ABSTRACT
Background
Even though previous systematic reviews have reported on the role of prenatal vitamin D on birth outcomes, its effect on child growth is poorly understood.
Objective
To synthesize a systematic summary of the literature on the effect of maternal vitamin D supplementation on the linear growth of under-five children.
Method
This study includes studies (both observational and interventional with a control group) that evaluated the effects of prenatal vitamin D status on child linear growth. The mean child length/length for age with 95% confidence interval (CI) was pooled as the weighted mean difference using a random-effects model. A funnel plot was used to assess potential publication bias.
Results
A total of 45 studies and 66 reports covering a total population of 44,992 (19,683 intervention or high vitamin D group, and 25,309 control or low vitamin D group) were analyzed. Studies spanned from 1977 to 2022. The pooled weighted mean difference was 0.4 cm (95% CI: 0.15–0.65). A subgroup analysis, based on vitamin D supplementation frequency, showed that mothers who supplemented monthly or less frequently had a 0.7 cm (95% CI: 0.2–1.16 cm) longer child. Supplementation with a dose of >2000 international units increased child length at birth. The weighted mean difference was 0.35 cm (95% CI: 0.11–0.58).
Conclusion
The evidence from this review shows that maternal supplementation of vitamin D is associated with increased birth length. This is apparent at higher doses, low frequency (monthly or less frequent), and during the second/third trimester. It appears that vitamin D supplementation during pregnancy is protective of future growth in under-five children. Clinical trials are needed to establish evidence of effectiveness for the frequency and dose of supplementation.
Responsible Editor
Jennifer Stewart Williams
Background
Vitamin D is a fat-soluble vitamin that increases the absorption of calcium, magnesium, and phosphate. It is used by the body for the development of calcified tissues and helps to prevent rickets [Citation1]. Due to the importance of vitamin D in the human body, its deficiency causing rickets was considered the ‘tip of the iceberg’ [Citation2]. Vitamin D deficiency also causes growth retardation in utero and during childhood, and skeletal deformities that lead to and exacerbate osteopenia, osteoporosis, and increase the risk of fracture [Citation2]. Vitamin D along with calcium plays an important role in the mineralization of bone and has a myriad of other benefits including the prevention of autoimmune diseases, decreased risk of cancer, hypertension, diabetes, and improved immunity [Citation3].
Vitamin D is a steroid hormone; its receptor is located in the nucleus, forming a complex with specific DNA sequences. Vitamin D causes the transcription of a large number of genes, some of which are proteins that promote growth, including growth hormone and insulin-like growth factor-1 [Citation4–7].
There is controversy regarding adequate or optimal levels of serum vitamin D to prevent adverse health consequences. The US Institute of Medicine defined adequate vitamin D in pregnant women as a serum concentration greater than 50 nanomoles per liter (nmol/L) (20 nanograms per milliliter (ng/ml)) [Citation8]. Others argued that the value should be raised to 75 nmol/L (30 ng/ml) [Citation9,Citation10], but the burden remains high. Despite disagreements, inadequate vitamin D is classified as a deficiency at <25 nmol/L [Citation8] and an insufficiency at <50 nmol/L. Adequate vitamin D is generally defined as more than 50 nmol/L [Citation9].
Low vitamin D status varies in populations across the globe. Depending on the Food and Agricultural Organization world regions, the prevalence of serum 25(OH)D < 50 nmol/L ranges from 24% to 49% [Citation10]. Although vitamin D deficiency affects every individual at all levels, diet, supplement use, geographic latitude, cultural and lifestyle factors, and skin pigmentation are important factors. Infants, older individuals, pregnant and lactating women, and individuals having specific disease conditions like cancer are at particular risk of vitamin D deficiency [Citation11,Citation12]. Maternal vitamin D deficiency during pregnancy is also a critical global public health problem, with variations across countries. For example, deficiency in pregnancy has been reported as 81% in Nepal [Citation13], and over 90% in Guizhou, China [Citation14], and Saudi Arabia [Citation15]. A pooled result from a study in African countries reported a prevalence of almost 44% in mothers and newborns [Citation16].
Some countries specify a recommended dietary intake during pregnancy. For example, in the USA, Australia, New Zealand, and Canada, the recommended dietary adequate intake of vitamin D for pregnant women is 200 International Units (IU)/day [Citation17,Citation18]. The UK recommends 400 IU/day during pregnancy [Citation19].
Children less than 5 years old are among the most-affected population segment in terms of vitamin D deficiency. A systematic review and meta-analysis covering countries in the African continent reported the prevalence of vitamin D deficiency at 49% and 25% in newborns and children, respectively, based on a cutoff value of <50 nmol/L [Citation16]. The vitamin D status of infants depends on maternal vitamin D status, the intake of breast milk, and its vitamin content. In India, almost 93% of healthy infants were found to be vitamin D deficient [Citation20].
In the first 6–8 weeks of postnatal life, the vitamin D status of infants is mainly dependent on placental transfer in utero [Citation21]. In most infants, the acquired vitamin D stores are depleted by approximately 8 weeks of age [Citation22]. Thereafter, the infant’s vitamin D supplement is derived from diet, sunlight, and supplementation. Human milk contains an insufficient amount for maintaining optimal vitamin D levels, especially if exposure to sunlight is limited [Citation23]. Exclusively, breastfed infants have hypovitaminosis D due to the poor content of human milk [Citation24,Citation25]. In exclusively breastfed infants, 6 weeks to 6 months postnatal is a critical window for addressing vitamin D deficiency [Citation26].
Since the early 1980s, there have been many vitamin D supplementation trials conducted during pregnancy. However, the interpretation of the results has been complicated by factors such as the type, duration, and dose of supplementation [Citation27]. Systematic reviews have been conducted previously to evaluate the effects of prenatal vitamin D status on the different health outcomes of children. Previous systematic reviews [Citation28–31] investigated the effect of prenatal vitamin D supplementation on birth outcomes. In these studies, the effect of prenatal vitamin D on child growth has remained largely unknown. Despite numerous original studies on maternal vitamin D and child linear growth, comprehensive scientific evidence is lacking. In this review, we ask the question: ‘what effect does maternal vitamin D status have on linear growth in children under the age of five?’ The findings of this synthesis will help inform the scientific community about priority research areas for vitamin D supplementation in child growth.
Methods
This systematic review and meta-analysis was conducted to synthesize existing evidence on the role of maternal gestational vitamin D supplementation/status in the linear growth of under-five children.
Search strategy
The search strategy was performed in three stages. In the first stage, relevant Medical Subject Heading (MeSH) and other terms were identified in the literature. In the second phase, full searches were conducted in PubMed, Ovid Embase, and Google Scholar. In the third phase, the bibliographies of relevant studies and university websites were searched to see the presence of eligible studies. The following terms were used to search for relevant articles. The population terms were combined using OR, and the PICO components were combined using AND. MeSH Terms and Asterisk were applied. Population terms were maternal, gestation*, prenatal, antenatal, pregnancy, child, children, under-five, preschool, infant, newborn, and ‘0–59 months’; intervention terms were vitamin D [MeSH Terms], ‘vitamin D’, cholecalciferol, ‘vitamin D3’, ergocalciferol, and alfacalcidol; and outcome was searched using growth disorders [MeSH Terms], ‘linear growth’, stunted, stunting, ‘height for age’, length, ‘length for age’, ‘short stature’, and growth. Filters were used in some databases. This study included studies published from inception to 22 February 2022.
Study selection
The search included both observational and interventional studies. Interventional/observational studies were required to have a control or comparison group. The outcome (child growth) was extracted as mean length at different age groups or as length for age (LFA)/height for age (HFA) from both interventional and observational studies. Some studies had supplementation in addition to vitamin D (e.g. calcium). We included such studies provided that the intervention and control groups differed only in terms of vitamin D. There was no restriction on when the supplementation/measurement took place, i.e. during the first, second, or third trimester. Childhood growth was evaluated for infants or children under the age of 5 years.
Studies were excluded if the women had multiple pregnancies, pregnancy complications, chronic illnesses, or a child with developmental disorders. We did not include review articles (scoping, narrative, meta-analysis), non-English articles, or conference proceedings and articles where full texts were unavailable. Two authors (AAT and WD) screened the searched articles using title and abstracts. Disagreements were solved by the third author (TAZ).
Outcome
The primary outcome of this meta-analysis was child linear growth measured by length/height, height for age, or length for age evaluated at different time points in under-five children.
Data extraction
Two independent authors (AAT and WD) extracted the data. Data extraction sheets containing relevant study characteristics and study outcomes were drafted into Covidence software. Disagreements were resolved by the third author (TAZ). Relevant information collected included author(s), publication year, study period, design, country, sample size, study outcomes, baseline maternal vitamin D status, initiation of supplementation, the dose of vitamin D, frequency of supplementation, duration of supplementation, maternal serum vitamin D concentration, child length/height, mean age, HFA/LFA, as well as the time of outcome evaluation in the experimental and comparison group.
Quality assessment
The risk of bias for included clinical trials was judged by the Cochrane Collaboration Risk of Bias Tool [Citation32], for reporting of sequence generation, allocation concealment, use of blinding of participants and personnel, loss to follow-up, and other biases. The methodological quality of the observational studies was assessed using the Newcastle Ottawa Scale [Citation33], and the risk of bias in individual studies was rated as low, unclear risk, and high risk.
Data analysis
Data analysis was dependent on the reporting system of the primary studies. Means of child length/height or length for age were pooled as weighted mean difference (WMD) in supplemented/high vitamin D and un-supplemented/low vitamin D groups. Some studies reported multiple treatment groups or reported deficient and insufficient vitamin D levels in observational studies. In both cases, the intervention group or deficient and insufficient vitamin D level sample size, mean length, and standard deviations were pooled [Citation34].
Since there are studies that report child growth parameters at different time points, the WMD was calculated at different time points as well. We reported WMD with a 95% confidence interval (CI) using random effects, and the inverse variance method. Statistical heterogeneity was measured by I2 static, and we consider percentages of around I2 = 25%, I2 = 50%, and I2 = 75% as low, medium, and high heterogeneity, respectively [Citation35]. Subgroup analysis was conducted to identify potential sources of clinical and methodological heterogeneity. This was performed on different variables, including study design, study area (continent), the dose of supplementation, trimester of pregnancy, subject recruitment time, and frequency of supplementation. To detect the robustness of the results, a sensitivity analysis was conducted by sequential elimination of each study from the pool. Potential publication bias was assessed using funnel plots, and where possible, Egger’s regression test was performed. The p-value ≤ 0.05 cut-point was used to declare statistical significance. The STATA software (Version 16, StataCorp, Texas, USA) was used for all analyses.
Results
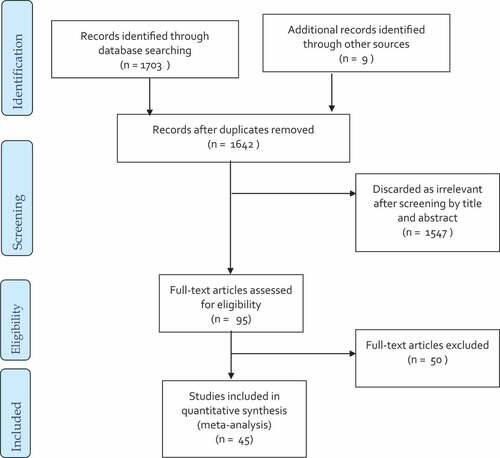
Overall, 1703 studies were identified through database searches, and nine additional articles were retrieved from the bibliographies of the included studies. Seventy duplicates were removed, and the remaining 1642 articles were screened by title and abstract, which resulted in the exclusion of 1547 irrelevant articles. Full-text screening was performed on 95 studies, and data for 45 studies were extracted for this meta-analysis. depicts the various exclusions and selection procedures.
The flow chart shows the stages of screening as well as numbers of articles excluded and included. The exclusion criteria included studies without a comparison group (18 articles), no outcome (13), giving wrong intervention (6), incomplete outcome (3), articles without full text or full text was unavailable (2), authors’ replies (2), intervention given with other nutrients (2), duplicate (1), studies investigating non-healthy children (1), review (1), and non-English language (1) articles were excluded after full-text screening. This sums up a total exclusion of 50 studies.
Characteristics of included studies
A total of 45 studies and 66 reports were included in this meta-analysis. Twenty-five clinical trials and 20 observational studies with a total population of 44,992 (19,683 either intervention or high vitamin D group, and 25,309 control or low vitamin D group) have been analyzed. Included studies reported the outcome at different time points, including birth (40 studies 23 interventional [Citation36–58] and 17 observational [Citation59–75]), 1 month (three studies) [Citation36,Citation54,Citation76], 3 months (five studies) [Citation36,Citation42,Citation54,Citation76,Citation77], 6 months (four studies) [Citation42,Citation60,Citation76,Citation77], 9 months (four studies) [Citation42,Citation62,Citation76,Citation77], 1 year (five studies) [Citation46,Citation55,Citation74,Citation76,Citation77] and five studies reported length for age [Citation45,Citation46,Citation55,Citation78,Citation79]. The clinical trials were conducted between the years 1977 and 2015. The vast majority were randomized, and two-thirds were carried out in low- and middle-income countries such as Iran, India, and Bangladesh. Recruitment began as early as 10 weeks and finished as late as 32 weeks. Almost all clinical trials found that the baseline maternal vitamin D concentration was insignificant. A description of the included clinical trials is given in .
Table 1. Characteristics of included interventional studies.
The design of observational studies was either cohort or cross-sectional. Maternal vitamin D levels were measured from 9 weeks after conception to full term. The definition of low and high vitamin D levels varied between studies. One study did not report the cut points, while another simply labeled vitamin D levels as adequate or inadequate. Three studies failed to provide length/height measurements at birth, although they were added subsequently, e.g. at 6 or 9 months post-birth. lists the characteristics of the included observational studies.
Table 2. Characteristics of included observational studies.
Meta-analysis
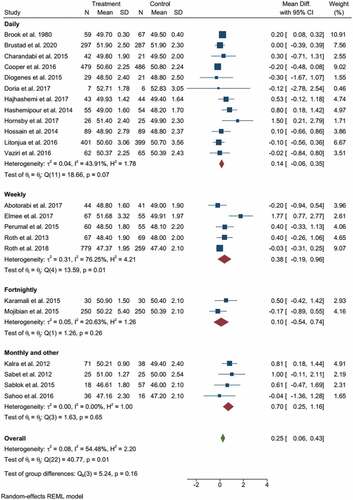
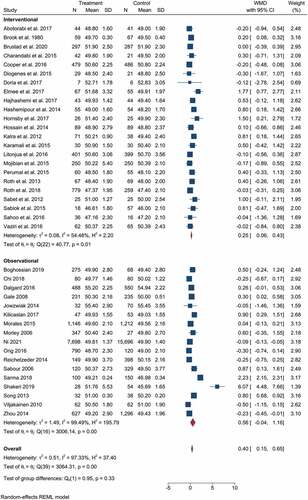
The pooled results from clinical trials and observational studies indicated the beneficial effect of vitamin D supplementation/higher vitamin D status during pregnancy for the linear growth of children. The pooled effect size from 23 clinical trials and 17 observational studies had a WMD of 0.4 cm birth length with a (95% CI: 0.15–0.65), and I2 statistics of 97.33%. Children whose mothers were supplemented with various doses of vitamin D during pregnancy, or had sufficient vitamin D, showed a significant increase in birth length (p-value < 0.001), indicated in .
Figure 2. The forest plot shows the effect of vitamin D supplementation/high vs low on birth length. The graph indicates the overall important effect of the vitamin to promote linear growth. The first subgroup represents clinical trials, and the second includes observational studies.
Subgroup analysis showed that prenatal vitamin D supplementation had a significant effect on childbirth length. shows that mothers who took vitamin D supplements had longer children with WMD = 0.25 cm (95% CI: 0.06–0.43 cm) and I2 static = 54.48%.
According to the findings of observational studies, there is no statistically significant difference in birth length between mothers with high and low levels of vitamin D. WMD = 0.56 cm (95% CI: −0.04 cm to 1.16 cm) (). The pooled analysis also indicated significant heterogeneity, with I2 = 99.49%. Neither subgroup analysis based on study area (developing vs developed), design (cohort vs cross-sectional), or vitamin D category (the authors’ criteria for classifying high and low) produced significant results or significantly reduced heterogeneity.
Subgroup analysis based on the frequency of supplementation indicated the significant effect of intermittent supplementation (monthly or less frequent) on childbirth length. Mothers who supplemented monthly or less frequently had a 0.7 cm longer child with (95% CI: 0.25−1.16 cm) of I2 = 0.00%, given in . Subgroup analysis with the dose of supplementation also revealed that supplementation with a dose of >2000 IU contributed to child length at birth, WMD = 0.35 cm (95% CI: 0.11−0.58 cm), and I2 = 49.82%; given in the supplementary file, SFigure 1.
Overall, higher maternal vitamin D or Vitamin D supplementation from 20 weeks of gestation had a significant positive effect on birth length. Subgroup analysis from the clinical trials indicated a significant effect of vitamin D supplementation either below or above 20 weeks of gestation. Clinical trials that supplemented vitamin D less than 20 weeks of gestation had a greater effect size (WMD, 0.38 cm vs 0.17 cm) (see the supplementary file, SFigure 2).
As previously stated, some studies report child length after birth. summarizes these findings. As can be seen, maternal vitamin D had a significant effect on child length at 3 months. Aside from this overall effect, observational studies at 6 months and both interventional and observational studies separately at 9 months reported a positive influence of higher maternal vitamin D levels (see ). In contrast to what we saw in this meta-analysis, observational studies revealed a negative effect of higher maternal vitamin D on child growth at 12 months of age (WMD = −0.05 cm (95% CI: −0.06 cm to −0.04 cm), I2 = 0.00%) (). The forest plots for these outcomes are included in the supplementary file (SFigure 5−SFigure 10).
Table 3. The role of maternal vitamin D on child linear growth beyond birth disaggregated by study design.
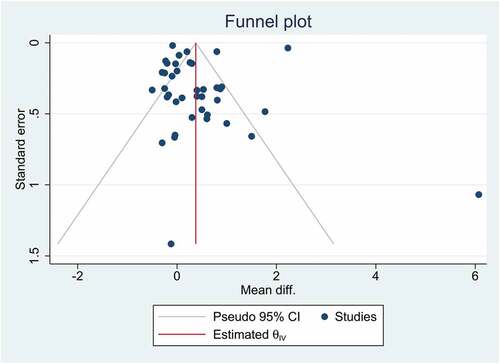
Publication bias and small study effects
shows a funnel plot for visual inspection of publication bias. In addition, the Eggers regression test was used to detect publication bias and small-study effects. According to the findings, there was no publication bias and small-study effects (p-value = 0.2414).
Discussion
Results from the pooled analysis of clinical trials and observational studies indicated beneficial effects of vitamin D supplementation in pregnancy on the linear growth of children. Children whose mothers were supplemented with various doses of vitamin D during pregnancy or who already had sufficient vitamin D showed a significant increase in birth length (p-value < 0.001). Previous systematic reviews highlighted the beneficial effects of vitamin D supplementation or higher levels of vitamin D during pregnancy on preeclampsia, preterm birth, small for gestational age [Citation80], birth weight and length, gestational diabetes [Citation81], cesarean section [Citation82], enhanced cognitive development, and lower risk of attention deficit hyperactivity disorder and autism-related traits later in life [Citation83]. Other studies [Citation26,Citation84] have questioned the role of prenatal vitamin D supplementation in the risk of cesarean section, gestational diabetes, stillbirth, neonatal death, and child respiratory health.
Overall, maternal vitamin D supplementation appears to increase child length at birth. Although there are no previous comprehensive meta-analyses to compare with the current findings, a few studies evaluated the growth-promoting effect of vitamin D as a secondary outcome. Bi et al. reported significantly greater height at 3 months, 9 months, and 12 months, but not at 6 months [Citation82]. A meta-analysis from four clinical trials indicated that the LFA z-score was higher in infants at 1 year in the vitamin-D-supplemented group compared with the control [Citation85]. Vitamin D supplementation at a higher dose and on an intermittent basis was found to be more beneficial than a lower dose (2000 IU) and daily or weekly supplement. Daily vitamin D is often inadequate to treat vitamin D deficiency due to compliance [Citation83]. Despite the scarcity of studies on pregnant women, various studies have stressed the importance of large single doses of vitamin D in different populations. In their review that investigated the effects of single, large doses of vitamin D on serum concentrations and other health outcomes, Kearns et al. [Citation86] came to the conclusion that a single vitamin D3 dose ≥300,000 IU was most effective at improving vitamin D status for up to 3 months in adults. In line with this finding, Boonen et al. [Citation87] reported cholecalciferol 100,000 IU was a safe, effective, and simple way to increase serum vitamin D for up to 2 months in the elderly. Other studies have found that daily, weekly, and monthly administrations of the daily equivalent of 1000 IU of vitamin D3 provide equal efficacy and safety profiles, with intermittent supplementation still being preferred [Citation29,Citation88].
The effect of maternal vitamin D on child growth was significant when initiated or measured at >20 weeks of gestation. Similar findings were reported in previous meta-analyses on different outcomes [Citation82]. Vitamin D supplementation increased birth weight only in the group with therapy initiated late (≥20 weeks’ gestation). Evidence that higher maternal vitamin D levels in later trimesters were associated with better outcomes suggests the need to monitor maternal vitamin D beyond the first trimester. Higher maternal vitamin D in the first trimester is not necessarily an indication of subsequent status during pregnancy. This was shown in clinical trials where the initiation of supplementation of vitamin D at less than 20 weeks of gestation had a greater effect size (WMD, 0.38 cm vs 0.17 cm) (supplementary file, SFigure 3). This underscores the importance of continuous vitamin D monitoring considering the plasma half-life.
This study adds to our existing knowledge of maternal vitamin D and its role in child development. Our review includes both interventional and observational studies. The risk of bias and methodological quality of included studies are summarized in supplementary files (STable 1 and STable 2). The focus was on linear growth as an outcome to provide us with a comprehensive understanding of this issue. This study also demonstrated the role of various factors such as supplementation dose, time of initiation, frequency of supplementation, and trimester. The findings suggest that the focus should be on higher vitamin D doses, earlier initiation, and sustained adequate levels, as well as less frequent supplementation.
In light of all of this, the following limitations should be considered when interpreting the results. First, there is significant heterogeneity. The included studies differed in many ways, including the populations studied, ethnicity, geographic factors, maternal vitamin D dose and cutoff points, clinical settings, the timing of intervention and/or measurement, and baseline maternal factors such as socioeconomic indicators.
Second, even though the objective of the meta-analysis was to assess linear growth in under-five children, there were a limited number of reports after birth. Few studies reported length at 1 month (3), 3 months (5), 6 months (4), and 1 year (5), and five studies reported length for age. The effect of maternal vitamin D on child growth beyond 12 months of age was not incorporated due to the lack of available studies. Despite our initial concept of the source of vitamin D in children, during the first 6 to 8 weeks of postnatal life, the vitamin D status of infants is mainly dependent on placental transfer in utero [Citation21]. As previously noted, stores are depleted by approximately 8 weeks of age [Citation22], after which time the infant’s vitamin D is dependent on diet, sunlight, and supplementation. This temporal relationship was not established in the data due to lack of available studies.
Third, another critical issue in this meta-analysis is adherence. This paper signified the importance of monthly or less frequent supplementation rather than daily or weekly. We hypothesized that this might be due to adherence. However, this was not confirmed here due to limited information regarding adherence. A prospective cohort study hypothesized that a 5000 IU daily supplement is superior to the 200,000 IU stat supplement and recommended that randomized control trials be conducted in order to confirm this hypothesis [Citation89]. A controlled trial reported that a single 5 mg dose of vitamin D given orally during the seventh month of pregnancy provided effective prophylaxis for vitamin D deficiency over 1000 IU daily supplement [Citation90]. A review also suggested that prenatal vitamin D supplementation with a higher dose could be reformulated due to several factors, the major one being adherence [Citation27]. The evidence on adherence is mixed. Daily supplementation has been shown to have poor adherence [Citation91]. In a 2001 study of protease inhibitor regimen adherence among HIV patients, for example, true adherence via electronic monitors was 63%, while pill count indicated 83% adherence [Citation92]. These findings support evidence of the poor adherence but also call the verification methods into question.
Conclusion
The evidence suggests that prenatal vitamin D supplementation in higher doses (>2000 IU), low frequency (monthly or less frequently), and later gestation (>20 weeks) is positively associated with higher child length/height. There is, however, a need for further evidence from clinical trials, not only comparing different doses and frequencies but also investigating adherence. In summary, the evidence to date suggests that consistent and adequate levels of vitamin D during pregnancy are critical for children’s growth.
Ethics and consent
Not applicable.
Paper context
Prenatal intake of vitamin D has positive effects on birth outcomes. Vitamin D supplementation during pregnancy is associated with higher birth length and the effects are more evident at higher doses, low frequency, and in later stages of pregnancy. Consistent and adequate vitamin D levels are crucial in pregnancy. Future clinical trials are needed to evaluate frequency, dose, and adherence.
Supplemental Material
Download MS Word (1.5 MB)Acknowledgments
TAZ’s time was covered by a fellowship funded by the TRAIN@Ed Marie Skłodowska-Curie Actions program.
Disclosure statement
None of the authors have any competing interest.
Supplemental data
Supplemental data for this article can be accessed online at https://doi.org/10.1080/16549716.2022.2102712.
Additional information
Funding
Notes on contributors
Addis Alem
AAT conceived the study and drafted the manuscript. All authors contributed substantially to the methodology, analysis, and interpretation of data. All authors read and approved the final version of the manuscript. AAT will be responsible for the accuracy or integrity of any part of the work.
References
- Sizar O, Khare S, Goyal A, et al. Vitamin D Deficiency. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 cited 2022 Feb 24]. Available from 2022 Feb 24. http://www.ncbi.nlm.nih.gov/books/NBK532266/
- Holick MF. Vitamin D Deficiency. N Engl J Med. 2007;357:266–15.
- Khazai N, Judd SE, Tangpricha V. Calcium and vitamin D: skeletal and extraskeletal health. Curr Rheumatol Rep. 2008;10:110–117.
- Bikle DDVDM. Mechanism of action, and clinical applications. Chem Biol. 2014;21:319–329.
- Christakos S, Dhawan P, Verstuyf A, et al. Vitamin D: metabolism, molecular mechanism of action, and pleiotropic effects. Physiol Rev. 2016;96:365–408.
- Fernández-Cancio M, Andaluz P, Torán N, et al. Vitamin D stimulates growth hormone-insulin-like growth factor (GH-IGF) gene axis expression and potentiates gh effect to reverse the inhibition produced by glucocorticoids in human growth plate chondrocytes. HRP. 2007;67:204–205.
- Ameri P, Giusti A, Boschetti M, et al. Vitamin D increases circulating IGF1 in adults: potential implication for the treatment of GH deficiency. Eur J Endocrinol. 2013;169:767–772.
- Institute of Medicine (US). Committee to review dietary reference intakes for vitamin D and calcium. In: Ross AC, Taylor CL, Yaktine AL, et al., editors. Dietary reference intakes for calcium and vitamin D. Washington (DC): National Academies Press (US). 2011 cited 2022 Jun 16. Available from: http://www.ncbi.nlm.nih.gov/books/NBK56070/
- Giustina A, Adler RA, Binkley N, et al. Consensus statement from 2nd international conference on controversies in Vitamin D. Rev Endocr Metab Disord. 2020;21:89–116.
- Cashman KD. 100 YEARS OF VITAMIN D: global differences in vitamin D status and dietary intake: a review of the data. Endocr Connect. 2022;11:cited 2022 Mar 16: Available from https://ec.bioscientifica.com/view/journals/ec/11/1/EC-21-0282.xml.
- Amrein K, Scherkl M, Hoffmann M, et al. Vitamin D deficiency 2.0: an update on the current status worldwide. Eur J Clin Nutr. 2020;74:1498–1513.
- Roth DE, Abrams SA, Aloia J, et al. Global prevalence and disease burden of vitamin D deficiency: a roadmap for action in low- and middle-income countries. Ann N Y Acad Sci. 2018;1430:44–79.
- Shrestha D, Budhathoki S, Pokhrel S, et al. Prevalence of vitamin D deficiency in pregnant women and their babies in Bhaktapur, Nepal. BMC Nutr. 2019 May 29;5:31.
- Hong-Bi S, Yin X, Xiaowu Y, et al. High prevalence of vitamin D deficiency in pregnant women and its relationship with adverse pregnancy outcomes in Guizhou, China. J Int Med Res. 2018;46:4500–4505.
- Al-Wassia H, Abo-Ouf N. Prevalence of vitamin D deficiency in mother–infant pairs in a tertiary hospital in the west coast of Saudi Arabia. J Clin Neonatol. 2016;5:243.
- Mogire RM, Mutua A, Kimita W, et al. Prevalence of vitamin D deficiency in Africa: a systematic review and meta-analysis. Lancet Glob Health. 2020;8:e134–142.
- Institute of Medicine (US). Standing committee on the scientific evaluation of dietary reference intakes. Dietary reference intakes for calcium, phosphorus, magnesium, vitamin D, and fluoride. Washington (DC): National Academies Press (US). 1997 cited 2022 Jun 19. Available from: http://www.ncbi.nlm.nih.gov/books/NBK109825/
- National Health and Medical Research Council of Australia. Vitamin D [Internet]. National Health Medical Research Council. 2014 cited 2022 Jun 19. Available from: https://www.nrv.gov.au/nutrients/vitamin-d
- Davies SC, Jewell T, McBride M, et al. Vitamin D—advice on supplements for at risk groups. London: Department of Health; 2012. p. 1–4.
- Kumar RK, Das H, Girish SV, et al. Prevalence of vitamin D deficiency among newborns. Indian Pediatr. 2020;57:258–259.
- Hillman LS, Haddad JG. Human perinatal vitamin D metabolism I: 25-Hydroxyvitamin D in maternal and cord blood. J Pediatr. 1974;84:742–749.
- Ala-Houhala M. 25-Hydroxyvitamin D levels during breast-feeding with or without maternal or infantile supplementation of vitamin D. J Pediatr Gastroenterol Nutr. 1985;4:220–226.
- Hollis BW, Roos BA, Draper HH, et al. Vitamin D and its metabolites in human and bovine milk. J Nutr. 1981;111:1240–1248.
- Dawodu A, Agarwal M, Hossain M, et al. Hypovitaminosis D and vitamin D deficiency in exclusively breast-feeding infants and their mothers in summer: a justification for vitamin D supplementation of breast-feeding infants. J Pediatr. 2003;142:169–173.
- Balasubramanian S, Ganesh R. Vitamin D deficiency in exclusively breast-fed infants. Indian J Med Res. 2008;127:250–255.
- Tareke AA, Hadgu AA, Ayana AM, et al. Prenatal vitamin D supplementation and child respiratory health: a systematic review and meta-analysis of randomized controlled trials. World Allergy Organ J. 2020;13:100486.
- Dror DK, Allen LH. Vitamin D inadequacy in pregnancy: biology, outcomes, and interventions. Nutr Rev. 2010;68:465–477.
- Tous M, Villalobos M, Iglesias L, et al. Vitamin D status during pregnancy and offspring outcomes: a systematic review and meta-analysis of observational studies. Eur J Clin Nutr. 2020;74:36–53.
- Maugeri A, Barchitta M, Blanco I, et al. Effects of Vitamin D supplementation during pregnancy on birth size: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2019;11:442.
- Fang K, He Y, Mu M, et al. Maternal vitamin D deficiency during pregnancy and low birth weight: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2021;34:1167–1173.
- Hollis BW, Wagner CL. Substantial vitamin D supplementation is required during the prenatal period to improve birth outcomes. Nutrients. 2022;14:899.
- Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011 cited 2020 May 5;343: Available from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3196245/
- Wells G, Wells G, Shea B, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2014. cited 2022 Jun 25. Available from: https://www.semanticscholar.org/paper/The-Newcastle-Ottawa-Scale-NOS-for-Assessing-the-Wells-Wells/c293fb316b6176154c3fdbb8340a107d9c8c82bf
- Rücker G, Cates CJ, Schwarzer G. Methods for including information from multi-arm trials in pairwise meta-analysis. Res Synth Methods. 2017;8:392–403.
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558.
- Diogenes MEL, Bezerra FF, Rezende EP, et al. Calcium plus vitamin D supplementation during the third trimester of pregnancy in adolescents accustomed to low calcium diets does not affect infant bone mass at early lactation in a randomized controlled trial. J Nutr. 2015;145:1515–1523.
- Hajhashemi M, Khorsandi A, Haghollahi F. Comparison of sun exposure versus vitamin D supplementation for pregnant women with vitamin D deficiency. J Matern Fetal Neonatal Med. 2019;32:1347–1352.
- Brustad N, Garland J, Thorsen J, et al. Effect of high-dose vs standard-dose vitamin D supplementation in pregnancy on bone mineralization in offspring until age 6 years: a prespecified secondary analysis of a double-blinded, randomized clinical trial. JAMA Pediatr. 2020;174:419–427.
- Litonjua AA, Carey VJ, Laranjo N, et al. Effect of prenatal supplementation with vitamin D on asthma or recurrent wheezing in offspring by age 3 years: the VDAART randomized clinical trial. JAMA. 2016;315:362–370.
- Hashemipour S, Ziaee A, Javadi A, et al. Effect of treatment of vitamin D deficiency and insufficiency during pregnancy on fetal growth indices and maternal weight gain: a randomized clinical trial. Eur J Obstet Gynecol. 2014;172:15–19.
- Elmee P, Shahrokh Taghavi S. Effect of vitamin D supplementation during pregnancy on birth weight: a case-control study. Iran J Neonatology IJN. 2017;8:27–30.
- Kalra P, Das V, Agarwal A, et al. Effect of vitamin D supplementation during pregnancy on neonatal mineral homeostasis and anthropometry of the newborn and infant. Br J Nutr. 2012;108:1052–1058.
- Karamali M, Beihaghi E, Mohammadi AA, et al. Effects of high-dose vitamin D supplementation on metabolic status and pregnancy outcomes in pregnant women at risk for pre-eclampsia. Horm Metab Res. 2015;47:867–872.
- Cooper C, Harvey NC, Bishop NJ, et al. Maternal gestational vitamin D supplementation and offspring bone health (MAVIDOS): a multicentre, double-blind, randomised placebo-controlled trial. Lancet Diabetes Endocrinol. 2016;4:393–402.
- Sahoo SK, Katam KK, Das V, et al. Maternal vitamin D supplementation in pregnancy and offspring outcomes: a double-blind randomized placebo-controlled trial. J Bone Miner Metab. 2017;35:464–471.
- Roth DE, Perumal N, Mahmud AA, et al. Maternal vitamin D3 supplementation during the third trimester of pregnancy: effects on infant growth in a longitudinal follow-up study in Bangladesh. J Pediatr. 2013;163:1605–1611.e3.
- Hossain N, Kanani FH, Ramzan S, et al. Obstetric and neonatal outcomes of maternal vitamin D supplementation: results of an open-label, randomized controlled trial of antenatal vitamin d supplementation in Pakistani women. J Clin Endocrinol Metab. 2014;99:2448–2455.
- Perumal N, Al Mahmud A, Baqui AH, et al. Prenatal vitamin D supplementation and infant vitamin D status in Bangladesh. Public Health Nutr. 2017;20:1865–1873.
- Sablok A, Batra A, Thariani K, et al. Supplementation of vitamin D in pregnancy and its correlation with feto-maternal outcome. Clin Endocrinol (Oxf). 2015;83:536–541.
- Mohammad-Alizadeh-Charandabi S, Mirghafourvand M, Mansouri A, et al. The effect of vitamin D and calcium plus vitamin D during pregnancy on pregnancy and birth outcomes: a randomized controlled trial. J Caring Sci. 2015;4:35–44.
- Mojibian M, Soheilykhah S, Zadeh MAF, et al. The effects of vitamin D supplementation on maternal and neonatal outcome: a randomized clinical trial. Iran J Reprod Med. 2015;13:687–696.
- Sabet Z, Ghazi MS, Tohidi M, et al. vit D supplementation in pregnant Iranian women effects of maternal and neonatal vitamin D and parathyroid hormone status. Acta Endocrinologica-Bucharest. 2012;3:59–66.
- Hornsby E, Pfeffer PE, Laranjo N, et al. Vitamin D supplementation during pregnancy: effect on the neonatal immune system in a randomized controlled trial. J Allergy Clin Immunol. 2018;141:269–278.e1.
- Vaziri F, Dabbaghmanesh MH, Samsami A, et al. Vitamin D supplementation during pregnancy on infant anthropometric measurements and bone mass of mother-infant pairs: a randomized placebo clinical trial. Early Hum Dev. 2016;103:61–68.
- Roth DE, Morris SK, Zlotkin S, et al. Vitamin D supplementation in pregnancy and lactation and infant growth. N Engl J Med. 2018;379:535–546.
- Brooke OG, Brown IR, Bone CD, et al. Vitamin D supplements in pregnant Asian women: effects on calcium status and fetal growth. Br Med J. 1980;280:751–754.
- Thiele DK, Ralph J, El-Masri M, et al. Vitamin D3 supplementation during pregnancy and lactation improves vitamin D status of the mother–infant Dyad. J Obstet Gynecol Neonatal Nurs. 2017;46:135–147.
- Abotorabi S, Hashemi Poor S, Esmailzadehha N, et al. Effect of treatment with vitamin D on maternal and neonatal indices in pregnant women with hypocalcemia: a randomized controlled trial. Int J Pediatr. 2017;5:5733–5739.
- Boghossian NS, Koo W, Liu A, et al. Longitudinal measures of maternal vitamin D and neonatal body composition. Eur J Clin Nutr. 2019;73:424–431.
- Chi MZ, Zhu L, Zhang ZL, et al. The relationship between maternal serum vitamin D levels and infant neurodevelopment and anthropometry: a prospective observational study. J Nutr Sci Vitaminol (Tokyo). 2018;64:161–167.
- Dalgård C, Petersen MS, Steuerwald U, et al. Umbilical Cord Serum 25-hydroxyvitamin D concentrations and relation to birthweight, head circumference and infant length at age 14 days. Paediatr Perinat Epidemiol. 2016;30:238–245.
- Gale CR, Robinson SM, Harvey NC, et al. Maternal vitamin D status during pregnancy and child outcomes. Eur J Clin Nutr. 2008;62:68–77.
- Skowrońska-Jóźwiak E, Lebiedzińska K, Smyczyńska J, et al. Effects of maternal vitamin D status on pregnancy outcomes, health of pregnant women and their offspring. Neuroendocrinol Lett. 2014;35:367–372.
- Kılıcaslan AÖ, Kutlu R, Kilinc I, et al. The effects of vitamin D supplementation during pregnancy and maternal vitamin D levels on neonatal vitamin D levels and birth parameters. J Matern Fetal Neonatal Med. 2018;31:1727–1734.
- Morales E, Rodriguez A, Valvi D, et al. Deficit of vitamin D in pregnancy and growth and overweight in the offspring. Int J Obesity. 2015;39:61–68.
- Morley R, Carlin JB, Pasco JA, et al. Maternal 25-hydroxyvitamin D and parathyroid hormone concentrations and offspring birth size. J Clin Endocrinol Metab. 2006;91:906–912.
- Ni M, Zhang Q, Zhao J, et al. Relationship between maternal vitamin D status in the first trimester of pregnancy and maternal and neonatal outcomes: a retrospective single center study. BMC Pediatr. 2021;21:330.
- Ong YL, Quah PL, Tint MT, et al. The association of maternal vitamin D status with infant birth outcomes, postnatal growth and adiposity in the first 2 years of life in a multi-ethnic Asian population: the growing up in Singapore towards healthy outcomes (GUSTO) cohort study. Br J Nutr. 2016;116:621–631.
- Reichetzeder C, Chen H, Föller M, et al. Maternal vitamin D deficiency and fetal programming - lessons learned from humans and mice. Kbr. 2014;39:315–329.
- Sabour H, Hossein-Nezhad A, Maghbooli Z, et al. Relationship between pregnancy outcomes and maternal vitamin D and calcium intake: a cross-sectional study. Gynecological Endocrinol. 2006;22:585–589.
- Sarma D, Saikia UK, Das DV. Fetal Skeletal Size and Growth are relevant biometric markers in vitamin D deficient mothers: a North East India prospective cohort study. Indian J Endocrinol Metab. 2018;22:212–216.
- Shakeri M, Jafarirad S. The relationship between maternal vitamin D status during third trimester of pregnancy and maternal and neonatal outcomes: a longitudinal study. Ijrm. 2019;17:33.
- Song SJ, Si S, Liu J, et al. Vitamin D status in Chinese pregnant women and their newborns in Beijing and their relationships to birth size. Public Health Nutr. 2013;16:687–692.
- Viljakainen HT, Saarnio E, Hytinantti T, et al. Maternal vitamin D status determines bone variables in the newborn. J Clin Endocrinol Metab. 2010;95:1749–1757.
- Zhou J, Su L, Liu M, et al. Associations between 25-hydroxyvitamin D levels and pregnancy outcomes: a prospective observational study in southern China. Eur J Clin Nutr. 2014;68:925–930.
- Leffelaar ER, Vrijkotte TGM, van EM. Maternal early pregnancy vitamin D status in relation to fetal and neonatal growth: results of the multi-ethnic Amsterdam born children and their development cohort. Br J Nutr. 2010;104:108–117.
- Brooke OG, Butters F, Wood C. Intrauterine vitamin D nutrition and postnatal growth in Asian infants. Br Med J (Clinical Research Ed). 1981;283:1024.
- O’Callaghan KM, Shanta SS, Fariha F, et al. Effect of maternal prenatal and postpartum vitamin D supplementation on offspring bone mass and muscle strength in early childhood: follow-up of a randomized controlled trial. Am J Clin Nutr. 2022;115:770–780.
- Eckhardt CL, Gernand AD, Roth DE, et al. Maternal vitamin D status and infant anthropometry in a US multi-centre cohort study. Ann Hum Biol. 2015;42:217–224.
- Wei SQ, Qi HP, Luo ZC, et al. Maternal vitamin D status and adverse pregnancy outcomes: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2013;26:889–899.
- Pérez-López FR, Pasupuleti V, Mezones-Holguin E, et al. Effect of vitamin D supplementation during pregnancy on maternal and neonatal outcomes: a systematic review and meta-analysis of randomized controlled trials. Fertil Steril. 2015;103:1278–1288.e4.
- Bi WG, Nuyt AM, Weiler H, et al. Association between vitamin D supplementation during pregnancy and offspring growth, morbidity, and mortality: a systematic review and meta-analysis. JAMA Pediatr. 2018;172:635–645.
- Boonen S, Vanderschueren D, Haentjens P, et al. Calcium and vitamin D in the prevention and treatment of osteoporosis – a clinical update. J Intern Med. 2006;259:539–552.
- De‐Regil LM, Palacios C, Lombardo LK, et al. Vitamin D supplementation for women during pregnancy. Cochrane Database of Systematic Reviews [Internet]. 2016. cited 2022 Apr 14. Available from: https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD008873.pub3/full
- Ma K, Wei SQ, Bi WG, et al. Effect of vitamin D supplementation in early life on children’s growth and body composition: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2021;13:524.
- Kearns MD, Alvarez JA, Large TV. Single-Dose, oral vitamin D supplementation in adult populations: a systematic review. Endocr Pract. 2014;20:341–351.
- Ilahi M, Armas LA, Heaney RP. Pharmacokinetics of a single, large dose of cholecalciferol. Am J Clin Nutr. 2008;87:688–691.
- Akács I, Tóth BE, Szekeres L, et al. Randomized clinical trial to comparing efficacy of daily, weekly and monthly administration of vitamin D3. Endocrine. 2017;55:60–65.
- Bokharee N, Khan YH, Wasim T, et al. Daily versus stat vitamin D supplementation during pregnancy; A prospective cohort study. PLoS One. 2020;15:e0231590.
- Mallet E, Gügi B, Brunelle P, et al. Vitamin D supplementation in pregnancy: a controlled trial of two methods. Obstet Gynecol. 1986;68:300–304.
- Mortensen C, Tetens I, Kristensen M, et al. Adherence and barriers to the vitamin D and calcium supplement recommendation at Danish nursing homes: a cross-sectional study. BMC Geriatr. 2022;22:27.
- Liu H, Golin CE, Miller LG, et al. A comparison study of multiple measures of adherence to HIV protease inhibitors. Ann Intern Med. 2001;134:968–977.