel case-control studies. HIV Med. 2013;14(1):40–48. https://doi.org/10.1111/j.1468-1293.2012.01057.x
- Neto L, das Neves MB, Ribeiro-Rodrigues R, et al. Dyslipidemia and fasting glucose impairment among HIV patients three years after the first antiretroviral regimen in a Brazilian AIDS outpatient clinic. Braz J Infect Dis. 2013;17(4):438–443.
- Sarkar S, Brown TTJE. Diabetes in people living with HIV. Endotext [Internet]. 2019:1–36.
- Noubissi EC, Katte J-C, Sobngwi E. Diabetes and HIV. Curr Diab Rep. 2018;18(11):125.
- Buzón-Martín L. Weight gain in HIV-infected individuals using distinct antiretroviral drugs. AIDS Rev. 2020;22(3):158–167.