ABSTRACT
2-Bromo-2-perfluoroalkyl acids are converted into the corresponding esters by a reaction of alcoholysis in the 2-chloroethanol. The action of zinc on perfluoroalkylated bromo-esters resulted in the bromine reduction products formation via zinc organic intermediates. When heated at 100°C, for 48 h, in the presence of an excess of zinc, the bromo-esters produce the symmetrical bis(vinyl perfluoroalkyl ester) ethoxide compounds, resulting from the BrZnF elimination reaction, followed by a dimerization reaction.
GRAPHICAL ABSTRACT
1. Introduction
In recent years, a particular interest is accorded to the synthetic methods of highly fluorinated organic compounds. The perfluoroalkyl chains constitute an important source of reagents for these syntheses. These chains have particular chemical [Citation1,Citation2], physical [Citation3–6] and biological [Citation7–10] properties. Their introduction into organic molecules gives them particular properties compared to their non-fluorinated homologues [Citation11].
The organoperfluoroalkyl compounds have a great number of applications in different fields [Citation12–16]. Such compounds are used as surfactants [Citation17–20], agrochemicals [Citation21,Citation22], pharmaceutical [Citation23–27], biological [Citation28] and medical agents [Citation29,Citation30]. While they are detected in human bodies [Citation31,Citation32], municipal water and solid wastes in different industrial areas around the world [Citation33–35] and considered as high polluter for environment and toxic compounds [Citation36,Citation37], perfluoroalkyl acids are interesting compounds used as tensioactifs [Citation38–40], fire-fighting foam [Citation41–43], fluoropolymers [Citation44] and other important applications.
In organic synthesis, some perfluoroalkylated compounds can be easily transformed into their corresponding organometallic derivatives [Citation45,Citation46]. However, their chemical reactivity is relatively complex [Citation47]. Many studies describe different positive [Citation48,Citation49] and negative [Citation50] results on their reactivity, adopting different reaction conditions [Citation48–50].
The perfluoroalkyl carboxylic acids are described as starting material for the synthesis of esters [Citation51–53] and heterocyclic compounds [Citation54,Citation55]. In particular, the 2-bromo-2-perfluoroalkyl acids and esters are described in Reformatsky reaction, conducing to the β-hydroxyesters [Citation56–60] and water elimination reaction products [Citation61].
We have previously described the action of zinc and vinyl zinc bromide on the 2-bromo-2-perfluoroalkylethanoic acid and the methyl 2-bromo-2-perfluoroalkylethanoate compounds [Citation52]. As a continuation, we describe herein an attempt of the synthesis of some perfluoroalkylated lactones [Citation54], via an intramolecular cyclization reaction, using the 2-chloroethyl 2-bromo-2-perfluoroalkylethanoate substrate with the zinc.
2. Results and discussion
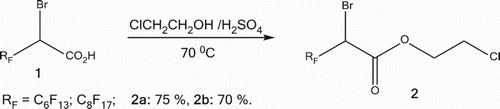
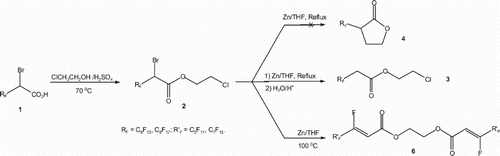
The 2-bromo-2-perfluoroalky acids 1 [Citation51] are transformed into the corresponding 2-chloroethyl 2-bromo-2-perfluoroalkylethanoates 2, in good yields, by an alcoholysis reaction with the 2-chloroethanol in acidic medium for 48 h ().
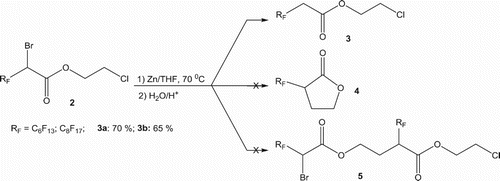
Compounds 2 are reacted with activated zinc for 6 h at 70°C, in tetrahydrofuran (THF), then hydrolysed. The reaction leads exclusively to the corresponding 2-chloroethyl 2-perfluoroalkylethanoates 3, resulting from the reduction reaction of the bromine atom in middle yields ().
The zinc atom inserts between carbon and bromine atoms, yielding the bromozincic organometallic intermediate. This nucleophilic intermediate is unable to act on the electrophilic chloro-substituted carbon atom. So, the expected perfluoroalkylated lactones 4 are not obtained even by modifying some reaction conditions (zinc amount, temperature and reaction duration).
This result may be explained on the basis of the weak bromozincic carbon atom (CHZnBr) nucleophilicity [Citation62] to act on the chloro-substituted carbon atom, neither via an intramolecular nucleophilic substitution (SNi) to produce the heterocyclic five-membered lactones 4, nor via an intermolecular nucleophilic substitution reaction to yield the dimeric compounds 5 ().
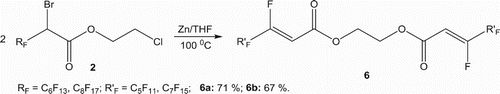
The reaction of the 2-chloroethyl 2-bromo-2-perfluoroalkylethanoate 2 in the presence of a zinc excess, in THF at 100 °C for 48 h, conduce to the symmetrical bis(vinyl perfluoroalkyl ester) 6 () as unique product, resulting from a trans BrZnF elimination reaction, followed by an ethane molecule elimination than an O-alkylation ().
Scheme 3. Effect of a zinc excess on the 2-chloroethyl 2-bromo-2-perfluoroalkylethanoates 2 at 100°C.
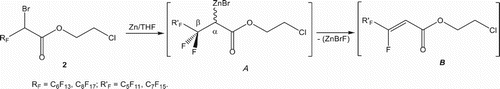
The formation of the esters 3 as well as the dimeric compounds 6 show that the reaction involves in all cases the bromozincic intermediate A (). While fluorine atom elimination reaction is not well frequent, because strength and the short length of the carbon-fluorine covalent single bond, such reaction occurs on compounds 2. The high and prolonged heat, of perfluotoalkyleted bromoester 2, conduces exclusively, through an elimination reaction of (BrZnF) to the Z geometrical configuration isomer B, [Citation63] highly stabilized from a conjugated system. The geometrical structure of the bromozincic intermediate A () is in favour of a trans elimination, because the β-carbon atom is bonded to two fluorine atoms (CF2α). So, regardless the absolute configuration of the α-carbon atom, in both cases, there is always a fluorine atom in the trans position to the ZnBr living group ().
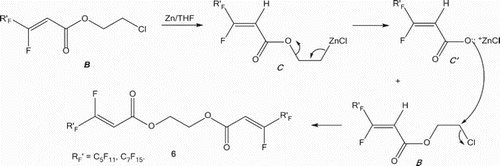
The presence of a zinc excess in the reaction with the ester 2, in the above-described reaction conditions, gives rise also to a second zinc insertion reaction between chlorine and carbon atoms. As a result, the intermediate B transforms into the intermediate C (). This latter transforms in its turn into the corresponding ionic acid salt intermediate C′, via an elimination reaction of an ethene molecule. The anionic species intermediate C′ acts on the intermediate B, yielding the corresponding symmetric bis(vinyl perfluoroalkyl) ethoxide compound 6 ().
The NMR spectroscopic data confirm the obtained structures. In particular, the 1H NMR spectra show a doublet at 6.05 ppm, corresponding to the two enonic protons (=CH), coupled with the single vinylic fluorine atom (=CF, 3JHFtrans ∼ 31 Hz). The 19F NMR spectra are in agreement with the 1H NMR spectra and show a signal corresponding to two vinylic fluorine atoms at −108 ppm. The 13C NMR spectra show a singlet at ∼106 ppm, attributed to the enonic (HC=) carbon atoms and a doublet of multiplet at 152 and 156 ppm, relative to fluorinated vinylic carbon atoms (=CF) with a coupling constant 1JFC ∼ 285 Hz.
3. Conclusion
The 2-chloroethyl 2-bromo-2-perfluoroalkylethanoates 2 are synthesized by an alcoholysis reaction of 2-bromo-2-perfluoroalkyl acids 1 in the 2-chloroethanol. Reacted with the zinc in middle conditions, the bromo-esters 2, yield the bromine atom reduction products 3 and no trace of the desired perfluoroalkyl lactones 4 is detected. Such results justify again the low reactivity of the zincic carbon atom to act as a nucleophile on the primary chloro-substituted carbon atom. The prolonged heat of the same reagents 2 with a zinc excess, yield the bis(vinyl perfluoroalkyl) oxyethylene 6 as exclusive products.
The highly fluorinated obtained compounds 2-perfluoroalkyl acids 3 and bis(vinyl perfluoroalkyl ester) ethoxides 6, among many others applications, may have interesting surface properties [Citation64–66] and can also undergo some interesting reactions, particularly in the synthesis of organofluorinated heterocyclic compounds [Citation67,Citation68].
4. Experimental
1H, 13C and 19F NMR spectra were realized in CDCl3 on a Bruker AC 300 spectrometer at 300, 75 and 282 MHz, respectively, for 1H, 13C (TMS) and 19F (C6F6). IR spectra were determined with PerkinElmer Paragon 1000 PC in CHCl3 spectrometer. HRMS spectra were realized on a Finnigan MAT 95 mass spectrometer in chemical ionization mode (CI). The used silicagel is of Merck 7734 type. The HRMS spectra were realized in the Institut National de Recherches et d’Analyses Physico-Chimiques (INRAP), Sidi Thabet 2020 Tunisia. The 2-bromo-2-perfluoroalkyl acids 1 were prepared according to the method described in Ref. [Citation51].
4.1. Synthesis of 2-chloroethyl 2-bromo-2-perfluoroalkylethanoates 2
A solution of 0.05 mmol of 2-bromo-2-perfluoroalkyl acid 1, dissolved in 4 mL of 2-chloroethanol and 0.1 mL of concentrated H2SO4 is refluxed for 48 h. The mixture is cooled and diluted with 20 mL of water. The crude is extracted with (3 × 50 mL) diethyl ether, then dried over MgSO4. The diethyl ether is evaporated and the residue is distilled, yielding ester 2 as yellowish liquid.
2-Chloroethyl 2-bromo-3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluorooctanoate: 2a
Bp = 64/0.01°C/mmHg; IR (CHCl3): νC=O = 1756 cm−1; 1H NMR (CDCl3), δ: 3.71 (t., 2H, CH2–Cl), 4.50 (t., 2H, CH2–O), 4.74 (q., 1H, CHBr); 13C NMR (CDCl3), δ: 165.0 (d., C = O), 110.0–120.0 (m., C6F13), 65.0 (s., CH2–O), 41.0 (s., –CH2–Cl) and 40.0 (t., CHBr, 2 JCF = 63.1 Hz); 19F NMR (CDCl3), δ: −84.3 (t., 3F, CF3, 3 JFF = 7.9 Hz), −115.2 (m., ABSyst, 2F, CF2α, 2 JFF = 279.2 Hz), −123.0 (m., ABSyst, 2F, CF2β, 2 JFF = 296.3 Hz), −125.0 (m., 2F, CF2γ), −126.1 (m., 2F, CF2δ), −129.5 (m., 2F, CF2ω); HRMS: Calcd. for C10H5F13BrClO2: 517.89530, found 517.89559.
2-Chloroethyl 2-bromo-3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,10-heptadecafluorodecanoate: 2b
Bp = 89/0.01°C/mmHg; IR (CHCl3): νC=O = 1753 cm−1; 1H NMR (CDCl3), δ: 3.75 (t., 2H, CH2–Cl), 4.51 (t., 2H, CH2–O), 4.75 (q., 1H, CHBr); 13C NMR (CDCl3), δ: 165.0 (d., C = O), 105.0-120.0 (m., C6F13), 65.4 (s., CH2–O), 41.5 (s., –CH2–Cl) & 40.2 (t., CHBr, 2JCF = 62.6 Hz); 19F NMR (CDCl3), δ: -84.4 (t., 3F, CF3, 3JFF = 9.1 Hz), −114.9 (m., ABSyst, 2F, CF2α, 2JFF = 272.4 Hz), −121.3 (m., ABSyst, 2F, CF2β, 2JFF = 298.4 Hz), −123.2 (m., 6F, 3CF2γ,ε), −125.2 (m., 2F, CF2δ), −128.4 (m., 2F, CF2ω); HRMS: Calcd. for C12H5F17BrClO2: 617.88900, Found 617.88943.
4.2. Synthesis of 2-chloroethyl 2-perfluoroalkylethanoate 3
(4 mmol) of 2-chloroethyl 2-bromo-2-perfluoroalkylethanoate 2 dissolved in dry THF (20 mL) is added slowly via a dropping funnel to 0.26 g (4.0 mmol) of activated zinc. The mixture is heated at 70°C under vigorously stirring for 6 h. The reaction progress is controlled by TLC. At the end of the reaction, the solids are filtered off. The filtrate is hydrolysed with 15 mL of water then extracted with diethyl ether (3 × 50 mL). The solution is dried on Na2SO4, then the solvent is evaporated. The product is purified by chromatography using the mixture petroleum ether/diethyl ether (70/30). The pure compounds 3 are obtained as yellowish liquids.
2-Chloroethyl 3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluorooctanoate: 3a
IR (CHCl3): νC=O = 1750 cm−1; 1H NMR (CDCl3), δ: 3.13 (t., 2H, CH2, 3 JHF = 17.6 Hz), 3.72 (t., 2H, Cl–CH2), 4.44 (m., 2H, O–CH2); 13C NMR (CDCl3), δ: 163.0 (s., C = O), 110.0–120.0 (m., C6F13), 65.8 (s., CH2–O), 39.7 (s., –CH2–Cl) and 35.0 (s., CH2–CO); 19F NMR (CDCl3), δ: −82.9 (t., 3F, CF3), −113.1 (m., 2F, CF2α), −121.9 (m., 2F, CF2β), −123.6 (m., 4F, 2CF2δ), −127.0 (m., 2F, CF2ω); HRMS: Calcd. for C10H6F13O2Cl: 439.98487, found 439.98524.
2-Chloroethyl 3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,10-heptadecafluorodecanoate: 3b
IR (CHCl3): νC=O = 1750 cm−1; 1H NMR (CDCl3), δ: 3.18 (t., 2H, CH2, 3 JHF = 17.6 Hz), 3.75 (t., 2H, CH2–Cl), 4.48 (m., 2H, CH2–O); 13C NMR (CDCl3), δ: 163.3 (s., C = O), 110.0–120.0 (m., C8F17), 65.1 (s., CH2–O), 40.7 (s., –CH2–Cl) and 35.5 (t., 1C, CH2–CF2, 2 JCF = 86.1 Hz); 19F NMR (CDCl3), δ: −82.7 (t., 3F, CF3), −113.0 (m., 2F, CF2α), −121.1 (m., 6F, 3CF2), −123.6 (m., 4F, 2CF2), −126.9 (m., 2F, CF2); HRMS: Calcd. for C12H6F17O2Cl: 539.97848, found 539.97877.
4.3. Synthesis of bis(allylperfluoroalkyl) ethoxide 6
0.26 g (4 mmol) of activated zinc powder and 10 mL of dry THF under nitrogen atmosphere, (4 mmol) of 2-chloroethyl 2-bromo-2-perfluoroalkylethanoate 2 are placed in a 100 mL erlenmeyer. The mixture is vigorously stirred during 48 h at 100°C, then cooled, filtered and diluted with 100 mL of diethyl ether. The solution is washed with water (2 × 50 mL) then dried on MgSO4. The solvents are evaporated and the obtained crude is purified on chromatographic column using the mixture petroleum ether/diethyl ether (70/30). Compounds 6 are isolated as yellowish solids.
(2Z,2’Z)-ethane-1,2-diyl bis(3,4,4,5,5,6,6,7,7,8,8,8-dodecafluorooct-2-enoate): 6a
Mp = 67°C; 1H NMR (CD3Cl3), δ: 6.01 (d., 2H, 2CH = CF, 3 JHF = 31.1 Hz), 4.50 (s., 4H, 2CH2–O); 13C NMR (CD3Cl3), δ: 160.9 (s., 2C, C = O), 152.3 and 156.2 (d.m., 2C, 2FC=, 1 JCF = 285.1 Hz), 105.7 (s., 2C, 2CH=), 110.0–120.0 (m., 10C, C7F15), 65.0 (s., 2C, 2CH2–O); 19F NMR (CDCl3), δ: −82.6 (t., 6F, 2CF3), −108.3 (m., 2F, 2CF=), −120.4 (m., 4F, 2CF2), −124.4 (m., 8F, 4CF2), −127.9 (m., 4F, 2CF2); HRMS: Calcd. for C18H6F24O4: 741.98828, found 741.98853.
(2Z,2’Z)-ethane-1,2-diyl bis(3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,10-hexadecafluorodec-2-enoate): 6b
Mp = 85°C; 1H NMR (CD3Cl3), δ: 6.08 (d., 2H, CF = CH, 3 JHF = 31.4 Hz), 4.60 (s., 4H, 2CH2–O); 13C NMR (CDCl3), δ: 163.5 (s., 2C, C = O), 152.3 and 156.2 (d.m., 2C, 2FC=, 1 JCF = 286.7 Hz), 105.7 (s., 2C, 2CH=), 110.0–120.0 (m, 14C, C7F15), 65.1 (s., 2C, CH2–O); 19F NMR (CDCl3) δ: −82.5 (t., 6F, 2CF3), −108.3 (m., 2F, 2CF=), −120.4 (m., 4F, 2CF2), −123.6 (m., 8F, 4CF2), −124.4 (m., 8F, 4CF2), −127.8 (m., 4F, 2CF2); HRMS: Calcd. for C22H6F32O4: 941.97551, Found 941.97589.
Supplementary_Figures.docx
Download MS Word (121.9 KB)Acknowledgements
This article is dedicated to Professors Baklouti Ahmed and Chaabouni Mohamed Moncef.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Nejib Hussein Mekni http://orcid.org/0000-0003-2924-3551
References
- Tomashenko OA, Grushin VV. Aromatic trifluoromethylation with metal complexes. Chem Rev. 2011;111(8):4475–4521.
- Liu T, Shen Q. Progress in copper-mediated formation of trifluoromethylated arenes. Eur J Org Chem. 2012;34:6679–6687.
- Tang XQ, Hu CM. Synthesis of 3-perfluoroalkyl-, including 3-trifluoromethyl-, substituted pyrazoles from perfluoroalkylacetylenes. J Fluor Chem. 1995;73(2):129–131.
- Deyama K, Tomoda H, Muramatsu H, et al. 3,4,9,10-Perylenetetracarboxdiimides containing perfluoroalkyl substituents. Dyes Pigm. 1996;30(1):73–78.
- Papagni A, Campiglio P, Campione M, et al. Synthesis and physical properties of polyfluoro-acridines bearing perfluoroalkyl Chains. J Fluor Chem. 2008;129(4):294–300.
- Adam C, Yang L, Cockroft SL. Partitioning solvophobic and dispersion forces in alkyl and perfluoroalkyl cohesion. Angew. Chem Int Ed. 2015;54(4):1164–1167.
- Furuya T, Kamlet AS, Ritter T. Catalysis for fluorination and trifluoromethylathylation. Nature. 2011;437(7348):470–477.
- Liang T, Neumann CN, Ritter T. Introduction of fluorine and fluorine-containing functional groups. Angew Chem Int Ed. 2013;52:8214–8264.
- O’Hara F, Baxter RD, O’Brien AG, et al. Preparation and purification of zinc sulfinate reagents for drug discovery. Nat Protoc. 2013;8(6):1042–1047.
- Riess JG. Fluorous micro-and nanophases with a biomedical perspective. Tetrahedron. 2002;8:4113–4131.
- Takai T, Takagi T, Baba T, et al. Highly fluorinated C18 fatty acids: synthesis and interfacial properties. J Fluor Chem. 2004;125(12):1959–1964.
- Kirsch P. Modern fluoroorganic chemistry. Weinheim: Wiley-VCH; 2013.
- Hiyama T, Kanie K, Kusumoto T, et al. Organofluorine compounds: chemistry and applications. Berlin: Springer; 2000.
- Kotthoff M, Muller J, Jurling H, et al. Perfluoroalkyl and polyfluoroalkyl substances in consumer products. Environ Sci Pollut Res. 2015;22(19):14546–14559.
- Uneyama K. Organofluorine chemistry. Oxford: Wiley-Blackwell; 2006.
- Chambers RD. Fluorine in organic chemistry. Oxford: Wiley-Blackwell; 2004.
- Schuster T, Schellenberger S, Friedrich R, et al. Branched fluorinated amphiphiles based on carbohydrates. J Fluor Chem. 2013;154:30–36.
- Jaoued Grayaa N, Boughariou-Charrada B, Hedhli A. Synthesis and the structure to property relationship of monoperfluoroalkyl polyethylene glycol. J Surfactants Deterg. 2014;17(4):767–772.
- Kawase T, Ankyu T, Oida T. Synthesis and surface properties of CO2H type gemini surfactant having semifluoroalkyl group as hydrophobic group. Tenside, Surfactants Deterg. 2012;49(6):498–507.
- Khalfallah A, Boughariou B, Hedhli A. Synthesis and surface-active properties of F-alkylated polar loops. J Surfactants Deterg. 2012;16(2):191–196.
- Giornal F, Pazenok S, Rodefeld L, et al. Synthesis of diversely fluorinated pyrazoles as novel active agrochemical. J Fluor Chem. 2013;152:2–11.
- Khrizanforov M, Gryaznova T, Sinyashin O, et al. Aromatic perfluoroalkylation with metal complexes in electrocatalytic conditions. J Organomet Chem. 2012;718:101–104.
- Shen Y, Han J, Sun X, et al. Facile synthesis of both perfluoroalkyl and phosphonate substituted trans-1,5-benzodiazepine and its derivatives via a one-pot catalyst-free process. Tetrahedron. 2015;71(23):4053–4060.
- Barata-Vallejo S, Postigo A. Metal-mediated radical perfluoroalkylation of organic compounds. Coord Chem Rev. 2013;257(21–22):3051–3069.
- Oka N, Murakami R, Kondo T, et al. Stereocontrolled synthesis of dinucleoside phosphorothioates using a fluorous tag. J Fluorine Chem. 2013;150:85–91.
- Geiger S, Xiao DJ, Shankar A. Positive association between perfluoroalkyl chemicals and hyperuricemia in children. Am J Epidemiol. 2013;177(11):1255–1262.
- Wang Z, Sun T, Chen J, et al. Convenient synthesis of perfluoroalkyl substituted 2-oxopyridine-fused 1,3-diazaheterocycles via a one-pot three component reaction. Tetrahedron. 2013;69(21):4270–4275.
- Selzer D, Abdel-Mottaleb MMA, Haha T, et al. Finite and infinite dosing: difficulties in measurements, evaluation and predictions. Adv Drug Deliv Rev. 2013;65:278–294.
- Martin-Cantalejo Y, Saez B, Monterde MI, et al. Synthesis and biological activity of new bispyridinium salts of 4,4’-bispyridyl-5,5’-perfluoroalkyl-2,2’-bisoxazoles. Eur J Med Chem. 2011;46(11):5662–5667.
- Sirisha B, Narsaiah B, Yakaiah T, et al. Synthesis and theoretical studies on energetics of novel N- and O- perfluoroalkyl triazole tagged thienopyrimidines – their potential as adenosine receptor ligands. Eur J Med Chem. 2010;45(5):1739–1745.
- Bao J, Lee YL, Chen PC, et al. Perfluoroalkyl acids in blood serum samples from children in Taiwan. Environ Sci Pollut Res. 2014;21(12):7650–7655.
- Yan H, Cousins IT, Zhang C, et al. Perfluoroalkyl acids in municipal landfill leachates from China: occurrence, fate during leachate treatment and potential impact on groundwater. Sci Total Environ. 2015: 524–525. 23–31.
- Idris A, Inanc B, Hassan MN. Overview of waste disposal and landfills/dumps in Asian countries. J Mater Cycles Waste Manag. 2004;6:104–110.
- Eggen T, Moeder M, Arukwe A. Municipal landfill leachates: a significant source for new and emerging pollutants. Sci Total Environ. 2010;408(21):5147–5157.
- Yan H, Zhang CJ, Zhou Q, et al. Short- and long-chain perfluorinated acids in sewage sludge from Shanghai, China. Chemosphere. 2012;88(11):1300–1305.
- Watkins DJ, Wellenius GA, Butler RA, et al. Association between serum perfluoroalkyl acids and LINE-1 DNA methylation. Environ Int. 2014;63:71–76.
- Lau C. Perfluoroalkyl acids: recent research highlights. Reprod Toxicol. 2012;33(4):405–409.
- Baba T, Takai K, Takagi T, et al. Effect of perfluoroalkyl chain length on monolayer behavior of partially fluorinated oleic acid molecules at the air-water interface. Chem Phys Lipids. 2013: 172–173: 31–39.
- Zuczek C, Gerardin-Charbonnier C, Rocca S, et al. Réactivité des acides 2-perfluoroalkyléthanoı¨ques: préparation de tensioactifs originaux et application à la formulation d’émulsions-gels. J Fluor Chem. 1999;99:41–49.
- Robert A, Tondre C. Solubilization of water in binary mixtures of fluorocarbons and nonionic fluorinated surfactants: existence domains of reverse microemulsions. J Colloid and Interface Sci. 1984;98(2):515–522.
- Laitinen JA, Koponen J, Koikkalainen J, et al. Firefighter’s exposure to perfluoroalkyl acids and 2-butoxyethanol present in firefighting foams. Toxicol Lett. 2014;231(2):227–232.
- Bourgeois A, Bergendahl J, Rangwala A. Biodegradability of fluorinated fire-fighting foams in water. Chemosphere. 2015;131:104–109.
- Gewurtz SB, Bhavsar SP, Petro S, et al. High levels of perfluoroalkyl acids in spot fish species downstream of firefighting training facility at Hamilton international airport, Ontario, Canada. Environ Int. 2014;67:1–11.
- DesMarteau DD, Lu C. Synthesis of N,N-bis(trifluoromethyl)amino difluoromethylene trifluorovinyl ether. J Fluor Chem. 2011;132(12):1194–1197.
- Davis CR, Burton DJ, Organozinc Reagent P, et al. Oxford: University Press, 1999, pp. 57–76.
- Erdik E. Transition metal catalyzed reactions of organozinc reagents. Tetrahedron. 1992;48(44):9577–9648.
- Mekni NH. Syntheis of new bis(3-perfluoroalkyl-1H-pyrazole) polyoxyethylene. J Fluor Chem. 2014;168:75–80.
- Mekni N, Baklouti A. Synthesis of new 1-substituted 4-perfluoroalkyl tetrazol-5-ones. J Fluor Chem. 2008;129(11):1073–1075.
- Mekni NH. A simple and efficient intramolecular 1,3-dipolar azidoalkyne cycloaddition: synthesis of 6-perfluoroalkylated fused exo-bicyclic 1,2,3-triazolo-1,4-oxazines. J Fluor Chem. 2016;186:97–100.
- Miller WT, Bergman E, Fainberge AH. Perfluoroalkylzinc compounds. I. The preparation and properties of perfluoroalkylzinc halides. J Am Chem Soc. 1957;79(15):4159–4164.
- Ould Amanetoullah A, Chaabouni MM, Baklouti A. Synthesis of F-alkyl α-hydroxy acids and esters from F-alkyl epoxides and F-alkyl α-bromo acids and esters from F-alkyl bromohydrins. J Fluor Chem. 1997;84(2):149–153.
- Jedidi Yaich B, Ould Amanatouallah A, Chaabouni MM, et al. Réactivité D’α-bromoacides et esters F-alkyles: synthèse de F-alkyl acides éthanoïques et F-alkyl acides 3-fluoroprop-2-enoiques. J Fluor Chem. 2002;114(1):47–50.
- Jedidi B, Yaich B, Debbabi K, et al. Synthèse d’une nouvelle série de f-alkyl α-bromoesters oligoéthoxylés monoalkylés. J Soc Chim Tun. 2006;8:199–202.
- Chehidi I, Ould Amanetoullah A, Chaabouni MM, et al. Synthesis of 5-(perfluoroalkylmethyl)-1,3-dioxalan-4-ones. J Fluor Chem. 2010;131(1):66–69.
- Kanishchev OS, Sanselme M, Bouillon JP. Hetero-diels-alder reactions of perfluoroalkyl thioamides with electtron-rich 1,3-diennes: synthesis of new 2-aminosubstituted-3,6-dihyddro-2H-thiopyrans and related compounds. Tetrahedron. 2013;69(4):1322–1336.
- Ocampo R, Dolbier WR. The refformatsky reaction in organic synthesis. Recent advances. Tetrahedron. 2004;60(42):9325–9374.
- Ishihara T, Mima K, Konno T, et al. Highly efficient and stereoselective route to threo- and erythro-α-alkylated α-fluoro-β-hydroxy esters via radical allylation reaction. Tetrahedron Lett. 2002;43(19):3493–3497.
- Burton DJ, Easdon JC. The α,α-difluoro reformatsky reagent: pregeneration and structural determination. J Fluor Chem. 1988;38(1):125–129.
- Ocampo R, Dolbier WR, Paredes R. Synthesis of 1,1-difluoroalkenes via αα-difluoro-β-lactones. J Fluor Chem. 1988;88(1):41–50.
- Li Y, Drew MGB, Welchman EV, et al. Syntheses of ethyl 3-deoxy-3,3-difluoro-D-arabino-heptulosonate and analogues. Tettrahedron. 2004;60(31):6523–6531.
- Cheguillaume A, Lacroix S, Marchand-Brynaert J. A practical synthesis of 2,2-difluoro-3-amino-propanoic acid (α,α-difluoro-β-alanine). Tetrahedron Lett. 2003;44(11):2375–2377.
- Burton DJ, Yang ZY. Fluornated organometallics: perfluoroalklyl and functionalized perfluoroalkyl organometallic reagents in organic synthesis. Tetrahedron. 1992;48(2):189–275.
- Mekni N, Hedhli A, Baklouti A. F-alkylation of bis(allyl) polyoxyethylene ethers. J Fluor Chem. 2002;114(1):43–46.
- Ozbay S, Erbil HY. Superhydrophobic and oleophobic surfaces obtained by graft copolymerization of perfluoroalkyl ethyl acrylate onto SBR rubber. Colloids Surf A Physicochem Eng Asp; 42015(81):537–546.
- Kawase T, Ankyu T, Oida T. Synthesis and surface properties of CO2H type gemini surfactant having semifluoroalkyl group as hydrophobic group. Tenside Surfact Det. 2012;49(6):498–507.
- Zhang SS, Cao SK, Wand S, et al. Synthesis of well-defined α-fluorinated alkyl ester, ω-carboxyltelechelic polystyrenes and fabrication of their hydrophobic highly ordered porous films and microspheres. RSC Adv. 2015;5:91140–91146.
- Langlais M, Kulai I, Coutelier O, et al. Straightforward xanthate-mediated synthesis of functional γ-thiolactones and their application to polymer synthesis and modification. Macromolecules. 2017;50(9):3524–3531.
- Gladow D, Reissig HU. Synthesis of perfluoroalkyl-substituted γ-lactones and 4,5-dihydropyridazin-3(2H)-one via donor-acceptor cyclopropanes. Helv Chim Acta. 2012;95:1818–1830.