ABSTRACT
Background
A low body mass index (BMI) at the start of treatment for rifampicin- or multidrug-resistant tuberculosis (MDR/RR-TB) is associated with poor treatment outcomes and may contribute to delayed sputum culture conversion, thereby prolonging the period of potential transmission to others. Whether the relative importance of low BMI in predicting treatment outcomes differs by HIV status is unclear.
Objectives
We evaluated the association between low BMI and two dependent variables, sputum culture conversion and end-of-treatment outcome, among patients receiving treatment for MDR/RR-TB in Lesotho, a setting with a high prevalence of HIV infection.
Methods
Secondary data from a prospective cohort of patients initiating a longer (18–20 months) treatment containing bedaquiline and/or delamanid under routine programmatic conditions in Lesotho were analysed. Risk ratios and differences were adjusted for potential confounders using multivariable logistic regression, and estimates were stratified by HIV status.
Results
Of 264 patients, 105 and 250 were eligible for culture conversion and end-of-treatment analyses, respectively. Seventy-one per cent of patients (74/105) experienced culture conversion within six months, while 74% (184/250) experienced a favourable end-of-treatment outcome. Low BMI was associated with a lower frequency of culture conversion at six months among those who were not living with HIV (relative risk [RR]: 0.50 [95% CI: 0.21, 0.79]); this association was attenuated among those living with HIV (RR: 0.88 [95% CI: 0.68, 1.23]). A low BMI was moderately associated with a lower frequency of treatment success (RR = 0.89 [95% CI: 0.77, 1.03]), regardless of HIV status.
Conclusions
Low BMI was common and associated with the frequency of six-month culture conversion and end-of-treatment outcomes. The association with culture conversion was more pronounced among those not living with HIV. Addressing the myriad factors that drive low BMI in this setting could hasten culture conversion and improve end-of-treatment outcomes. This will require a multipronged approach focused on alleviating food insecurity and enabling prompt diagnosis and treatment of HIV and TB.
Responsible Editor Jennifer Stewart Williams
Background
Despite the recent improvements in the treatment of multidrug- or rifampicin-resistant and resistant tuberculosis (MDR/RR-TB), not all patients experience treatment success [Citation1,Citation2]. Even among patients receiving all-oral and/or shortened regimens, those living with HIV or advanced TB experience a higher risk of unfavourable treatment outcomes. Low body mass index (BMI) indicates advanced disease and may therefore correlate with unsuccessful treatment [Citation3]. Low BMI is also a proxy for malnutrition, including micronutrient and protein energy deficiencies, which may impact immune system functioning and, thereby, the response to treatment [Citation4]. Because malnutrition and advanced TB may occur simultaneously or singularly, the underlying cause of the low body weight may be challenging to disentangle. Regardless of the mechanism, low BMI may serve as a marker of risk or vulnerability, identifying patients needing additional care and support.
Sputum culture conversion is a marker of treatment response; delayed or no culture conversion identifies patients at high risk for an unfavourable end-of-treatment outcome [Citation5,Citation6]. Studies from diverse settings, including two meta-analyses, have suggested that low BMI is an important prognostic factor for delayed culture conversion and poor end-of-treatment outcomes among patients undergoing treatment for MDR/RR-TB [Citation3,Citation7–13]. While some of these studies were conducted in settings with a high HIV prevalence [Citation10,Citation12], whether the relative importance of low BMI differs according to HIV status with regard to these outcomes remains unclear.
Lesotho is a small, landlocked country surrounded by the Republic of South Africa. The country’s population is estimated at 2.2 million, with 57% living on less than one dollar a day and about 80% living in rural areas [Citation14]. TB incidence is 650 per 100,000 population, and the estimated proportion of cases that are MDR/RR-TB is 11.8% across new and previously treated cases [Citation15]. In Lesotho, the tuberculosis epidemic is fuelled by HIV (one in five adults aged 15 to 49 lives with HIV) [Citation16]. It co-occurs alongside frequent malnutrition and food insecurity arising from El Niño-induced drought. Over 57% of the population lives below the poverty line [Citation14].
Given the synergistic epidemics of tuberculosis, HIV, food insecurity, and poverty in Lesotho, low BMI may constitute a particularly important warning sign for patients who present to care for MDR/RR-TB. Among patients treated for MDR/RR-TB with a longer regimen containing bedaquiline and/or delamanid, we examined whether a low BMI predicted culture conversion within six months of treatment or end-of-treatment success and whether these relationships were modified by HIV status.
Methods
Data resource, target population, and study sites
This secondary analysis relied on data from the prospective endTB observational cohort (NCT03259269) of patients initiating longer (18–20 month) treatment for MDR/RR-TB with a regimen containing bedaquiline and/or delamanid under routine programmatic conditions. Data from standardised forms were entered into an electronic medical record. The current analysis was restricted to patients from Lesotho who had documented MDR/RR-TB and consented to participate in the observational cohort. For analyses of culture conversion, we additionally required a positive baseline culture taken before the initiation of treatment [Citation17,Citation18]. For the end-of-treatment (EOT) outcome analyses, patients were excluded if their EOT outcome was not evaluated (i.e. in the case of transfer out of the country). All patients in Lesotho received a monthly food package including 50 kg of maize meal, 20 kg of sorghum, 2 kg of beans, 2 kg of peas, 1 g of sugar, 5 kg of vegetable oil, and 1 g of powdered milk. This support was intended for one person and provided to enable and encourage treatment adherence and improve patients’ nutritional status.
Dependent variables and definitions
Sputum culture conversion within the first six months of treatment was defined as two consecutive negative cultures collected at least 15 days apart, one before 180 days of treatment and one before 210 days of treatment [Citation19–21]. The second outcome was favourable EOT outcomes. In accordance with WHO guidelines, using an algorithm, we calculated the EOT outcomes based on the available culture results at the end of treatment [Citation22].
Independent variables and definitions
The primary independent variable was BMI, which was classified into underweight (<18.5), normal weight (18.5 to 24.9), and overweight (≥25). We considered potential confounding by demographic characteristics (i.e. age and sex), substance use (i.e. alcohol use, tobacco use, injection drug use, and non-injection drug use), comorbidities (i.e. anaemia, HIV infection, diabetes mellitus or glucose intolerance, hepatitis B virus, hepatitis C virus), and TB-related characteristics (i.e. bilateral disease, cavitary disease, fibrosis, sputum culture, sputum smear, and baseline resistance profile).
Statistical analysis included descriptive statistics, bivariable analyses, and multivariable logistic regression. From the list of potential confounders identified a priori, we identified those associated with BMI and the outcomes of interest in these data and adjusted for these potential confounders. Besides the list of potential confounders, we adjusted for resistance profile at baseline and numbers of likely effective Group A drugs in the multivariable analyses. We created an interaction term between BMI and HIV status to examine whether the impact of BMI on treatment outcome differed by HIV status. The missing indicator method was used to handle missing data. In the case of model nonconvergence, we used bootstrapping with 500 random samples to calculate the risk ratio (RR), risk difference (RD), and 95% confidence intervals (CI) based on the predicted probabilities.
We conducted a secondary analysis to differentiate between underweight and severely underweight. For the analysis of culture conversion, we conducted a sensitivity analysis, which consisted of a time-to-event analysis in which we censored patients who died or became lost to follow-up before the six months of treatment. Analyses were conducted using SAS software version 9.4 (SAS Institute, Inc., Cary, NC), and the forest plot was created using the ‘forestplot’ package in R version 4.1.0 (The R Foundation).
Research ethics
The study protocol for the endTB observational cohort was approved by the ethics review committees for the three consortium partners (i.e. Partners In Health, Medecins Sans Frontieres, and Interactive Research and Development) and in each country where the study was conducted. In Lesotho, approval was granted by the National Health Research Ethics Committee (REF: 11; 61-2016; March 2015). Study participants provided written informed consent for inclusion in the observational cohort.
Results
Overview
Of the 2,789 patients enrolled in the endTB observational cohort, 264 (9%) were from Lesotho. A total of 105 and 250 patients were eligible for culture conversion analyses and end-of-treatment analyses, respectively. Seventy-one per cent of patients (74/105) experienced culture conversion within six months, while 74% (184/250) shared a favourable EOT outcome. Across both analytic groups, patients tended to be male and married. More than half were underweight, and a majority were living with HIV ().
Table 1. Baseline characteristics of patients initiating treatment for rifampicin- or multidrug-resistant tuberculosis in Lesotho, 2015–2018.
Culture conversion within six months
In univariable analyses, being underweight (crude RR = 0.75 [95% CI]: 0.58, 0.99]; in Appendix) was associated with a lower probability of culture conversion. Adjusting for potential confounders (i.e. age, marital status, anaemia, HIV infection, resistance profile at baseline, and numbers of likely effective Group A drugs), being underweight was associated with a similar reduction in the probability of culture conversion within six months (RR: 0.76 [95% CI: 0.59, 0.97]; RD: −0.19 [95% CI: −0.34, −0.03]; , ).
Figure 1. Forest plot of the adjusted relative risk of low BMI on culture conversion within six months or favourable EOT outcomes, stratified by HIV status.
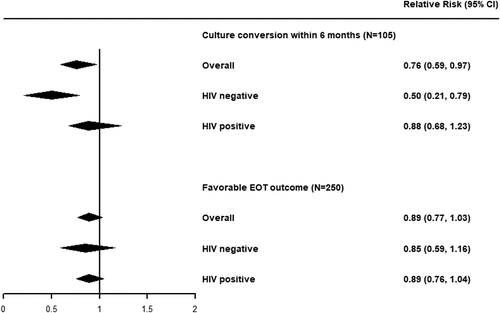
Table 2. Multivariable analyses of low BMI and culture conversion and favourable treatment outcome among patients initiating treatment for rifampicin- or multidrug-resistant tuberculosis in Lesotho, 2015–2018.
We found potential evidence for effect modification by HIV status. Among patients without HIV, being underweight was associated with a sizeable reduction in the frequency of six-month culture conversion (RR: 0.50 [95% CI: 0.21, 0.79]; RD: −0.50 [95% CI: −0.79, −0.21]; , ). This relationship was attenuated for patients living with HIV (RR: 0.88 [95% CI: 0.68, 1.23]; RD: −0.08 [95% CI: −0.25, 0.15]; , ).
In the secondary analyses, which differentiated between underweight and severely underweight, the results were consistent and did not affect the interpretation ( in Appendix). Being severely underweight (RR: 0.78 [95% CI: 0.57, 1.01]; RD: −0.18 [95% CI: −0.37, 0.01]) or underweight (RR: 0.78 [95% CI: 0.57, 1.01]; RD: −0.18 [95% CI: −0.36, 0.00]) was negatively associated with culture conversion within six months. Among patients who were HIV negative, being severely underweight (RR: 0.52 [95% CI: 0.00, 1.00]; RD: −0.48 [95% CI: −1.00, 0.00]) or underweight (RR: 0.49 [95% CI: 0.00, 0.83]; RD: −0.52 [95% CI: −1.00, −0.17]) was associated with a reduction in the risk of six-month culture conversion. This association was less pronounced in patients living with HIV (severely underweight: RR: 0.89 [95% CI: 0.60, 1.23]; RD: −0.08 [95% CI: −0.31, 0.14]; underweight: RR: 0.93 [95% CI: 0.64, 1.33]; RD: −0.05 [95% CI: −0.27, 0.20]).
Sensitivity analyses, in which patients who died or were lost to follow-up before six months of the treatment were censored, yielded similar results. Patients who were underweight experienced a lower rate of culture conversion overall (HR: 0.44 [95% CI: 0.26, 0.78]) ( in Appendix); however, this impact tended to be stronger among patients who were HIV-negative (HR: 0.20 [95% CI: 0.07, 0.56]) as compared to those living with HIV (HR: 0.60 [95% CI: 0.31, 1.14]).
Favourable EOT outcomes
In univariable analyses, being underweight (crude RR: 0.89 [95% CI: 0.76, 1.04]) or overweight (crude RR: 1.10 [95% CI: 0.90, 1.33]; in Appendix) was not associated with the probability of favourable outcomes.
In multivariable analyses adjusted for age at study drug start, HIV infection, diabetes, at least one other comorbidity, bilateral disease, cavity disease, fibrosis, and positive culture at baseline, we found moderate evidence that patients with TB who were underweight were less likely to have a favourable outcome after treatment (RR: 0.89 [95% CI: 0.77, 1.03]; RD: −0.09 [95% CI: −0.19, 0.02]). The impact of being underweight was similar among those who were (RR: 0.85 [95% CI: 0.59, 1.16]; RD: −0.11 [95% CI: −0.35, 0.11]) and were not living with HIV (RR: 0.89 [95% CI: 0.76, 1.04]; RD: −0.09 [95% CI: −0.20, 0.03]) (, ).
The results of secondary analyses were also consistent with the main findings ( in Appendix). We found that patients who were severely underweight (RR: 0.79 [95% CI: 0.66, 0.94]; RD: −0.17 [95% CI: −0.29, −0.04]) or underweight (RR: 0.92 [95% CI: 0.77, 1.08]; RD: −0.07 [95% CI: −0.19, 0.06]) at baseline suffered from a lower probability of a favourable outcome. These results were similar, regardless of HIV status.
Discussion
Low BMI was common in this cohort of patients treated for MDR/RR-TB in Lesotho, most of whom also lived with HIV. Low BMI was strongly associated with a lower frequency of culture conversion at six months and moderately associated with a lower frequency of treatment success. This is similar to a study by Park et al., where low BMI was an independent risk factor for failure to achieve sputum culture conversion within three months among MDR/RR-TB patients [Citation2,Citation8,Citation23]. While the impact of low BMI on favourable EOT outcomes appeared to be similar among those who were and were not living with HIV, low BMI seemed to be a more important predictor of culture conversion among patients who were not living with HIV than those living with HIV.
The high prevalence of low BMI in this population suggests that targeted interventions that effectively prevent and/or mitigate this condition could impact culture conversion and treatment success. The development of such interventions requires understanding the factors driving low BMI in this setting, which are likely multifactorial. These include food insufficiency and advanced HIV and/or TB disease, all common among patients treated for MDR/RR-TB in Lesotho. A large part of the Basotho population experiences chronic food insecurity and/or nutrient deficiency, especially residents of rural areas where subsistence farming is common despite unfavourable agricultural conditions [Citation24,Citation25]. Advanced HIV infection and delayed TB diagnosis or treatment may also be important contributors. In Lesotho, migration in pursuit of economic opportunities is common and may interfere with consistent antiretroviral treatment in the absence of differentiated programmes to ensure mechanisms for treatment retention [Citation26,Citation27]. Traditional beliefs may contribute to tuberculosis treatment delays in Lesotho as some patients prefer to seek healthcare from traditional healers first and only present at the hospital when their clinical conditions have significantly deteriorated [Citation28]. Traditional healers have been successfully engaged to promote HIV care in Lesotho and could likewise be engaged to facilitate prompt care for tuberculosis [Citation29]. Health system challenges and centralised services may also contribute to delayed linkage to care and treatment initiation. Based on Lesotho’s national drug-resistant TB guidelines, all bacteriologically confirmed drug-resistant TB patients should be referred to a specialised hospital for treatment initiation [Citation30,Citation31]. Centralised approaches may lead to delays along the care cascade, resulting in morbidity, mortality, and ongoing TB transmission [Citation32,Citation33]. An approach where care and treatment are decentralised to the district hospitals where patients are initiated on treatment once diagnosed may be a solution to delayed treatment initiation among MDR/RR-TB patients in the country. As a result, the country is currently implementing a decentralisation strategy for the care and treatment of all drug-resistant TB patients.
The finding that low BMI was particularly harmful for individuals not living with HIV was unexpected. Rather, we hypothesised that low BMI could be more harmful in the context of HIV due to impaired immune functioning. While this unanticipated finding may be due to chance, an alternative explanation is that low BMI reflects social and medical histories that may vary by HIV status. For example, low BMI may be more likely to represent advanced TB disease among individuals without HIV than those living with HIV. A low BMI resulting from advanced HIV may be less likely than a low BMI resulting from advanced TB disease to impact TB-related outcomes. A second example is that patients with HIV may be more likely than those without HIV to have low BMI due to food insufficiency (and, therefore, may be more likely to respond to monthly nutritional support packages provided during TB treatment) [Citation34]. The major limitation of this analysis was an inability to discern between different contributors to low BMI. Future analyses could incorporate markers of food insecurity, recent weight loss, and/or qualitative in-depth interviews to elucidate the prevailing causes of low BMI and identify promising interventions.
Among the other limitations of this analysis is the lack of information on baseline HIV control and its correlates (i.e. adherence to antiretroviral therapy, time on antiretroviral therapy) which may influence BMI and TB treatment outcome. Additionally, while we hypothesise that treatment delays may have contributed to the high prevalence of low BMI at baseline, we did not have data on the onset of symptoms to confirm this hypothesis.
In conclusion, low BMI was common among patients treated with MDR/RR-TB and appeared to impact the frequency of six-month culture conversion and favourable EOT outcomes. Addressing the multiple factors that drive low BMI in this setting will require a multipronged approach focused on alleviating food insecurity and enabling prompt diagnosis and treatment of HIV and TB to hasten culture conversion and improve end-of-treatment outcomes.
Author contributions
The manuscript was conceived by L.O., B.O., C.M., L.M., M.M., and M.F. C.Z. conducted analyses with contributions by B.O. Study design was done by all authors, and first draft was written by L.O. with support from M.F. All authors interpreted study results and approved the final version.
Paper context
A low BMI at the start of treatment for rifampicin- or multidrug-resistant tuberculosis (MDR/RR-TB) is associated with poor treatment outcomes and may contribute to late sputum culture conversion, thereby prolonging the period of potential transmission to others. Whether the relative importance of low BMI in predicting treatment outcomes differs by HIV status is unclear. Given the synergistic epidemics of tuberculosis, HIV, food insecurity, and poverty in Lesotho, low BMI may constitute a particularly important warning sign for patients who present to care for MDR/RR-TB. Among patients treated for MDR/RR-TB with a longer regimen containing bedaquiline and/or delamanid, we examined whether a low BMI predicted culture conversion within six months of treatment or end-of-treatment success and whether these relationships were modified by HIV status.
Ethics and Consent
The endTB Observational Study protocol was approved by central ethics review committees for each consortium partner, and local ethical approval was obtained in all endTB countries. Participants provided written informed consent for inclusion in the observational cohort.
Additional information regarding study methods, including eligibility criteria, definitions, and statistical analyses, can be found in the online supplement.
Acknowledgments
We acknowledge and thank our funder, Unitaid, for financing the endTB project, including the observational study. Great appreciation to all the patients who participated in the endTB observational study and to the clinicians and programme staff at Partners In Health (PIH), Lesotho as well as the Lesotho National Tuberculosis and Leprosy Program (NTLP) for all your efforts in ending TB. We thank endTB staff at Partners In Health (PIH), Médecins Sans Frontières (MSF), Epicentre, and Interactive Research and Development (IRD) for making this project a success. We thank Marguerite Curtis and Alyson Nunez for their administrative support for this publication.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Belachew T, Yaheya S, Tilahun N, Gebrie E, Seid R, Nega T, et al. Multidrug-resistant tuberculosis treatment outcome and associated factors at the university of Gondar comprehensive specialized hospital: a Ten”>ten-year retrospective study. Infect Drug Resist [Internet]. 2022 [cited 2023 Oct 26];15:2891–10. doi: 10.2147/IDR.S365394
- Ali MH, Alrasheedy AA, Kibuule D, Godman B, Hassali MA, Ali HMH. Assessment of multidrug-resistant tuberculosis (MDR-TB) treatment outcomes in Sudan; findings and implications. Expert Rev Anti Infect Ther [Internet]. 2019 Nov 2 [cited 2023 Oct 26];17: 927–937. doi: 10.1080/14787210.2019.1689818
- Campbell JR, Chan ED, Falzon D, Trajman A, Keshavjee S, Leung CC, et al. Low body mass index at treatment initiation and rifampicin-resistant tuberculosis treatment outcomes: an individual participant data meta-analysis. Clin Infect Dis [Internet]. 2022 Dec 19 [cited 2023 Oct 26];75:2201–2210. 10.1093/cid/ciac322
- Sinha P, Davis J, Saag L, Wanke C, Salgame P, Mesick J, et al. Undernutrition and tuberculosis: public health implications. J Infect Dis [Internet]. 2019 May 5 [cited 2023 Oct 26];219:1356. doi: 10.1093/infdis/jiy675
- Kurbatova EV, Cegielski JP, Lienhardt C, Akksilp R, Bayona J, Becerra MC, et al. Evaluation of sputum culture conversion as a prognostic marker for end-of-treatment outcome in patients with multidrug-resistant tuberculosis. Lancet Respir Med [Internet]. 2015 Mar 1 [cited 2023 Oct 26];3:201. doi: 10.1016/S2213-2600(15)00036-3
- Holtz TH, Sternberg M, Kammerer S, Laserson KF, Riekstina V, Zarovska E, et al. Time to sputum culture conversion in multidrug-resistant tuberculosis: Predictors and relationship to treatment outcome. Ann Intern Med. 2006 May 2;144:650–659. doi: 10.7326/0003-4819-144-9-200605020-00008
- Tang S, Tan S, Yao L, Li F, Li L, Guo X, et al. Risk factors for poor treatment outcomes in patients with MDR-TB and XDR-TB in China: retrospective multi-center investigation. PLoS One [Internet]. 2013 Dec 5 [cited 2023 Oct 26];8:e82943. doi: 10.1371/journal.pone.0082943
- Park HO, Kim SH, Moon SH, Byun JH, Kim JW, Lee CE, et al. Association between body mass index and sputum culture conversion among South Korean patients with multidrug resistant tuberculosis in a tuberculosis referral hospital. Infect Chemother [Internet]. 2016 [cited 2023 Oct 26];48:317. doi: 10.3947/ic.2016.48.4.317
- Van LH, Phu PT, Vinh DN, Son VT, Hanh NT, Nhat LTH, et al. Risk factors for poor treatment outcomes of 2266 multidrug-resistant tuberculosis cases in Ho Chi Minh City: a retrospective study. BMC Infect Dis [Internet]. 2020 Feb 22 [cited 2023 Oct 26];20. doi: 10.1186/s12879-020-4887-1.
- Assemie MA, Alene M, Petrucka P, Leshargie CT, Ketema DB, Time to sputum culture conversion and its associated factors among multidrug-resistant tuberculosis patients in Eastern Africa: a systematic review and meta-analysis. Int J Infect Dis [Internet]. 2020 Sep 1 [cited 2023 Oct 26];98:230–236. doi: 10.1016/j.ijid.2020.06.029
- Putri FA, Burhan E, Nawas A, Soepandi PZ, Sutoyo DK, Agustin H, et al. Body mass index predictive of sputum culture conversion among MDR-TB patients in Indonesia. Int J Tuberc Lung Dis. 2014 May 1;18:564–570. doi: 10.5588/ijtld.13.0602
- Wakjira MK, Sandy PT, Mavhandu-Mudzusi AH, Anupurba S. Treatment outcomes of patients with MDR-TB and its determinants at referral hospitals in Ethiopia. PLoS One [Internet]. 2022 Feb 1 [cited 2023 Oct 26];17: e0262318. doi: 10.1371/journal.pone.0262318
- Velayutham B, Nair D, Kannan T, Padmapriyadarsini C, Sachdeva KS, Bency J, et al. Factors associated with sputum culture conversion in multidrug-resistant pulmonary tuberculosis. Int J Tuberc Lung Dis. 2016 Dec 1;20:1671–1676. doi: 10.5588/ijtld.16.0096
- Lesotho | World Food Programme [Internet]. [cited 2023 Oct 26]. Available from: https://www.wfp.org/countries/lesotho
- World Health Organization. Lesotho TB profile [Internet]. [cited 2023 Oct 26]. Available from: https://worldhealthorg.shinyapps.io/tb_profiles/?_inputs_&entity_type=%22country%22&lan=%22EN%22&iso2=%22LS%22
- Lesotho | UNAIDS [Internet]. [cited 2023 Oct 26]. Available from: https://www.unaids.org/en/regionscountries/countries/lesotho
- Tefera KT, Mesfin N, Reta MM, Sisay MM, Sisay Tamirat K, Akalu TY Treatment delay and associated factors among adults with drug resistant tuberculosis at treatment initiating centers in the Amhara regional state, Ethiopia. [cited 2023 Oct 26].
- Shi W, Davies Forsman L, Hu Y, Zheng X, Gao Y, Li X, et al. Improved treatment outcome of multidrug-resistant tuberculosis with the use of a rapid molecular test to detect drug resistance in China. Inter J Infect Dis [Internet]. 2020 Jul 1 [cited 2023 Oct 26]; 96:390–397. doi: 10.1016/j.ijid.2020.04.049
- Rodriguez CA, Brooks MB, Aibana O, Mitnick CD, Franke MF. Sputum culture conversion definitions and analytic practices for multidrug-resistant TB. Int J Tuberc Lung Dis [Internet]. 2021 Jul 7 [cited 2023 Oct 26];25: 596. doi: 10.5588/ijtld.21.0090
- Rodriguez CA, Lodi S, Horsburgh CR, Bastard M, Hewison C, Huerga H, et al. Selection bias in multidrug-resistant tuberculosis cohort studies assessing sputum culture conversion. PLoS One [Internet]. 2022 Nov 1 [cited 2023 Oct 26];17:e0276457. doi: 10.1371/journal.pone.0276457
- Franke MF, Khan P, Hewison C, Khan U, Huerga H, Seung KJ, et al. Culture conversion in patients treated with bedaquiline and/or delamanid. Am J Respir Crit Care Med [Internet]. 2021 Jan 1 [cited 2023 Oct 26];203:111–119. doi: 10.1164/rccm.202001-0135OC
- Zeng C, Mitnick CD, Hewison C, Bastard M, Khan P, Seung KJ, et al. Concordance of three approaches for operationalizing outcome definitions for multidrug-resistant TB. Int J Tuberc Lung Dis [Internet]. 2023 Jan 1 [cited 2023 Oct 26];27:34. doi: 10.5588/ijtld.22.0324
- Bade AB, Mega TA, Negera GZ. Malnutrition is associated with delayed sputum culture conversion among patients treated for MDR-TB. Infect Drug Resist [Internet]. 2021 [cited 2023 Oct 26];14:1659. doi:10.2147/IDR.S293461.
- Verschuur J, Li S, Wolski P, Otto FEL. Climate change as a driver of food insecurity in the 2007 Lesotho-South Africa drought. Sci Rep [Internet]. 2021 Dec 1 [cited 2023 Oct 26];11. doi: 10.1038/s41598-021-83375-x
- Himmelgreen DA, Romero-Daza N, Turkon D, Watson S, Okello-Uma I, Sellen D. Addressing the HIV/AIDS—food insecurity syndemic in sub-Saharan Africa. Afr J AIDS Res [Internet]. 2009 Dec [cited 2023 Oct 26];8:401–412. doi: 10.2989/AJAR.2009.8.4.4.1041
- Bygrave H, Kranzer K, Hilderbrand K, Whittall J, Jouquet G, Goemaere E, et al. Trends in loss to follow-up among migrant workers on antiretroviral therapy in a community cohort in Lesotho. PLoS One [Internet]. 2010 [cited 2023 Oct 26];5:e13198. doi: 10.1371/journal.pone.0013198
- Faturiyele I, Karletsos D, Ntene-Sealiete K, Musekiwa A, Khabo M, Mariti M, et al. Access to HIV care and treatment for migrants between Lesotho and South Africa: a mixed methods study. BMC Public Health [Internet]. 2018 May 29 [cited 2023 Oct 26];18. doi: 10.1186/s12889-018-5594-3.
- Seleteng Kose L, Moteetee A, Van Vuuren S. Ethnobotanical survey of medicinal plants used in the Maseru District of Lesotho. J Ethnopharmacol. 2015 Jul 21;170:184–200. doi: 10.1016/j.jep.2015.04.047
- Furin J. The role of traditional healers in community-based HIV care in rural Lesotho. J Community Health [Internet]. 2011 Oct 4 [cited 2023 Oct 26];36: 849–856. doi: 10.1007/s10900-011-9385-3
- Katende B, Esterhuizen TM, Dippenaar A, Warren RM. Rifampicin resistant tuberculosis in Lesotho: diagnosis, treatment initiation and outcomes. Sci Rep [Internet]. 2020 Dec 1 [cited 2023 Oct 26];10. doi: 10.1038/s41598-020-58690-4
- Mabote N, Mamo M, Nsakala B, Lanje S, Mwanawabene NR, Katende B, Linkage to care and treatment outcomes for patients diagnosed with drug-susceptible tuberculosis using Xpert MTB/RIF assay in Thaba-Tseka district in Lesotho. IJID Regions [Internet]. 2022 Dec 1 [cited 2023 Oct 26];5:33. doi: 10.1016/j.ijregi.2022.08.010
- Ho J, Byrne AL, Linh NN, Jaramillo E, Fox GJ. Decentralized care for multidrug-resistant tuberculosis: a systematic review and meta-analysis. Bull World Health Organ [Internet]. 2017 Aug 8 [cited 2023 Oct 26];95:584. Available from: /pmc/articles/PMC5537756/
- Khan U, Lotia-Farrukh I, Akhtar A, Khowaja SN, Khan S, Madhani F, et al. Re-evaluating the merits of decentralization as a core strategy for effective delivery of drug-resistant tuberculosis care in Pakistan. Health Policy Plan [Internet]. 2022 Oct 1 [cited 2023 Oct 26];37:979–989. doi: 10.1093/heapol/czac038
- Ojo T, Ruan C, Hameed T, Malburg C, Thunga S, Smith J, et al. HIV, tuberculosis, and food insecurity in Africa—a syndemics-based scoping review. Int J Environ Res Public Health [Internet]. 2022 Feb 1;19:1101. [cited 2023 Oct 26]10.3390/ijerph19031101
Appendix
Table A1. Univariate logistic regressions of baseline variables and culture conversion within six months among patients initiating treatment for rifampicin- or multidrug-resistant tuberculosis in Lesotho, 2015–2018 (N = 105).
Table A2. Multivariate analysis of four-group BMI and culture conversion within six months among patients initiating treatment for rifampicin- or multidrug-resistant tuberculosis in Lesotho, 2015–2018 (N = 105).
Table A3. Multivariate Cox regression of BMI and culture conversion within six months among patients initiating treatment for rifampicin- or multidrug-resistant tuberculosis in Lesotho, 2015– 2018 (N = 105).
Table A4. Univariate logistic regressions of baseline variables and favourable EOT outcome among patients initiating treatment for rifampicin- or multidrug-resistant tuberculosis in Lesotho, 2015–2018 (N = 250).
Table A5. Multivariate analysis of four-group BMI and favourable EOT outcome among patients initiating treatment for rifampicin- or multidrug-resistant tuberculosis in Lesotho, 2015–2018 (N = 250).
