ABSTRACT
The ongoing Zika virus (ZIKV) outbreak in Latin America, the Caribbean, and the Pacific Islands has underlined the need for a coordinated research network across the whole region that can respond rapidly to address the current knowledge gaps in Zika and enhance research preparedness beyond Zika. The European Union under its Horizon 2020 Research and Innovation Programme awarded three research consortia to respond to this need. Here we present the ZikaPLAN (Zika Preparedness Latin American Network) consortium. ZikaPLAN combines the strengths of 25 partners in Latin America, North America, Africa, Asia, and various centers in Europe. We will conduct clinical studies to estimate the risk and further define the full spectrum and risk factors of congenital Zika virus syndrome (including neurodevelopmental milestones in the first 3 years of life), delineate neurological complications associated with ZIKV due to direct neuroinvasion and immune-mediated responses in older children and adults, and strengthen surveillance for birth defects and Guillain–Barré Syndrome. Laboratory-based research to unravel neurotropism and investigate the role of sexual transmission, determinants of severe disease, and viral fitness will underpin the clinical studies. Social messaging and engagement with affected communities, as well as development of wearable repellent technologies against Aedes mosquitoes will enhance the impact. Burden of disease studies, data-driven vector control, and vaccine modeling as well as risk assessments on geographic spread of ZIKV will form the foundation for evidence-informed policies. While addressing the research gaps around ZIKV, we will engage in capacity building in laboratory and clinical research, collaborate with existing and new networks to share knowledge, and work with international organizations to tackle regulatory and other bottlenecks and refine research priorities. In this way, we can leverage the ZIKV response toward building a long-term emerging infectious diseases response capacity in the region to address future challenges.
Responsible Editor Nawi Ng, Umeå University, Sweden
Background
The 21st century has seen an unparalleled number of emerging infections – the latest is the Zika virus (ZIKV) epidemic in Latin America and South Pacific Islands [Citation1]. The unusual neurotropism of this flavivirus resulting in clusters of neurodevelopmental birth defects, further compounded by clusters of severe neurological disease in adults, triggered the World Health Organization (WHO) to declare a public health emergency of international concern (PHEIC) in February 2016. The ongoing ZIKV outbreak has exposed the challenges associated with the implementation of urgently needed research in the Latin American region, and has underlined the need for a coordinated research network across the whole region that can rapidly respond to emerging threats. Such a network would not only facilitate research to investigate ZIKV but also be available to respond rapidly to any future emerging threats in Latin America.
For this reason, in January 2016 the European Commission announced a funding call, with the specification to set up a research network across the Latin America region to facilitate, coordinate and implement urgent research against the current ZIKV outbreak, and eventually lay the foundation for a preparedness research network against any future emerging severe infectious threats. The scope of the call can be found in : Call Topic SC1-PM-22-2016. The submission deadline was 28 April 2016; on 21 October 2016 the European Commission formally announced that three consortia were awarded: ZikaPLAN (www.zikaplan.tghn.org), ZIKAction (www.zikaction.org), and ZIKAlliance (www.zikalliance.tghn.org). Here we present ZikaPLAN, the composition of the consortium and its objectives and research design.
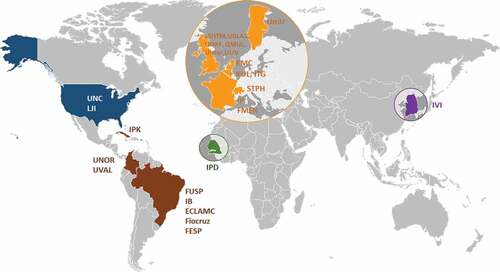
The ZikaPLAN initiative combines a multinational and interdisciplinary consortium consisting of 25 institutional partners in Latin America, North America, Africa, Asia, and various academic centers in Europe. The institutions are listed in , and the geographic distribution of the consortium is shown in . The hosting institution is Umeå University, Sweden and the Scientific Coordinator and Scientific Co-coordinator are Professors Annelies Wilder-Smith and Eduardo Massad respectively with Dr Raman Kaur Preet as the Executive Director. The project, the consortium, and its main components have been designed with these dual, complementary purposes in mind ().
The consortium consists of experts with a broad range of expertise covering virology, entomology, arboviral diseases, clinical trial expertise, birth defect surveillance, clinical cohort studies, pediatrics, neurology, qualitative research, and communications. Many of these experts already collaborated in a previous European Union (EU)-funded consortium called DengueTools [Citation2], and many experts in this consortium serve as advisors to the WHO related to Zika. Even prior to the EU research funding, many consortium members had already conducted Zika related work and published extensively on Zika (check under ‘publications’ at: https://zikaplan.tghn.org).
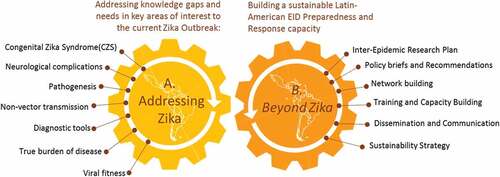
(A) Addressing ZIKA: we will address the current knowledge gaps in ZIKV as requested in the call topic: association with congenital syndromes and neurological complications, clinical spectrum of disease, determinants of severe disease, pathogenesis, and in particular neuro-pathogenesis, nonvector and vector transmission, diagnostics innovation and evaluation, burden of disease, and risk factors for geographic spread; birth defect surveillance, social science for communication strategies with affected communities, novel personal preventive measures, and modeling on vector control and vaccine strategies for evidence-informed policies.
(B) Preparing for beyond Zika: While addressing Zika, we will position the various activities and results as a springboard toward building a sustainable Latin American network capable of rapidly launching concerted, large-scale research responses to emerging infectious diseases outbreaks of significant (potential) impact to the region. This way, we can leverage the Zika response toward building a long-term emerging infectious diseases (EID) response capacity in the region beyond the 4-year project period.
Concept and methodology
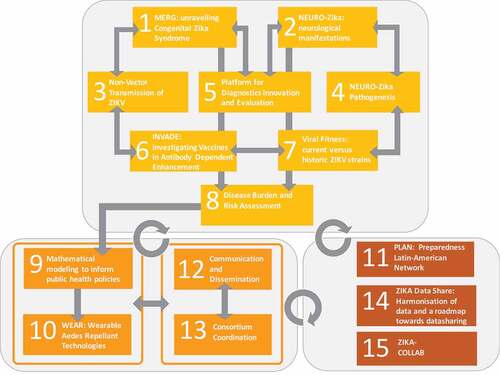
The project’s work plan is built on 15 interoperating work packages (WP). The structural design of ZikaPLAN reflects the ambition to address the urgent research gaps (WP 1–8), identify short- and long-term solutions (WP 9–10), and build a single sustainable Latin American EID Preparedness and Response capacity, WP 11. Communications and consortium coordinated are handled in WP 12 and 13 respectively. The project collaborates and interlinks with the two other EU-funded Zika consortia, ZIKAction and ZIKAlliance on protocol development, data sharing, joint data-analysis plans, and biobanking, and aims to develop commonalities for the dissemination of research findings (WP 14–15). presents the schematic work plan by WP. summarizes the primary research objectives of WP 1–12.
Description of WP
WP 1 Microcephaly Epidemic Research Group (MERG): unraveling congenital Zika syndrome
WP 1 aims to address the main concern of ZIKV infections that led to the declaration of a PHEIC: adverse fetal outcomes. This work package is conducted by the Brazilian MERG [Citation3], which was established in response to the first alert of microcephaly in Recife/North-East Brazil in 2015 (University of Pernambuco, London School of Hygiene and Tropical Medicine), and is devoted to the clinical recognition of congenital Zika syndrome. MERG recruits pregnant women presenting with rash and laboratory-confirmed ZIKV infection in a number of sites in Brazil. Its objectives are to determine the absolute risk of miscarriage, still birth, microcephaly, and other clinical manifestations of adverse outcomes to fetuses born to pregnant women with ZIKV infection by gestational age; and to define the full spectrum of congenital Zika syndrome at birth and the subsequent manifestations and neurodevelopmental milestones in the first 3 years of life [Citation4–Citation14]. This will help develop an improved case definition of congenital Zika syndrome for surveillance purposes and birth-defect registries, and help us understand the extent of disability and the social and economic burden to affected families.
WP 2 neuro-Zika: neurological manifestations of Zika
The ‘Neuroviruses Emerging in the Americas Study’ (NEAS) is a multicentre study in the Americas (NCT03206541) to establish a comprehensive registry of the clinical, radiologic, and paraclinical profile of patients with new onset of neurological diseases associated with Zika, dengue and chikungunya viruses (http://www.neasstudy.org/en/home/) with a network involving more than 10 hospitals in Colombia. NEAS was initiated by Prof. Carlo Pardo-Villamizar from Johns Hopkins University, US, and has already resulted in various publications [Citation15–Citation17]. The NEAS network will be enhanced by various additional sites in Brazil. The main objective is to define the full spectrum of neurological complications due to ZIKV by using a case-control study design. This potential spectrum includes immunologically mediated illness, for example Guillain–Barré syndrome (GBS) or acute disseminated encephalomyelitis (ADEM), and direct viral invasion of the nervous system for example meningoencephalitis and neuropathies. The International GBS outcome study (IGOS) https://gbsstudies.erasmusmc.nl/, based at Erasmus University, the Netherlands, will adapt some of its protocols to the Latin American context and make them widely available to neurologists and expand the network to increase the number of participating hospitals so that improved case-management protocols, larger sample sizes for biomarker studies, and a coordinated and harmonized effort for well-designed studies will be achieved that will benefit Latin America beyond the immediate response to ZIKV. Several investigators in this work package are members of the WHO Guideline Development Group for GBS and other neurological diseases associated with ZIKV, and are experts in the clinical care and management of patients with GBS and other neurological sequelae of central nervous systems (CNS) viral infection. The implementation of the research in this work package will strengthen the management of these conditions across Brazil and Colombia, working in conjunction with the WHO Expert Panel for ZIKV infections. For example, clinical guidelines for the harmonization of management of patients with Neuro-Zika across Latin America will be produced.
WP 3 nonvector transmission in humans and mice models
The incidence rate of ZIKV semen infection in male returning travelers with laboratory confirmed ZIKV infection will be determined as a proportion of the total number of included men with confirmed ZIKV infection. The replication fitness of ZIKV in semen will be explored by isolation of ZIKV virions in culture. The persistence of ZIKV in semen after acute infection will be analyzed semiquantitatively, and the incidence of secondary cases reported. Furthermore, this WP will establish a ZIKV mouse model for sexual transmission and study the pathway of entry of the virus via the vaginal route. Furthermore, a vertical transmission mice model will be developed to study whether an infected fetus contributes to higher viremia in the mother.
WP 4 neuropathogenesis
WP 4 will investigate pathomechanisms of neurotropism in the laboratory through a combination of approaches in virology, animal studies, neuroimmunology, cellular neuroscience, and host–virus interaction studies including neural receptors. Through close integration and specimen sampling through WP2 enhanced by mice models, the specific objectives are: (1) to define Zika virus tropism in the peripheral (PNS) and CNS in vitro and in vivo; (2) to determine the consequences for PNS and CNS cell health, function and survival of direct ZIKV infection, in vitro and in vivo; (3) to investigate intra- and intercellular trafficking of ZIKV within and between the periphery and the PNS and CNS; (4) to characterize the nature of ZIKV’s main cellular receptors on human neural cells, screening proteins, glycoproteins, proteoglycans, glycolipids, and thereby identify candidates used by ZIKV to attach to neural cells; (5) to determine if humoral factors from Zika-GBS patient and control sera are pathogenic to PNS and CNS cells in vitro and ex vivo; (6) to examine sera from Zika-GBS cases and controls for antigen targets known to be associated with conventional GBS, principally peripheral nerve glycolipids, using sensitive immunoassays; and lastly (7) to examine differential immune responses in patients with nonneurological ZIKV infection, patients with viral invasion of the CNS (e.g. encephalitis), and patients with autoimmune neurological disease. Understanding the intricate relationships between direct neuroinfection and postinfection-related neural autoimmunity, and exploring the scientific principles that underpin the clinical phenotypes associated with this dichotomization will have a profound impact on developing approaches to diagnosis, treatment, and vaccine development. We hope to identify relevant biomarkers, and possibly neural receptors – which could be potentially exploited for prognostic use (biomarkers) or therapeutic intervention (targeting neural receptors).
WP 5 platform for diagnostics innovation and evaluation
The platform for diagnostics innovation and evaluation is a major focus of ZikaPLAN. Regarding diagnostics innovation, we aim to identify the major type-specific and cross reactive B-cell epitopes on ZIKV for development of minimally cross-reactive IgM and IgG for more specific serological assays. We also hope to identify monoclonal antibodies to NS1 antigen for the potential development of a NS1 based assay – all with the aim to develop novel ZIKV diagnostic tests in accordance with WHO Target Product Profiles.
Our evaluation platform will be based upon well-characterized archived specimens obtained by informed consent, in accordance with the protocols developed by the WHO prequalification program for this purpose. All the evaluation sites will be compliant with the principles and practice of Good Clinical Laboratory Practice (GCLP). Assays that fulfill the target performance set by WHO will be selected to proceed to clinical performance studies, which will be conducted at our virtual network laboratories that include the Pan American Laboratory Network for Dengue and other arboviruses, RELDA (which comprises 18 laboratories in Latin America and five in the Caribbean), the WHO collaborating center for arboviral diseases in Senegal (IPD), ITG Antwerp, and the International Vaccine Institute Korea (IVI) – all with access to a geographic diversity of samples to test for crossreactivity against an array of diverse flaviviruses. Samples from these sites will form a virtual biobank with a strict code of governance on the use of samples to facilitate test development, evaluation, and research. A manual and standard operating procedures on sample collection and characterization will be developed to ensure harmonization of bio-banking procedures. This platform will be used for evaluation of commercially developed new diagnostic assays (at a fee) and will be free to the wider academic community under certain contractual conditions. The evaluation results will be provided to WHO for prequalification and regulatory authorities in countries for approval.
WP 6 INVADE: investigating vaccines in antibody-dependent enhancement
‘INVADE’ sets out to carry out T-cell and B-cell epitope mapping, and assess the role of flavivirus antibodies in protective and pathogenic immunity and the role of antibody-dependent enhancement (ADE) [Citation18]. In particular, the aim is to define the epitope breadth/repertoire and the targets of CD4 and CD8 responses; to derive sets of HLA class I and class II predicted epitopes covering the entire ZIKV proteome; to define phenotypes and T-cell subsets associated with CD4 and CD8 specific T cells; to identify the major type-specific and crossreactive B-cell epitopes on ZIKV, and to assess the role of flavivirus antibodies in protective and pathogenic immunity (e.g. related to prior dengue infections, dengue, and yellow fever vaccines). More than 2000 T-cell epitopes [Citation3,Citation4] and epitopes targeted by type-specific neutralizing, nonneutralizing, and possibly enhancing/autoimmune reactive antibodies will be identified, which may help develop vaccines and study potential mechanisms of immune pathogenesis responsible for severe neurological and fetal defects.
WP 7 viral fitness: current versus historic ZIKV
The aim of WP 7 is to investigate whether ZIKV emergence is associated with adaptive viral evolution in the vector, i.e. viral fitness differences that may explain the observed recent increase in ZIKV transmission by mosquitoes among humans. We will also address whether contemporary ZIKV result in more severe neurological disease, different tissue tropism, and higher viral loads in mice compared with historic ZIKV isolates.
WP 8 ‘disease burden and risk assessment’
This WP assembles a wealth of data obtained from various sources, including a longitudinal cohort study of 17,000 subjects aged 2–59 years in 14 different geographic locations in Brazil over 3 years, recruited through an ongoing Phase 3 trial by ZikaPLAN Partner Butantan. Seroprevalence studies will help to assess the asymptomatic to symptomatic ratio, the age- and gender-stratified attack rates, the clinical spectrum, and the impact of preceding dengue infections on the severity of illness. Documentation of the evolving epidemiology of ZIKV will be enriched by data from the Latin American network on birth-defect surveillance (ECLAMC). We will establish an ‘International Committee for Birth Defect Surveillance Tools for Infection Disease Response Preparedness’. This will consist of representatives from four birth-defect surveillance networks with expertise in this area: The European network of population-based congenital anomaly registries (EUROCAT), ECLAMC (Latin America), both of which are ZikaPLAN partners, and the International Clearinghouse for Birth Defects (ICBDSR), and Centre for Disease Control (US). A detailed inventory of Birth Defect Surveillance Tools will be made available on The Global Health Network (TGHN) web platform. A new Tablet App will be produced, tested, and used for the diagnosis and coding of birth defects in low-resource settings with good access to pediatricians. The App will contain atlas/picture material, diagrams created digitally by a medical illustrator, the International Classification of Disease Codes version 10, training videos, and guides. A specific ‘Zika module’ will also be created. The App will be an important part of the sustainability in terms of globally available tools for infectious disease preparedness and the surveillance of birth defects in low-resource settings.
The Swiss Tropical and Public Health Institute (Swiss TPH) will establish a smartphone-based sentinel surveillance system in travelers to Latin America and other Zika-affected countries in order to assess the incidence of Zika-/arboviral-related symptoms in travelers, trace and diagnose these travelers, and assess the risk of zika/arbovirus introduction to a vector-infested region of Europe (Barcelona, Spain). Swiss TPH will also develop baited filter-paper-based mosquito traps for the collection of mosquito saliva in tropical and subtropical countries (using international travelers), in order to assess the arbovirome of the local mosquito populations.
The results from our partners and this work package, further enhanced by literature reviews, will feed into mathematical modeling to document and predict burden of disease in Latin America. Complemented by data from GeoSentinel, Healthmap, and Bluedot, we will analyze the geographic spread of Zika infections beyond Latin America. Much of this will involve modeling spread via air passengers [Citation19,Citation20]. We will apply previously developed models for the risk of dengue spread via air passengers [Citation21–Citation23] to model the spread of ZIKV. We already modeled the risk of ZIKV infection during the Olympics [Citation24,Citation25] and provided input to the WHO IHR committee not to cancel the Olympics. We also published the most likely time of ZIKV introduction into Brazil based on mathematical modeling [Citation26].
WP 9 mathematical modeling to inform public health policies
Here, we will model best vector control strategies for high-population-density areas in Latin America. To this end, work on vectorial capacity of Aedes mosquitoes for Zika in temperate countries has already been conducted by the ZikaPLAN team [Citation27]. Modeling ecological, climate, and mobility patterns will assist in enhancing appropriate public health responses [Citation27–Citation29]. We will model vector-control strategies (Wolbachia/genetically modified mosquitoes) to inform optimal control bundles to mitigate future outbreaks, in particular in Latin American cities. Furthermore, this WP aims to establish mathematical models on the role of sexual transmission in countries without Aedes aegypti/albopictus mosquitoes [Citation30].
We will also build a community of practice related to vector control. This community of practice for vector-control groups will be called The Global Vector Hub and will be located within the TGHN digital platform to maximize visibility and uptake, and also to provide users with access to further tools and resources that can support their research and teams. The community of practice for vector control is focused on supporting and linking researchers working with mosquitoes through a digital infrastructure where they can share tools and resources in order to deliver training, raise capacity, and establish standards. Enhancing vector-control strategies through such a community of practice will benefit ZIKV control but also other mosquito-borne diseases.
WP 10 WEAR: wearable Aedes-repellant technologies
‘WEAR’ aims to develop personal protective measures for pregnant women (impregnated maternity clothing for example). We will investigate and develop novel detergents containing repellents that can be used during laundry to allow active repellents to be applied to clothing for protection and also investigate new wash-resistant technologies, including novel silica-shell, polymer fibers, and microencapsulated formulations to determine whether repellent active ingredients can be retained in fabrics for multiple washes. New plastic wearable repellent technologies will be developed and tested, including flip flops, wrist bands, and necklaces using a plastic silica technology for protection against Aedes spp. mosquitoes. Qualitative research will be conducted to assess the acceptability of such clothing and technologies.
WP 11 ZIKA collaborative establishment of a Latin American preparedness network (PLAN)
‘PLAN’ led by TGHN at Oxford University will ensure that the consortium, networks built, activities performed, and results obtained are leveraged into establishing a Latin American EID Preparedness and Response network in the region beyond Zika. To this purpose, we will work together with ZIKAlliance and ZIKAction in order to leverage synergies, in particular with regard to the clinical cohort studies. A specific platform will be set up to enhance the outreach to expand to new sites and to develop into an independent research network beyond the project time. This is called REDe (the Spanish and Portuguese term for network), which will be a shared and open space for information exchange and releasing research documents such as protocols and operating procedures, to ensure standard outcome measures are used, to optimize data sharing, and also to disseminate the research tools in order that others may use and adapt them. REDe is hence a research-capacity development and knowledge-exchange space for all three EU-funded Zika consortia and their clinical sites. The bottlenecks that we will identify throughout the project are likely to be somewhat similar to those encountered in Europe, and we will therefore also liaise with EU funded Platform for European Preparedness Against (Re-)emerging Epidemics (PREPARE) to incorporate lessons learned through their preapproval processes of epidemic clinical research protocols.
Research needs to be embedded into a response to an outbreak or emerging disease [Citation31], and to achieve this there needs to be local ability to undertake study design and operation to gather the high-quality evidence that is rapidly needed [Citation32]. TGHN will be harnessed as a respected online platform for research education, training, and resources for capacity building in Latin America as the host of the REDe network. The aim of REDe is to link together and work with experienced and inexperienced research sites across Latin American and the Caribbean to deliver knowledge, research tools, and training. The underlying philosophy is that if there is to be a locally led and rapid research response in an outbreak, then the local ability to undertake the collection of high-quality research data needs to be built up. A wide range of cross-cutting research resources and tools will be provided, such as free online seminars and courses, downloadable training kits, articles, templates and guidance notes, in particular linkage to IGOS, ECLAMC, and diagnostics. Harmonized web-based tools for birth-defect registries will be accessible. Other tools such as ‘SiteFinder’, an application that allows partners planning studies to find new sites to collaborate with, are also freely available and easily accessible. The training center already provides translated core courses such as Good Clinical Practice, GCLP, and Bioethics, as well as broader topics such as ‘Introduction to Clinical Research’ and ‘Children and Clinical Research’. Alongside these resources and training, there will be a whole set of regionally based activities and support systems to provide opportunities for research staff from a specific region to meet, learn, and share experiences.
WP 12 dissemination and communication
This WP will develop and implement a detailed communication and dissemination plan serving as the central consortium-wide guiding document for how we deal with dissemination and communication with our stakeholders. These stakeholders include international and national governmental organizations/health authorities/funders in the area of EID preparedness and response, e.g. WHO, PAHO, PREPARE, and Global Research Collaboration for Infection Disease Preparedness (GLoPID-R). Engagement with affected communities, in particular pregnant women, including social messaging of personal protection, will also be central to WP 12. Following qualitative research in North-East Brazil supportive, actionable messages will be developed that take data on burden of disease into account. These social messages will be based on an empirical understanding of people’s perceptions and concerns about Zika and will be built on the lessons learned from the Zika-related messages that have been used to date, in particular with those coordinated by WHO (http://www.who.int/emergencies/zika-virus/en/)
WP 13 consortium coordination and management
Umeå University, Sweden under WP 13 will coordinate all the collaborative efforts and facilitate communication within the consortium. It will set up an effective management framework to ensure progress toward its planned objectives reflecting our contractual commitments to the European Commission.
WP 14 Zika data share: harmonization of data and roadmap to data sharing
The aims of the work package are (1) to ensure harmonization of protocols and standardization of tools (including data dictionaries) for the multicentre pregnant women and children cohort studies across the three EU-funded Zika consortia (ZikaPLAN, ZIKAction, ZIKAlliance); (2) to conduct data sharing within the three consortia and to provide a roadmap toward wider data sharing. The pregnant women cohort is harmonized between ZIKAction and ZIKAlliance (as both recruit asymptomatic and symptomatic pregnant women), whereas ZikaPLAN recruits only women symptomatic ZIKV infection during pregnancy. The data garnered through these combined cohorts will also feed into the WHO-led ‘individual participant data meta-analysis (IPD-MA)’, which will combine de-identified, participant-level cohort data to identify and quantify the relative importance of different predictors of congenital Zika syndrome (CZS) (www.who.int/entity/reproductivehealth/projects/ZIKV.pdf). Individual participant data (IPD) allow for more consistent control of confounders and facilitate the assessment of effect measure modification and sensitivity analyses that would not have been possible if we did not combine the data obtained from the three EU-funded consortia and other consortia or research groups with similar cohort studies. Under the guidance of WHO, the use of IPD will allow us to fully explore subject-level sources of heterogeneity in the association between congenital ZIKV infection and pregnancy complications, and will facilitate the identification of the subgroups of women at the highest risk of experiencing ZIKV-related pregnancy complications.
WP 15 ZIKA-COLLAB
The overall objective is to enhance the output of the respective EU-funded Zika consortia through shared management structures and communications activities, create and maintain joint management and oversight structures, and address ethical and information governance issues, ensuring ethical and safe care and treatment of animals and use of patient data and samples for research across and potentially outside of participating consortia. We will organize integrated communications across consortia, including the creation of a Communications Oversight Board, planning of joint meetings, and organization of cross-consortia working groups to ensure clear, coherent messages that integrate all communication and dissemination activities. Ethics is an integral part of research from beginning to end, and ethical compliance is pivotal to achieve real research excellence (http://ec.europa.eu/research/participants/data/ref/fp7/89888/ethics-for-researchers_en.pdf). Within the European regulatory framework, research ethics is based on the explicit European commitment to human rights. We therefore established a joint Ethics Advisory Board for the three EU-funded consortia, which will provide oversight and advice over ethical issues pertaining to our human and animal studies.
Outlook
The speed in research response after the declaration of the PHEIC in 2016 was unprecedented. As the ZIKV outbreak is rapidly waning in 2017, it is even more significant that the Consortium should further capitalize on the REDe platform established in WP 11 and the experience gained through this ZIKV research response, in order to evolve into a network capable of rapidly launching a research response to future severe infectious outbreaks caused by emerging pathogens.
Box 1. Call topic SC1-PM-22-2016
Box 2. List of institutions in ZikaPLAN
Box 3. Primary research objectives for WP 1–12
Ethics and consent
Ethics clearance will be sought in the institutional review boards of the countries where the research will take place
Paper context
The causal association between maternal Zika virus infection and birth defect has been established, but many other research gaps exist.
We will further define CZS, delineate neurological complications associated with Zika, investigate the role of sexual transmission, determinants of severe disease, and viral fitness, conduct burden of disease studies, and model outbreak responses. We will also build up research capacity in the region.
Acknowledgments
We acknowledge all our consortium members and many other members who participate in the ZikaPLAN project in different capacities: John Kinsman, Joacim Rocklöv, Niklas Arnberg (Umeå University, Sweden); Hannah Kuper, Chris Drakeley, Laith Yakob, Michael Gaunt (London School of Hygiene and Tropical Medicine, UK); Susan Barnett, Julia Edgar, Christopher Linington (University of Glasgow, UK); Joan Morris (Queen Mary University London, UK); Helen Dolk, Maria Loane (University of Ulster, UK); Susanne Kaptein (University of Leuven, Belgium); Bart C. Jacobs, Pieter A. van Doorn (Erasmus University Medical Centre Rotterdam, Netherlands); Claudia Romero-Vivas (Universidad del Norte, Colombia) Lyda Osorio, Beatriz Parra, Andreas Zea-Vera, Carlos-Pardo Villamizar (Universidad del Valle, Colombia); Sebastian Quesney, Koren Wolman-Tardy (Fondation Meriux, France); Lisa Ng, Lance Turtle, Anu Jacob, Michael Griffiths, Julian Hiscox (University of Liverpool, UK); Alessandro Sette (La Jolla Institute, USA); Aravinda de Silva, Stefan Matz (University of North Carolina at Chapel Hill, USA); Ralph Huits, Kevin K. Arien, Liselotte Cnops (Prins Leopald Instituut voor Tropische Geneeskunde, Belgium); Esper Kallas, Benedito Fonseca, Amilton Antunes Barreira (Fundacao de Apoio a Universidade de Sao Paulo, Brazil); (Instituto Butantan, Brazil); Ana Maria Bispo de Filippis, Patricia Brazil, David Brown (Fundacao Oswaldo Fiocruz, Brazil); Maria G. Guzman, Jose Pelegrino (Instituto Medicina Tropical Pedro Kouri, Cuba); Amadou Alpha Sall (Institut Pasteur de Dakar, Senegal); Christoph Hatz, Pie Muller, Andreas Neymer (Schweizerisches Tropen-und Public Health-Institut, Switzerland); In-Kyu Yoon (International Vaccine Institute, Korea); Democrito De Barros Miranda Filhos, Celina Turchi (Fundacao Universidade de Pernambuco, Brazil).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
A. Wilder-Smith
AWS wrote the manuscript; all other authors contributed in their respective field of expertise as work package leaders of ZikaPLAN.
References
- Wilder-Smith A, Gubler DJ, Weaver SC, et al. Epidemic arboviral diseases: priorities for research and public health. Lancet Infect Dis. 2017;17:e101–e106.
- Wilder-Smith A, Renhorn KE, Tissera H, et al. DengueTools: innovative tools and strategies for the surveillance and control of dengue. Glob Health Action. 2012;5.
- Souza WV, Araujo TV, Albuquerque Mde F, et al. Microcephaly in Pernambuco State, Brazil: epidemiological characteristics and evaluation of the diagnostic accuracy of cutoff points for reporting suspected cases. Cad Saude Publica. 2016;32:e00017216.
- Azevedo RS, Araujo MT, Martins Filho AJ, et al. Zika virus epidemic in Brazil. I. Fatal disease in adults: clinical and laboratorial aspects. J Clin Virol. 2016;85:56–10.
- Brunoni D, Blascovi-Assis SM, Osorio AA, et al. Microcephaly and other Zika virus related events: the impact on children, families and health teams. Cien Saude Colet. 2016;21:3297–3302.
- de Araujo TV, Rodrigues LC, de Alencar Ximenes RA, et al. Association between Zika virus infection and microcephaly in Brazil, January to May, 2016: preliminary report of a case-control study. Lancet Infect Dis. 2016;16:1356–1363.
- de Sa TH, Reis-Santos B, Rodrigues LC. Zika outbreak, mega-events, and urban reform. Lancet Glob Health. 2016;4:e603.
- do Rosário MS, de Jesus PA, Vasilakis N, et al. Guillain-Barre syndrome after Zika virus infection in Brazil. Am J Trop Med Hyg. 2016;95:1157–1160.
- Leal MC, Muniz LF, Ferreira TS, et al. Hearing loss in infants with microcephaly and evidence of congenital Zika virus infection – Brazil, November 2015–May 2016. MMWR Morb Mortal Wkly Rep. 2016;65:917–919.
- Magalhaes-Barbosa MC, Prata-Barbosa A, Robaina JR, et al. Trends of the microcephaly and Zika virus outbreak in Brazil, January–July 2016. Travel Med Infect Dis. 2016;14:458–463.
- Moura da Silva AA, Ganz JS, Sousa PD, et al. Early Growth and neurologic outcomes of infants with probable congenital Zika virus syndrome. Emerg Infect Dis. 2016;22:1953–1956.
- Leal MC, van der Linden V, Bezerra TP, et al. Characteristics of dysphagia in infants with microcephaly caused by congenital Zika virus infection, Brazil, 2015. Emerg Infect Dis. 2017;23(8):1253–1259.
- Magalhaes-Barbosa MC, Prata-Barbosa A, Robaina JR, et al. New trends of the microcephaly and Zika virus outbreak in Brazil, July 2016–December 2016. Travel Med Infect Dis. 2017;16:52–57.
- Vieira M, Cruz ACR, Barros ANM, et al. Guillain-Barre syndrome and dengue-like disease in 2015: temporal relationship in Piaui state and implications on Zika virus surveillance. Rev Inst Med Trop Sao Paulo. 2017;59:e22.
- Costello A, Dua T, Duran P, et al. Defining the syndrome associated with congenital Zika virus infection. Bull World Health Organ. 2016;94:406–A.
- Muñoz LS, Barreras P, Pardo CA. Zika virus-associated neurological disease in the adult: Guillain-Barré syndrome, encephalitis, and myelitis. Semin Reprod Med. 2016;34:273–279.
- Parra B, Lizarazo J, Jiménez-Arango JA, et al. Guillain-Barre syndrome associated with Zika virus infection in Colombia. N Engl J Med. 2016;375:1513–1523.
- Collins MH, McGowan E, Jadi R, et al. Lack of durable cross-neutralizing antibodies against Zika virus from dengue virus infection. Emerg Infect Dis. 2017;23:773–781.
- Massad E, Tan S-H, Khan K, et al. Estimated Zika virus importations to Europe by travellers from Brazil. Glob Health Action. 2016;9:31669.
- Quam MB, Wilder-Smith A. Estimated global exportations of Zika virus infections via travellers from Brazil from 2014 to 2015. J Travel Med. 2016;23:taw059.
- Lopez LF, Amaku M, Coutinho FA, et al. Modeling importations and exportations of infectious diseases via travelers. Bull Math Biol. 2016;78:185–209.
- Massad E, Rocklov J, Wilder-Smith A. Dengue infections in non-immune travellers to Thailand. Epidemiol Infect. 2013;141:412–417.
- Quam MB, Khan K, Sears J, et al. Estimating air travel-associated importations of dengue virus into Italy. J Travel Med. 2015;22:186–193.
- Massad E, Coutinho FA, Wilder-Smith A. The olympically mismeasured risk of Zika virus in Rio de Janeiro – authors’ reply. Lancet. 2016;388:658–659.
- Massad E, Coutinho FA, Wilder-Smith A. Is Zika a substantial risk for visitors to the Rio de Janeiro Olympic Games? Lancet. 2016;388:25.
- Massad E, Burattini MN, Khan K, et al. On the origin and timing of Zika virus introduction in Brazil. Epidemiol Infect. 2017;145:2303–2312.
- Rocklöv J, Quam MB, Sudre B, et al. Assessing seasonal risks for the introduction and mosquito-borne spread of Zika virus in Europe. EBioMedicine. 2016;9:250–256.
- Liu-Helmersson J, Stenlund H, Wilder-Smith A, et al. Vectorial capacity of Aedes aegypti: effects of temperature and implications for global dengue epidemic potential. PLoS One. 2014;9:e89783.
- Liu-Helmersson J, Quam M, Wilder-Smith A, et al. Climate change and Aedes vectors: 21st century projections for dengue transmission in Europe. EBioMedicine. 2016;7:267–277.
- Yakob L, Kucharski A, Hue S, et al. Low risk of a sexually-transmitted Zika virus outbreak. Lancet Infect Dis. 2016;16:1100–1102.
- Lang T. Ebola: embed research in outbreak response. Nature. 2015;524:29–31.
- Furtado T, Franzen S, van Loggerenberg F, et al. Strengthening neglected tropical disease research through enhancing research-site capacity: an evaluation of a novel web application to facilitate research collaborations. PLoS Negl Trop Dis. 2014;8:e3225.