ABSTRACT
Today and in the highly complex pharmaceutical industry, reverse logistics is a core capability for pharma companies that seek competitive advantages from the efficient management of returns and recalls, e.g. due to errors, expired stocks, quality-related or environmental issues, non-compliance and other consumer-related concerns. And yet in many developing countries where the pharma industry is a big part of the economy, the value of RL is not well understood. In this study, we examine the ambivalent impact of reverse logistics capabilities within the pharmaceutical supply chains in a developing country by focusing on four key reverse logistics capabilities, i.e. logistics information systems, process formalisation, flexibility, and top management support. The findings show that logistics information management systems, process formalisation, and flexibility significantly affect supply chain performance of pharmaceutical firms. However, the hierarchical regression found no significant moderating effect of top management support between reverse logistics capabilities and supply chain performance. Our subsequent discussion and implications for practice and future research are based on these findings.
Introduction
Until recently, reverse logistics was associated with the waste management process (Dekker et al. Citation2004). Today, the standard definition considers reverse logistics (RL) to be ‘the process of planning, implementing and controlling the efficient, cost-effective flow of raw materials, in-process inventory, finished goods and related information from the point of consumption to the point of origin for the purpose of recapturing or creating value or proper disposal’ (Jack, Powers, and Skinner Citation2010, 230). In the literature, the drivers of RL across several industries mostly include cost when firms recapture the value of returned products or reduce operating costs due to efficient reverse logistics systems (Bensalem & Kin, Citation2019: Jack, Powers, and Skinner Citation2010), environmental interests such as reducing the amount of waste and emissions (Olorunniwo & Li, Citation2011: Turrisi, Bruccoleri, and Cannella Citation2013), competitive pressure to satisfy customers (Vahabzadeh and Yusuff Citation2015), and now government regulations holding producers accountable for collection and recycling of their products and packages (Govindan and Bouzon Citation2018). Also the growing emphasis on sustainability, where firms seek to minimise their ecological footprint, reduce carbon emissions and enhance social responsibility throughout their supply chains, is pushing businesses into reverse logistics practices (Dabees et al. Citation2023).
In the pharmaceutical industry, which is the focal area of this study, RL is a salient process that largely aims to reduce customer risk both from an environmental perspective (e.g. product disposal, expired products, etc.) and a regulatory perspective (e.g. recalls, non-conformance, etc.). According to Aghalaya (Citation2012), efficient RL systems in the pharmaceutical industry, largely involving returns and recalls, are necessary because medicines are considered a high-value product that is critical to the health of consumers as well as the environment. Proper disposal of defect medicines can reduce environmental waste and pollution, thus maintaining acceptable environmental standards (Dabees et al. Citation2023). Yet in most developing countries, studies (such as Kwateng et al. Citation2014) show the pharma industry struggling with proper medicines management systems including cases of medicines being dubiously thrown back into the forward supply chain which is detrimental to public health. In Africa, for example. inefficient inventory management systems, the fragmented nature of pharmaceutical supply chains coupled with many uncoordinated actors and the lack of integration are some of the biggest setbacks for pharmaceutical firms. The study by Ali and Abdelsalam (Citation2017) in Egypt shows the lack of regulation and public awareness regarding the importance of RL as among the biggest challenges of pharma firms there. In South Africa, Makaleng and Lambert (Citation2021) found the issue of process-gatekeeping, resulting in damages during reverse flow in pharmaceutical firms. Vlachos (Citation2016) conclude that most of these institutional factors such as those listed by Ali and Abdelsalam (Citation2017) and Makaleng and Lambert (Citation2021) affect RL, not necessarily supply chain capabilities, which in turn affects firm performance, i.e. cost, and customer satisfaction.
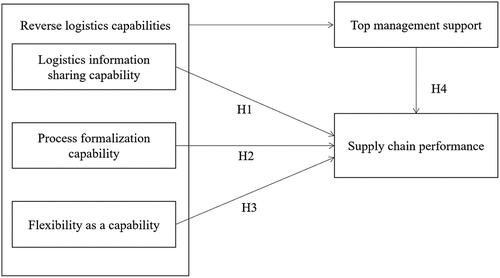
In this study, we set out to examine the ambivalent impact of reverse logistics capabilities within the supply chain: in particular, we examine the effect of information systems, process formalisation, and flexibility as some of the key reverse logistics capabilities on supply chain performance among pharmaceutical companies in Uganda.
The majority of studies on RL capabilities (such as Agrawal, Singh, and Murtaza Citation2015; Ha Citation2012; Li and Olorunniwo Citation2008; Autry Citation2005) have focused on RL in developed and high volumes industries such as the automobile industry, electrical, electronics, paper and plastics recycling among others. In this study, we focused on the pharmaceutical industry where environmental and waste disposal management implications due to high waste-to-product ratios have a significant impact on firm and supply chain performance. Moreover, pharmaceutical supply chains have relatively high demands for better information sharing, industrial process safety and flexibility that developing country pharma firms still grapple with (de Campos, Ten Caten, and de Paula Citation2021; Lima et al. Citation2022).
The paper is organised as follows. The next section presents the literature review section. Then the methodology is presented, while subsequent section presents the results. The final parts are the discussion and conclusion sections.
Review of the literature
The evolution of reverse logistics (RL) is traced back to the pre-industrial recycling and repurposing programs in post WWII when material shortages affected many manufacturers. Today, RL addresses issues of both the environment and natural resource depletion (Dabees et al. Citation2023). Further RL interest has been due to increased public awareness within industry and government, which now aims to address sustainability issues through closed-loop manufacturing (Ha Citation2012). The literature, however, offers a distinction between RL management and closed-loop manufacturing. Belvedere and Grando (Citation2017) consider closed-loop manufacturing the strategic intention of RL where RL focuses on taking waste out of the supply chain, while closed-loop manufacturing targets value recovery throughout the supply chain. Govindan and Soleimani (Citation2017) contends that closed loop economy renders the manufacturers fully responsible for the end-of-life products, which is technically an RL role responsible for the recovery and recycling of their products, i.e. that closed-loop supply chains use RL to feed products back into the supply chain.
Looking at the pharmaceutical industry, which is characterised by returns and recalls (Abbas and Farooquie Citation2013), argue that RL occurs mostly due to quality-related issues, for example non-compliance, adverse effects on the consumers, expired stocks and other quality-related defects. Other factors that could trigger goods to be returned or recalled also include errors in order fulfilment and changes in regulatory requirements (Lima et al. Citation2022).
In practice, RL in the pharma industry is more complicated due to uncertainty in planning and forecasting quantity, quality and timelines, yet it requires quick responsiveness and efficiency (Ha Citation2012). de Campos et al. (Citation2017) note that the demands of the highly competitive pharmaceutical industry require firms to continuously improve RL strategies and renew RL capabilities to match with every increasing complexity of medicine flows. Jack, Powers, and Skinner (Citation2010) argue that better RL performance is tied to the type and quality of logistics capabilities firms have. Turrisi, Bruccoleri, and Cannella (Citation2013) show that variability of reverse flows in a closed loop supply chains increases the serviceable inventory variance which in turn affects supply chain performance. In the next section, we go into detail about what theory infers in the RL capabilities context.
Reverse logistics capabilities – theory and hypotheses
According to Caldart (Citation2015), the capabilities of a firm are a set of resources and competencies that it relies on to survive in its market environment. This definition comes from the Resource-Based View Theory (RBV) of Jay Barney (Citation1996), which classifies resources as tangible (e.g. as facilities, money, equipment and employees etc.) and intangible (e.g. management skills, knowledge, reputation, brand image, and customer relationships etc.). The idea behind RBV is how firms efficiently utilise their resources to enable them to gain a competitive advantage (Hitt, Xu, and Carnes Citation2015). But in order to sustain the advantage over time, the resources must be unique and difficult to imitate or substitute by other firms’ resources (Barney Citation1996). Sirmon, Hitt, and Ireland (Citation2007) also suggested that unique inimitable resources alone cannot maintain a competitive advantage, but instead how firms bundle these resources to create capabilities and then leverage those capabilities is what makes them sustainable. Firms within the same industry are not identical, and their performance is subject to differ given the difference in capabilities possessed by each (Hawawini, Subramanian, and Verdin Citation2003).
Ha (Citation2012) identified three types of resources that are used to develop reverse logistics capabilities, namely, financial resources, technology resources and managerial resources. Jack, Powers, and Skinner (Citation2010) argued that while RL capabilities can enable retailers to enhance their return policies and improve their overall cost position, it is the resource commitments and contractual obligations that positively influence reverse logistics capabilities, which result in cost savings.
Reverse logistics processes are resource-intensive and require top management, given the nature of uncertainty and complexity. Daugherty et al. (Citation2005) examined how resource commitment and information technology capabilities impact RL performance and concluded that reverse logistics IT capabilities have a direct and positive impact on economic performance as well as service quality. Karbassi Yazdi et al. (Citation2020) assessed the critical success factors (CSFs) of RL for manufacturing companies in the healthcare industry and found that besides technological competencies and management attention, the company’s policies, work processes (ordering, transportation, etc.) and environment adaption are top CSFs for RL. Huang and Yang (Citation2014) examined the relationship between RL innovation and performance given the moderating effect of institutional pressure and concluded RL innovation positively correlates with economic performance. The innovative RL capabilities considered in their paper include customisation, formalisation, flexibility, information-related ability and cross-functional integration.
Based on the theoretical background, the study therefore addresses the importance of RL capabilities on supply chain performance where the literature in other industries (e.g. Bai and Sarkis Citation2013; Lima et al. Citation2022; Vlachos Citation2016) shows positive performance outcomes at the supply chain level, but these positive outcomes are mainly driven by institutional incentives and regulatory pressures.
Rather than the outside look into supply chain performance, this study focuses on the inward-looking, firm-level aspects of RL capability development and deployment, building on Vlachos’s (Citation2016) view that firms must find mechanisms of integrating the RL activities with the forward flow processes in what is called the closed-loop capability. This reduces transaction costs and enables more efficient in resource utilisation (Vlachos Citation2016).
In this study, we specifically focus on the following RL firm-level capabilities that appear to be important and yet have mixed results within the pharmaceutical industry in developing country context, i.e. the logistics information management capability. Both Daugherty, Myers, and Richey (Citation2002) and Lima et al. (Citation2022) show that information systems are central to RL strategies.
The process formalisation capability, according to de Campos, Ten Caten, and de Paula (Citation2021), enables different cost outcomes depending on which RL processes are being considered. The flexibility capability, according to Bai and Sarkis (Citation2013), generates different performance outcomes and helps manage uncertainties that arise in the RL networks. Finally, the role of top management in defining RL decisions, hence influencing institutional behaviour, is an integral part of RL (de Campos, Ten Caten, and de Paula (Citation2021). So, we propose the conceptual framework in as the research model of our paper, where the dependent variable supply chain performance is measured using the following constructs: reliability, responsiveness and agility.
The logistics information management capability (LIMC)
RL heavily relies on information technology and systems that facilitate information sharing with the aim of increasing visibility into the products throughout the reverse supply chain (Li and Olorunniwo Citation2008). According to Jack, Powers, and Skinner (Citation2010), RL capabilities include the accuracy and the availability of information, the process and timeliness of reverse logistics information, and the internal and external connectivity and usefulness of that information. These capabilities represent a bundle of information-related processes that enable a firm to manage better its reverse logistics activities which translates to cost savings.
Information systems and technology have proved to have a positive relationship with the cost and process effectiveness in reverse logistics (Huscroft Citation2010). An exploration study of RL practices in three companies by Li and Olorunniwo (Citation2008) confirmed that IT solutions allow effective information sharing with customers/suppliers, which enables swift material return authorisation and enables making correct decisions consistently in real-time. In other logistics research studies, Liu and Luo (Citation2012) show that information integration capabilities were positively associated with a higher level of competitive advantage and firm performance
This may not be different in the pharmaceutical industry in many developing countries where supply chain partners have invested in information systems to manage the flow of information, thus enhancing their supply chain performance (Ali and Abdelsalam Citation2017). de Campos, Ten Caten, and de Paula (Citation2021) argue that the lack of information technology limits quality assurance and leads to loss of product data in the RL flow, which directly affects supply chain performance. Therefore, LMIC is hypothesised to improve supply chain performance through better information and material flow. Thus:
H1:
Logistics information management capability in RL is positively related to supply chain performance.
The process formalization capability
Standardising processes and procedures has long been a practice used in the pharma industry as a mechanism for managing quality and process consistency in order to build routines and minimise errors and delays. Studies (Autry Citation2005; Genchev et al. Citation2010) have shown a positive effect of standardisation of reverse logistics processes, procedures and management roles on cost and process effectiveness to enable organisations to achieve a competitive advantage confirmed. One of the common difficulties observed with RL is the lack of standardisation of processes, which results in communication difficulties across the firm and with other supply chain actors. But formalisation remedies that good RL processes begin by simplifying and standardising returns policies and procedures which translate into fewer labour hours dedicated to returns processing as well as higher quality decisions (Rogers and Tibben-Lembke Citation1998). Formalisation can be achieved through written policies, defining roles and responsibilities, developing strategic and operational plans, defining objectives, processes standardisation and formalising communication systems (Genchev, Glenn Richey, and Gabler Citation2011).
Process formalisation and its positive effect on performance has been widely discussed in the logistics literature. Bowersox and Daugherty (Citation1992) identified formalisation as a key characteristic of leading-edge logistics organisations that can be used as a valuable tool for streamlining processes. Autry (Citation2005) examined the relationships between formalisation, liberal policies and related capabilities and the overall effectiveness of reverse logistics programs in the automotive aftermarket parts industry and found that RL process formalisation strengthened the relationship between returns handling capabilities and RL program effectiveness. Daugherty et al. (Citation2005) examined the influence of RL program design characteristics on the subsequent program performance and the differential influence of making versus buying RL program software and concluded that process formalisation did not significantly influence performance, especially for firms outsourcing software needs; it, however, influenced cost-effectiveness for firms that developed software in-house.
The benefits of formalising results in less task redundancy and increased processes control, which has a direct effect on organisational efficiency and therefore supply chain effectiveness. Thus:
H2:
Process formalization capability in RL is positively related to supply chain performance.
The flexibility capability
RL is characterised by uncertainties that could be internal or external in origin. This is mainly because it is a reactive process in response to external downstream partners and unique consumer requirements; therefore, firms do not proactively plan for it (Barad and Sapir Citation2003). As such, flexibility is regarded as a strategy for improving the responsiveness of the system to changes (Barad and Sapir Citation2003). Wang et al. (Citation2020) present a conceptual framework of reverse logistics capability in the pharmaceutical industry, where they identify five types of RL capabilities including technology, innovation capability, customisation capability, responsiveness capability and flexible operation capability. Some studies on logistics capabilities have indicated that the flexibility capability within the firm is positively associated with a higher level of competitive advantage (Liu and Luo Citation2012).
A framework for flexibility in RL is proposed by Bai and Sarkis (Citation2013) and subdivides flexibility into operational and strategic flexibilities. Operational flexibility includes product mix flexibility, volume flexibility, equipment flexibility, labour flexibility, supply flexibility and scheduling/routing flexibility across different reverse logistics processes. In contrast, strategic flexibilities are subdivided into network and organisation design flexibility dimensions. Both these two forms of flexibility represent the significance of flexible reverse logistics in building safety stock and managing uncertainty within the firm. However, within the supply chain context, several studies (e.g. Prater, Biehl, and Smith Citation2001) show that because of the coordination complexity across different product groups, distribution centres, supply chain actors and networks, high flexibility can have a boomerang effect in the form of higher information needs, additional distribution costs and hence high uncertainty. Thus:
H3:
The flexibility capability in RL is negatively related to supply chain performance.
Top management support
Top management responsibilities include the formulation of the strategy, communication of the strategy to firm members, and implementation. Formulating a proper RL strategy may create significant effects on performance because it supports firms in identifying the strategic roles of RL, eliminating ambiguity and clarifying priorities of resources for RL in the process of integrated supply chain management (Ha Citation2012).
Top management support enables the development and implementation of firm-specific and relational capabilities among supply chain partners for mutual benefits (Paulraj, Chen, and Lado Citation2012). Management support involves a commitment to resource allocation and alignment of the resources to strategic opportunities in order to leverage distinctive capabilities that result in a competitive advantage. Capabilities are learned skills and competencies that require repetition to perfect them, and while they are in pursuit of strategy, it is the task of management to continuously revisit the strategy and align it with expected future changes (Grant Citation1999). This enables the firm not only to meet the current competitive advantage but also to develop unique capabilities across its supply chain.
Top management support, leadership and commitment, therefore, play an important role in the organisation’s strategies, which ultimately impacts on its performance. Likewise, it is considered an essential antecedent in the implementation of supply chain management thus impacting supply chain performance (Mentzer et al. Citation2001). Thus:
H4:
Top management support positively moderates the relationship between reverse logistics capabilities and supply chain performance.
Supply chain performance
Studies (e.g. Beamon Citation1999; Shepherd, Günter, and MacBryde Citation2006; Sillanpää and Kohtamäki and Petri Helo Citation2015) indicate that measuring supply chain performance is an onerous task because of the difficulties in ascertaining at what level the metrics should apply. The most cross-cutting metrics include reliability, responsiveness, flexibility, quality, cost, innovativeness and efficiency.
In this study, supply chain performance metrics adopted from the supply chain operations reference (SCOR) model are conceptualised along five dimensions. Reliability, responsiveness and agility dimensions are customer focused, whereas costs and assets dimensions are internally focused.
It is deemed that internally focused supply chain performance measures of RL activities in the pharmaceutical industry may not always be appropriate. Instead, this study used customer-focused supply chain performance measures, emphasising reliability, responsiveness and agility. Besides Shepherd, Günter, and MacBryde (Citation2006) shows that unlike the plan, source, make and deliver dimensions of the SCOR model, the return aspect associated with customer satisfaction has very few performance measures.
The reliability attribute addresses the ability to perform tasks as required. Reliability focuses on the predictability of the outcome of a process. Typical metrics for the reliability attribute include on-time, the right quantity, and the right quality. The responsiveness attribute describes the speed at which tasks are performed. The average time associated with return processes, for examples include return cycle-time metrics. The agility attribute describes the ability to respond to external influences and the ability to respond to marketplace changes to gain or maintain competitive advantage. SCOR agility metrics include adaptability and overall value at risk (Gordon Citation1997).
Methods
Participants and instrumentation
The study is based on a survey of the key pharmaceutical companies, their suppliers and customers in Uganda. The questionnaire used included four sections: background information, reverse logistics capabilities, supply chain performance and top management support. The questionnaire was sent to pharmaceutical companies in the entire pharma supply chain, and these included manufacturers, wholesale pharmacies and retail pharmacies in the central region of Uganda. The target respondents were staff in different departments handling returns to their stores or manufacturing facilities. Multi-item scales measured RL capabilities. The tool was subjected to one pilot among a small group of 20 managers with pharmaceutical industry knowledge at a renowned pharma firm in Uganda to test for reliability, acceptability and check for any anomalies.
Data collection
Following the piloting round, modifications were made to the questionnaire. For example, the language was simplified for easy interpretation by the respondents. Also, in preparation of the main survey, a self-administered questionnaire with items measured on a five-point Likert scale was entered into the KoBoToolbox data collection tool and a link was sent to respondents. This enabled easy collection of data from various respondents during the COVID-19 pandemic and eliminated the submission of responses with missing items.
Reliability and validity
The research instrument was proofread by two experts in SCM to establish face validity. The Kaiser-Meyer-Olkin (KMO) measure of sampling adequacy was 0.898 and Bartlett’s test Chi-square of 2823.004 (P-value = 0.000), which suggested that factor analysis was appropriate. Construct validity tests were conducted using factor analysis utilising principal component analysis (PCA) with the varimax rotation method. Items loaded above 0.5, which is the minimum recommended value in research, were considered for further analysis. Therefore, the factor analysis results satisfied the criteria of construct validity, including both the discriminant validity (loading of at least 0.50, no cross-loading of items above 0.50) and convergent validity (total variance of eigen values greater than 1 was 76.5%). Reliability and consistency were examined by establishing internal consistency reliability of the measurement scales for the study variables as well as split-half reliability using Cronbach’s Alpha. All the reliability coefficients were above 0.70. The factor loadings and reliabilities are presented in below.
Table 1. Validity and reliability test for factors and items.
Findings
The background information of respondents
In this section, data are presented on the background information of the respondents, which included gender, age group, education level, length of service, department and category of organisation. All the results are based on the 102 responses.
shows that majority of the respondents were male (66.7%) and 33.3% of the respondents were female. 29.4% of the respondents were between 20 and 30 years of age, 20.6% were between 31 and 35 years of age, 17.6% were between 36 and 40 years of age, 17.6% were between 41 and 45 years of age and 14.7% were above 45 years of age. 72.5% of the respondents hold a bachelor’s degree, 20.6% have attained a master’s degree, 3.9% are diploma holders, 2.0% had undertaken a postgraduate diploma and 1.0% were PhD holders. The pharmaceutical industry has a highly experienced workforce with a period of service above 6 years illustrated by 61.8%, 24.5% of the respondents have served for a period of 3–6 years and 13.7% have served for a period below 3 years. Most of the respondents that participated in this study were from the regulatory affairs department represented by 40.2%, 32.4% were from the sales department, 11.8% were from the procurement department, 9.8% are working in the warehouse department and 5.9% respondents came from the marketing department. Most respondents are working in Retail outlets represented by 58.8%, 36.3% of respondents work in wholesale outlets and 4.9% work in manufacturing facilities.
Table 2. Demographic information of the respondents.
Empirical results from the quantitative analysis
The model results in above show that logistics information management capability had a positive and significant effect on supply chain performance in Ugandan pharmaceutical industry (B = 0.843, P-value <0.05). The model findings reveal that a unit increase in logistics information management capability results in an increase in supply chain performance by 0.843.
Table 3. Model findings on the effect of logistics information management capability on supply chain performance.
The results from the ANOVA table show that the model fits well with the data on logistics information management capability and supply chain performance. The results from the coefficient of determination (adjusted R-square) show that 60.9% of the variations in supply chain performance are explained by logistics information management capability, and other factors explain the remaining 39.1% of the variations. The results that were obtained revealed a significant effect since the P-value is below 0.05 level of significance; therefore, the null hypothesis (H1) was rejected in support of the alternative hypothesis, that there’s a significant effect of logistics information management capability on supply chain performance.
Process formalization capability in the pharmaceutical industry
The findings from the study show that there is a positive and statistically significant effect of process formalisation capability on supply chain performance in pharmaceutical companies in Uganda (B = 0.852, P-value <0.05). The evidence in shows that an increase in process formalisation capability by one unit significantly increases supply chain performance by 0.852.
Table 4. Model findings on the effect of process formalisation capability on supply chain performance.
The results from the ANOVA table show that the model fits well the data on the two variables since the P-value (0.000) for F-test is less than 0.05 level of significance (F = 109.285, P-value = 0.000). In addition, the model summary findings show that process formalisation capability accounts for 51.7% of the total variations in supply chain performance in pharmaceutical companies in Uganda and 48.3% of the variations are accounted for by other factors which were not included in the model.
The results that were obtained revealed a significant effect since the P-value, which is below 0.05 level of significance; therefore, the null hypothesis (H2) was rejected in support of the alternative hypothesis, that there’s a significant effect of process formalisation capability on supply chain performance.
The findings from the study show that there is a positive and statistically significant effect of flexibility capability on supply chain performance in pharmaceutical companies in Uganda (B = 0.802, P-value <0.05). The evidence in shows that an increase in flexibility capability by one unit significantly increases supply chain performance by 0.802. The results from the ANOVA table show that the model fits well the data on the two variables since the P-value (0.000) for F-test is less than 0.05 level of significance (F = 105.013, P-value = 0.000).
Table 5. Model findings on the effect of flexibility capability on supply chain performance.
In addition, the model summary findings show that flexibility capability accounts for 50.7% of the total variations in supply chain performance in pharmaceutical companies in Uganda, and 49.3% of the variations are accounted for by other factors, which were not included in the model.
The results that were obtained revealed a positive significant effect since the P-value is below 0.05 level of significance; therefore, the null hypothesis (H3) was rejected in support of the alternative hypothesis, that there’s a significant effect of flexibility capability on supply chain performance.
Supply chain performance in the pharmaceutical industry
The moderating effect of top management support on the relationship between reverse logistics capabilities and supply chain performance
The researcher examined the moderating effect of top management support on the relationship between reverse logistics capabilities and supply chain performance using the steps proposed by Baron and Kenny (Citation1986). To test for moderation, the researchers used hierarchical regression.
Model 1 in with top management support and reverse logistics capabilities as the independent variables and supply chain performance as the dependent variable produced F-change (2, 99) = 113.791 and P-value < 0.05. The model has revealed that top management support and reverse logistics capabilities have a statistically significant effect on supply chain performance. The variations in supply chain performance accounted for by top management support and reverse logistics capabilities is 69.1%, and 30.9% is accounted for by other factors.
Table 6. Model goodness of fit with supply chain performance as the dependent variable and reverse logistics capabilities, top management support, and interaction term as independent.
In model 2 in , top management support and reverse logistics capabilities were centred, and the interaction term (Top management support X Reverse logistics capabilities) was entered into the model. The R-change was 0.001, which was an increase in variations accounted for over model 1 though the increase was not statistically significant at the 5% level. The second model shows that the effect of top management support, reverse logistics capabilities and interaction term on supply chain performance was not statistically significant, F-change (1, 98) = 0.206 and P-value >0.05. The variations in supply chain performance accounted for by top management support, reverse logistics capabilities, and the interaction term was 68.8%, leaving 31.2% accounted for by other factors.
The results from the model coefficients in show that before including the interaction term in model 2, model 1 produced a reverse logistics capabilities model coefficient of 0.924, t-test value of 9.536, and was statistically significant (P-value <0.05). The model coefficient of top management support was 0.118 with t-test value of 1.749 and was not statistically significant at 5% level (P-value = 0.083).
Table 7. Model coefficients with supply chain performance as the dependent variable and reverse logistics capabilities, top management support and interaction term as independent.
After including the interaction term, the model coefficient of reverse logistics capabilities reduced to 0.835 with a t-test value of 3.801 and was statistically significant at a 5% level (P-value = 0.000). The model coefficient of top management support reduced to 0.054 with a t-test value of 0.343 and was not statistically significant at a 5% level (P-value = 0.732). The interaction term (Reverse logistics capabilities X Top management support) was not significant at a 5% level (P-value = 0.651, beta coefficient = 0.025). This indicates that there is no significant moderating effect of top management support on the relationship between reverse logistics capabilities and supply chain performance in the Ugandan pharmaceutical industry. Therefore, the null hypothesis (H4) was accepted that there is no significant moderating effect of top management support between reverse logistics capabilities and supply chain performance.
Discussions of findings
Earlier we set out to examine the ambivalent impact of reverse logistics capabilities within the pharmaceutical supply chains in developing country context. We identified and focused four most important reverse logistics variables based on the nature of the pharmaceutical supply chains in general and their significance on supply chain performance in the African pharma context. These included the logistics information management systems, process formalisation and flexibility capabilities and top management support as a moderating variable.
Most of the results corroborate the observations in the literature except for one important aspect. First, the study revealed that logistics information management capability had a positive significant effect on supply chain performance in the pharmaceutical industry. This research result is consistent with Huscroft (Citation2010), Jack, Powers, and Skinner (Citation2010), and Li and Olorunniwo (Citation2008). More specifically that pharmaceutical supply chains depend a lot on the collaboration of actors to share the right information about product flow and the right information system support that capability (de Campos, Ten Caten, and de Paula Citation2021). As proposed by de Campos et al. (Citation2023), information systems and information systems technology are extremely important for the performance of daily tasks, reducing errors and costs in the entire supply chain, easing manual identification and labelling which has an important logistic performance.
Notwithstanding the importance of logistics information systems within pharma supply chains, there was some evidence that companies still struggled with information integration across the entire industry, which posed challenges to supply chain visibility of medicines and other healthcare products: creating an opportunity for defective products to exist within the end-to-end supply chain which may cause danger to public health while compromising sustainable availability of quality medicines (Shashi and Gossett Citation2022). The information systems should not only be able to collect information but also should facilitate sharing this information with industry stakeholders to enable visibility and traceability of inventory within the return process (Daugherty et al. Citation2005; Daugherty, Myers, and Richey Citation2002).
Second, the process formalisation capability was found to have a significant effect on supply chain performance. This result lends support to previous empirical studies by Genchev et al. (Citation2010), Autry (Citation2005), which show that having documented procedures describing the handling of the return process enables the process to be managed efficiently and effectively since employees know what to do and when to do it and get well versed with the process, which eliminates irregularities in return handling. Autry (Citation2005) study of the relationship between reverse logistics capabilities and reverse logistics program effectiveness highlights the importance of formalisation on the relationship between reverse logistics effectiveness and firm performance. Defining roles and responsibilities and having formalised communication channels through well-organised reporting structures enables timely decision-making on the return process, which eliminates delays in removing defective products from the supply chain. Wang et al. (Citation2020) claim that RL process standardisation is a key attribute for innovation in the supply chain.
Third, the study revealed that flexibility capability had a significant effect on supply chain performance although it was the least predictor for variance in supply chain performance.
While the finding and observations are supported by other scholars such as Bai and Sarkis (Citation2013) and Liu and Luo (Citation2012) who regarded flexibility as a strategy for improving the responsiveness of the system to changes, it was also evident from the study that the studied pharmaceutical companies had not developed their own flexibility capabilities in terms of scheduling/routing and volume flexibilities: the majority were able to outsource the activities of reverse logistics to a third-party provider.
Due to the unpredictable nature of returns in the pharmaceutical industry, companies should develop flexibility capabilities to be in a position to handle the magnitude of the uncertainties given the detrimental effects it could have on the entire public health system (Wang et al. Citation2020). One factor that may explain the supposed RL inflexibility in the pharmaceutical industry in Africa was the limited collaboration across the supply chain and also the perceived complexity that RL infers across the different levels of the supply chain.
And finally, the study revealed no significant moderating effect on supply chain performance. This confirms that top management does not consider reverse logistics as an important strategy to supply chain performance, and therefore may not commit resources to the development of reverse logistics capabilities and does not directly involve themselves in the reverse logistics activities within pharmaceutical companies in Uganda. This is contradictory to the empirical evidence that considered top management support an important antecedent in the implementation of supply chain management, thus impacting supply chain performance (Mentzer et al. Citation2001). Both de Campos, Ten Caten, and de Paula (Citation2021) and de Campos et al. (Citation2023) place a strong emphasis on the role of good top management in articulating the strategic benefits of RL to internal actors and external supply chain actors.
The contradictory result, however, may be due to the fact that management of reverse logistics in pharmaceutical industries in many developing countries was based on the evidence from Uganda and is not considered a strategic activity which explains the significance of planning for handling returns.
Conclusion & recommendations
Based on the findings of this study, reverse logistics capabilities play an important part in attaining supply chain performance for many pharmaceutical firms in terms of reliability, responsiveness and agility. The study concludes that in order to manage returns appropriately, logistics information management, process formalisation and flexibility are necessary capabilities that guarantee better supply chain performance. It also concludes that the role of top management is not as significant as we assume – rather it is the reverse logistics capabilities that are important when discussing supply chain performance in a developing country context.
While outside the scope of this paper, the fragmented optimisation in RL strategies across the entire pharma industry was well noted because of the low collaboration and low information integration. This could, in part, explain the positive results on all the RL capabilities considered, i.e. logistics information management, process formalisation and flexibility in the supply chain in such a highly unstructured and emerging pharma industry. Thus, we interpreted that individual pharma companies optimised RL locally and at the individual firm and occasionally supply chain level (for the large firms), but not across the entire industry. The literature shows that limited interdependence of supply chain actors has a significant effect on the successful implementation of RL strategies irrespective of the level of development of the industry.
In general, the findings show that data adapted well to the structure of the conceptual model presented, and, in general, it offers a better understanding of the pharmaceutical supply chains in developing countries. Furthermore, the paper presents RL capabilities as an important resource to be exploited not just at the firm level and the supply chain levels but across the entire industry in a manner that adds value to customers and creates advantages over competitors.
Having confirmed the validity of the effect of reverse logistics capabilities on supply chain performance in the management of returns in the pharmaceutical industry, the study recommended that companies need to invest in developing logistics information management systems, process formalisation, and flexibility capabilities to enable them to manage returns more efficiently and effectively. The study also recommends further research on the optimisation of reverse logistics capabilities at the pharma industry level and perhaps firms to seek integration with forward logistics capabilities for better process optimisation.
Acknowledgments
We are deeply grateful to all those who played a role in bringing this paper this far. We would like to specifically thank Dr. George William Kajjumba (Southern Nevada Water Authority, NV, USA) for their invaluable feedback on the methods and results section of the paper.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Abbas, H., and J. A. Farooquie. 2013. “Return and Disposal of Unused Medicines: A Customer Perspective of Reverse Logistics.” International Journal of Business and Management Invention 2 (11): 59–66.
- Aghalaya, S. N., A. A. Elias, and R. K. Pati. 2012. “Analysing Reverse Logistics in the Indian Pharmaceuticals Industry: A Systems Approach. 26th Australian and New Zealand academy of management conference, Perth, December 5–7, 1–19.
- Agrawal, S., R. K. Singh, and Q. Murtaza. 2015. “A Literature Review and Perspectives in Reverse Logistics.” Resources, Conservation & Recycling 97:76–92. https://doi.org/10.1016/j.resconrec.2015.02.009.
- Ali, C., and A. Abdelsalam. 2017. “Analyzing Pharmaceutical Reverse Logistics Barriers: An Interpretive Structural Modeling Approach.” International Journal of Applied Logistics (IJAL) 7 (1): 16–48. https://doi.org/10.4018/IJAL.2017010102.
- Autry, C. W. 2005. “Formalization of Reverse Logistics Programs: A Strategy for Managing Liberalized Returns.” Industrial Marketing Management 34 (7): 749–757. https://doi.org/10.1016/j.indmarman.2004.12.005.
- Bai, C., and J. Sarkis. 2013. “Flexibility in Reverse Logistics: A Framework and Evaluation Approach.” Journal of Cleaner Production 47:306–318. https://doi.org/10.1016/j.jclepro.2013.01.005.
- Barad, M., & D. E. Sapir. 2003. “Flexibility in Logistic Systems—Modeling and Performance Evaluation.” International Journal of Production Economics 85 (2): 155–170. https://doi.org/10.1016/S0925-5273(03)00107-5.
- Barney, J. B. 1996. “The Resource-Based Theory of the Firm.” Organization Science 7 (5): 469–469. https://doi.org/10.1287/orsc.7.5.469.
- Baron, R. M., and D. A. Kenny. 1986. “The Moderator–Mediator Variable Distinction in Social Psychological Research: Conceptual, Strategic, and Statistical Considerations.” Journal of Personality & Social Psychology 51 (6): 1173.
- Beamon, B. M. 1999. “Measuring Supply Chain Performance.” International Journal of Operations & Production Management 19 (3): 275–292. https://doi.org/10.1108/01443579910249714.
- Belvedere, V., and A. Grando. 2017. “Sustainable Operations and Supply Chain Management.” Wiley series in operations research and management science. Chichester, West Sussex, UK: John Wiley & Sons Ltd.
- Bensalem, A., and V. Kin 2019. “A Bibliometric Analysis of Reverse Logistics from 1992 to 2017.” Supply Chain Forum: An International Journal 20 (1): 15–28.
- Bowersox, D. J., and P. J. Daugherty. 1992. “Logistics Leadership–Logistics Organizations of the Future.” Logistics Information Management 5 (1). https://doi.org/10.1108/09576059210011446.
- Caldart, A. A. 2015. “Capabilities and Capability Analysis.” Wiley Encyclopedia of Management 12:1–2. https://doi.org/10.1002/9781118785317.weom120006.
- Dabees, A., M. Barakat, S. S. Elbarky, and A. Lisec. 2023. “A Framework for Adopting a Sustainable Reverse Logistics Service Quality for Reverse Logistics Service Providers: A Systematic Literature Review.” Sustainability 15 (3): 1755. https://doi.org/10.3390/su15031755.
- Daugherty, P. J., M. B. Myers, and R. G. Richey. 2002. “Information Support for Reverse Logistics: The Influence of Relationship Commitment.” Journal of Business Logistics 23 (1): 85–106. https://doi.org/10.1002/j.2158-1592.2002.tb00017.x.
- Daugherty, P. J., R. G. Richey, S. E. Genchev, and H. Chen. 2005. “Reverse Logistics: Superior Performance Through Focused Resource Commitments to Information Technology.” Transportation Research Part E: Logistics & Transportation Review 41 (2): 77–92. https://doi.org/10.1016/j.tre.2004.04.002.
- de Campos, E. A. R., I. C. de Paula, C. S. T. Caten, K. P. Tsagarakis, and J. L. D. Ribeiro. 2023. “Logistics Performance: Critical Factors in the Implementation of End-Of-Life Management Practices in the Pharmaceutical Care Process.” Environmental Science and Pollution Research 30:29206–29228. https://doi.org/10.1007/s11356-022-24035-z.
- de Campos, E. A. R. D., I. C. D. Paula, R. N. Pagani, and P. Guarnieri 2017. “Reverse Logistics for the End-Of-Life and End-Of-Use Products in the Pharmaceutical Industry: A Systematic Literature Review.” Supply Chain Management: An International Journal 22 (4): 375–392.
- de Campos, E. A. R., C. S. Ten Caten, and I. C. de Paula. 2021. “End-Of-Use and End-Of-Life Medicines—Insights from Pharmaceutical Care Process into Waste Medicines Management.” Environmental Science and Pollution Research 28 (41): 58170–58188. https://doi.org/10.1007/s11356-021-14661-4.
- Dekker, R., M. Fleischmann, K. Inderfurth, and L. N. Van Wassenhove. 2004. Reverse Logistics: Quantitative Models for Closed-Loop Supply Chains, 436. 1st ed. Berlin, Heidelberg: Springer. https://doi.org/10.1007/978-3-540-24803-3.
- Genchev, S. E., R. Glenn Richey, and C. B. Gabler. 2011. “Evaluating Reverse Logistics Programs: A Suggested Process Formalization.” The International Journal of Logistics Management 22 (2): 242–263. https://doi.org/10.1108/09574091111156578.
- Genchev, S. E., T. D. Landry, P. J. Daugherty, and A. S. Roath. 2010. “Developing Reverse Logistics Programs: A Resource Based View.” Journal of Transportation Management 21 (1): 7–26. https://doi.org/10.22237/jotm/1270080120.
- Gordon, S. 1997. “Supply‐chain operations reference model (SCOR): the first cross‐industry framework for integrated supply‐chain management.” Logistics Information Management 10 (2): 62–67. https://doi.org/10.1108/09576059710815716.
- Govindan, K., and M. Bouzon. 2018. “From a Literature Review to a Multi-Perspective Framework for Reverse Logistics Barriers and Drivers.” Journal of Cleaner Production 187:318–337. https://doi.org/10.1016/j.jclepro.2018.03.040.
- Govindan, K., and H. Soleimani. 2017. “A Review of Reverse Logistics and Closed-Loop Supply Chains: A Journal of Cleaner Production Focus.” Journal of Cleaner Production 142:371–384. https://doi.org/10.1016/j.jclepro.2016.03.126.
- Grant, R. M. 1999. “The Resource-Based Theory of Competitive Advantage: Implications for Strategy Formulation.” Knowledge and Strategy, edited byM. H. Zack, 3–23. Butterworth-Heinemann. https://doi.org/10.1016/B978-0-7506-7088-3.50004-8 .
- Ha, N. V. 2012. “Development of Reverse Logistics–Adaptability and Transferability.” Published dissertation in Technische Universität Darmstadt–Germany. Accessed May 18, 2024. https://tuprints.ulb.tu-darmstadt.de/3220/1/NguyenHa_Dissertation_2012.pdf.
- Hawawini, G., V. Subramanian, and P. Verdin. 2003. “Is Performance Driven by Industry-Or Firm-Specific Factors? A New Look at the Evidence.” Strategic Management Journal 24 (1): 1–16. https://doi.org/10.1002/smj.278.
- Hitt, M. A., K. Xu, and C. M. Carnes. 2015. “Resource Based Theory in Operations Management Research.” Journal of Operations Management 41 (1): 77–94. https://doi.org/10.1016/j.jom.2015.11.002.
- Huang, Y. C., and M. L. Yang. 2014. “Reverse Logistics Innovation, Institutional Pressures and Performance.” Management Research Review 37 (7): 615–641. https://doi.org/10.1108/MRR-03-2013-0069.
- Huscroft, J. R., Jr. 2010. The Reverse Logistics Process in the Supply Chain and Managing Its Implementation. Auburn University. Accessed April 18, 2024. https://etd.auburn.edu/bitstream/handle/10415/2438/Huscroft_FINAL_Ver_3_Dissertation_TC_good.pdf;sequence=2.
- Jack, E. P., T. L. Powers, and L. Skinner. 2010. “Reverse Logistics Capabilities: Antecedents and Cost Savings.” International Journal of Physical Distribution & Logistics Management 40 (3): 228–246. https://doi.org/10.1108/09600031011035100.
- Karbassi Yazdi, A., P. F. Wanke, T. Hanne, and E. Bottani. 2020. “A Decision-Support Approach Under Uncertainty for Evaluating Reverse Logistics Capabilities of Healthcare Providers in Iran.” Journal of Enterprise Information Management 33 (5): 991–1022. https://doi.org/10.1108/JEIM-09-2019-0299.
- Kwateng, K. O., B. Debrah, D. V. Parker, R. N. Owusu, and H. Prempeh. 2014. “Reverse Logistics Practices in Pharmaceutical Manufacturing Industry: Experiences from Ghana.” Global Journal of Business Research 8 (5): 17–26.
- Li, X., and F. Olorunniwo. 2008. “Case Study an Exploration of Reverse Logistics Practices in Three Companies.” Supply Chain Management: An International Journal 13/5, 13 (5): 381–386. https://doi.org/10.1108/13598540810894979.
- Lima, P. A. B., F. C. M. Delgado, T. L. dos Santos, and A. P. Florentino. 2022. “Medications Reverse Logistics: A Systematic Literature Review and a Method for Improving the Brazilian Case.” Cleaner Logistics and Supply Chain 3:100024. https://doi.org/10.1016/j.clscn.2021.100024.
- Liu, L., and D. Luo. 2012. “Effects of Logistics Capabilities on Performance in Manufacturing Firms.” Contemporary Logistics 9:8–14. Accessed April 18, 2024. https://www.proquest.com/scholarly-journals/effects-logistics-capabilities-on-performance/docview/1355493538/se-2.
- Makaleng, M. S. M., and K. R. Lambert. 2021. “Evaluation of Reverse Logistics in Challenges within the Manufacturing Pharmaceutical Companies.” Emerging Science Journal 5 (4): 486–496. https://doi.org/10.28991/esj-2021-01291.
- Mentzer, J. T., J. S. Keebler, N. W. Nix, C. D. Smith, Z. G. Zacharia, C. D. Smith, and Z. G. Zacharia. 2001. “Defining Supply Chain Management.” Journal of Business Logistics 22 (2): 1–25. https://doi.org/10.1002/j.2158-1592.2001.tb00001.x.
- Olorunniwo, F. O., and X. Li. 2011. “An Overview of Some Reverse Logistics Practices in the United States.” Supply Chain Forum: An International Journal 12 (3): 2–9.
- Paulraj, A., I. J. Chen, and A. A. Lado. 2012. “An Empirical Taxonomy of Supply Chain Management Practices.” Journal of Business Logistics 33 (3): 227–244. https://doi.org/10.1111/j.0000-0000.2012.01046.x.
- Prater, E., M. Biehl, and M. A. Smith. 2001. “International Supply Chain Agility - Tradeoffs Between Flexibility and Uncertainty.” International Journal of Operations & Production Management 21 (5/6): 823–839. https://doi.org/10.1108/01443570110390507.
- Rogers, D. S., and R. S. Tibben-Lembke. 1998. Going Backwards: Reverse Logistics Trends and Practices. Pittsburgh, PA: RLEC Press.
- Shashi, M., and K. Gossett. 2022. “Exploring Strategies to Leveraging the Blockchain in Pharmaceutical Supply Chains.” International Journal Research Analysis Review 9:284–290. http://dx.doi.org/10.1729/Journal.31224.
- Shepherd, C., H. Günter, and J. MacBryde. 2006. “Measuring Supply Chain Performance: Current Research and Future Directions.” International Journal of Productivity & Performance Management 55 (3/4): 242–258. https://doi.org/10.1108/17410400610653219.
- Sillanpää, I., and M. Kohtamäki and Petri Helo. 2015. “Empirical Study of Measuring Supply Chain Performance.” Benchmarking: An International Journal 22 (2): 290–308. https://doi.org/10.1108/BIJ-01-2013-0009.
- Sirmon, D. G., M. A. Hitt, and R. D. Ireland. 2007. “Managing Firm Resources in Dynamic Environments to Create Value: Looking Inside the Black Box.” Academy of Management Review 32 (1): 273–292. https://doi.org/10.5465/amr.2007.23466005.
- Turrisi, M., M. Bruccoleri, and S. Cannella. 2013. “Impact of Reverse Logistics on Supply Chain Performance.” International Journal of Physical Distribution & Logistics Management 43 (7): 564–585. https://doi.org/10.1108/IJPDLM-04-2012-0132.
- Vahabzadeh, A. H., and R. B. M. Yusuff. 2015. “A Content Analysis in Reverse Logistics: A Review.” Journal of Statistics and Management Systems 18 (4): 329–379. https://doi.org/10.1080/09720510.2014.927605.
- Vlachos, I. P. 2016. “Reverse Logistics Capabilities and Firm Performance: The Mediating Role of Business Strategy.” International Journal of Logistics: Research & Applications 19 (5): 424–442. https://doi.org/10.1080/13675567.2015.1115471.
- Wang, M., P. Shi, B. Wang, and G. Chow. 2020. “Reverse logistics capability for sustainable development in a pharmaceutical industry : a conceptual framework from a logistics management perspective.” 10th International Conference on Operations and Supply Chain Management (OSCM): Technology, People, and Innovation in Supply Chain, December 14–16.