ABSTRACT
Date palm phytoplasma disease (Wijam) is an emerging economical threat to the production of dates in the western provenance of Saudi Arabia. Disease symptoms were first observed in Al-Madinah region in 2014; a total of 525 healthy and symptomatic samples were collected from 34 date palm cultivars, were analysed by PCR and dot-blot hybridization. Nested PCR (nPCR) and sequencing results confirmed the presence of 16S rDNA phytoplasma, scoring 98% similarity to the 16SrI phytoplasma. Hybridization results showed that 17.4% of the collected samples were positively infected, the highest percentage of infection was found in city farms, reaching up to 13.4%. Furthermore, 16 date palm cultivars did not show any infection, while the other 18 cultivars had different percentages of infectivity. This finding may partially explain the sudden decline of date production in Al-Madinah region and suggests an alert to investigate disease control management procedures.
1. Introduction
Phytoplasmas are plant pathogens that belong to the Mollicutes class of prokaryotes, which are characterized by their small microscopic size and polymorphic shape due to the absence of cell-wall [Citation1]. The obligate plant parasite colonizes the phloem tissue of plants and can spread by vegetative propagation [Citation2] or transmitted by sap-feeding insect vectors [Citation3,Citation4]. Although yellowing symptoms of phytoplasma-infected plants were reported more than 100 years ago [Citation5], the causing pathogen was first described in 1967 [Citation6]. The wide-range of susceptible host plants makes phytoplasma a serious economical threat to agriculture [Citation1,Citation7], where infected plants show leaf yellowing, streaking, reduced yield, stunting and death in advanced severe infections [Citation8].
Date palms (Phoenix dactylifera L.)-phytoplasma is classified under the genus Candidatus, with a genome size ranging between 0.530 and 1.350 Mbp [Citation9] and species are distinguished by its 16S rRNA gene [Citation10]. The Wijam disease symptoms were reported in the eastern region of Saudi Arabia in 1945 [Citation11], Candidatus phytoplasma asteris, a member of the 16SrI group, has been identified as the causing agent of Wijam symptoms in 2002 [Citation12,Citation13] Reports have shown that Wijam disease resulted in a loss of more than 30–40% of date production and the death of many palm trees [Citation14]. More recently, 16SrII group members were found in symptomatic date palms of Alkharj, Riyadh and Qassim regions of Saudi Arabia but neither 16SrI nor 16SrII were found at Al-Madinah region [Citation15].
The dates of Al-Madinah provenance, west of Saudi Arabia, embrace a special religious value on top of its economical and nutritious value, favoured by visiting pilgrims over any other dates [Citation16]. The local date market contains more than 150 different date varieties [Citation17], including the most favourable, Ajwa and Anbara [Citation18,Citation19]. A sudden decline in the production of dates was observed between the years of 2013 and 2015, associated with the emergence of yellowing symptoms, the annual production was reduced from 128,807 to 73,900 tons, respectively [Citation20,Citation21]. More than 3.6 million date palm trees, planted in an area of 163,307 hectares, in Al-Madinah provenance [Citation21] are in threat, moreover, the primary income of Al-Madinah farmers is at risk. Although new agricultural technologies are being applied in Saudi Arabia, date production is lower than other producing countries [Citation22]. The date palms of the region face serious pest and disease threats, which are responsible for the low production and also the lack of disease management measures is another factor affecting the productivity of date palms in the region [Citation13,Citation23].
In this paper, we report the occurrence of date palm phytoplasma disease as well as its distribution in Al-Madinah provenance, using both PCR and dot-blot hybridization. The percentage of infection, distribution of phytoplasma in Al-Madinah region, susceptible and tolerant date palm cultivars are discussed.
2. Materials and methods
2.1. Sample collection
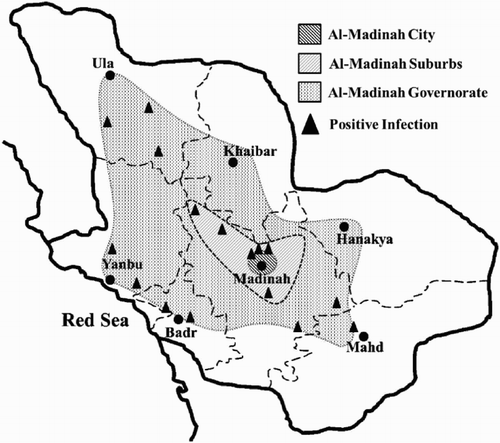
A survey was conducted in Al-Madinah provenance during 2015–2016, where it was divided into three sectors; the city, suburbs and governorate. Date palm farms at each sector were surveyed based on coordinates from north to east, south and west ().
Figure 1. Location of sampling. Map of Al-Madinah provenance divided into three sectors; Al-Madinah city, Suburbs and governorate. The black triangles showing the locations of infected farms found in the area. The topography of the western side of the city and suburbs is mountainous and no date palm farms are found in these areas.
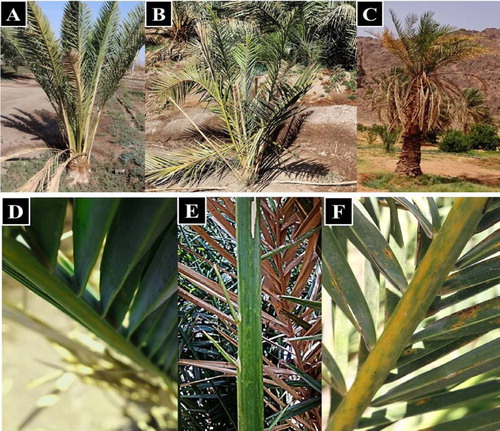
A total of 525 palm leaf samples were collected from 60 farms and 34 different date palm cultivars. Symptomatic date palm leaves, showing yellow streaks and stunting of inner new-grown leaves (), considering the morphological characteristics of date palm varieties [Citation19], in a range from sever to mild and symptomless leaves were also collected from the same locations. Samples were immediately processed or kept in −20°C prior to analysis.
Figure 2. Symptoms of date palm phytoplamsa infection. (A) Date palm showing mild yellowing symptoms, (D) pale yellow streaks on leaves. (B) Date palm showing medium yellowing symptoms, (E) yellow streaks shown on leaf. (C) Date palm showing sever yellowing and leaf stunting symptoms, (F) the yellow streaks on leaves of severely diseased date palm.
2.2. DNA extraction, PCR and sequencing
Total DNA was extracted from symptomatic and healthy leaves by the modified Dellaporta method [Citation24]. In brief, 100 mg of leaf samples were ground to fine powder in liquid nitrogen using knote pestles in a 2 ml tube and 500 µl of extraction buffer was added (50 mM EDTA, 100 mM Tris-HCl, 500 mM NaCl, 10 mM β-mercaptoethanol), 33 µl of 20% SDS was added, vortexed and incubated at 65°C for 10 min. Next, 160 µl of 5 M potassium acetate and 700 µl of PCI (Phenol: Chloroform: Isoamyl-alcohol 24:24:1) were added, vortexed, kept on ice for 5 min then centrifuged for 10 min at 5000 rpm. Clarified top layer was transferred to a new tube and 1/10 volume of sodium acetate and 2.5 volumes of ice-cold ethanol were added, kept in −80°C for 30 min followed by centrifugation at 15,000 rpm for 15 min to precipitate nucleic acids. Pellet was washed twice with 70% cold ethanol and air-dried then re-suspended in 50 µl distilled water.
The first round of PCR was performed in a 50 µl reaction using R16mF2/R16mR1 primers [Citation25], where 2 µl of DNA extract were used as a template, 2 µl of each primer (10 pmol), 5 µl of 2.5 mM dNTP mix, 5 µl MgCl2 (25 mM), 5 µl of 10X Taq reaction buffer, 5 units of Taq DNA polymerase (Promega™, USA) and completed the volume to 50 µl with deionized water. Thermal cycler parameters were set to one cycle at 94°C for 2 min; 35 cycles of 94°C for 1 min, 58°C for 1 min and 72° for 1 min, followed by a final extension at 72°C for 10 min. Resulting PCR products were diluted 1:50 with deionized water and 2 µl were used as a template for the 50 µl nPCR with fU5/rU3 primer pair [Citation26] and same PCR parameters.
Resulting nPCR products were analysed on 1% agarose gel, excised and cleaned using Invitrogen gel extraction kit, following the manufacturer instructions. A total of 30 cleaned nPCR products were sequenced and analysed using http://ncbi.nlm.nih.gov online tools. A representative sequence was submitted to genebank.
2.3. Dot-blot hybridization
Total DNA extracted from all date palm samples was analysed by non-radioactive hybridization, where 5 µl of DNA were blotted on a Nylon membrane (Amersham GE™, USA), air-dried, denatured on 0.5 M NaOH soaked filter papers for 5 min, followed by soaking the membranes in 1M Tris (pH 7.4) for 5 min, 2X SSC for another 5 min and finally in 95% ethanol. The membranes were allowed to dry at room temperature and DNA was bound to membrane in 80°C oven for 2 h. Labelled probe was prepared from the nPCR product of 16S rDNA with AlkPhos direct labelling kit (Amersham GE™, USA) following the manufacturer instructions. Membranes were hybridized with the labelled probe at 50°C overnight, washed and treated with CDP star signal stimulation reagent, then exposed to X-Omat (Kodak™, USA) films for 4 h. Films were developed, fixed and scanned as illustrated in .
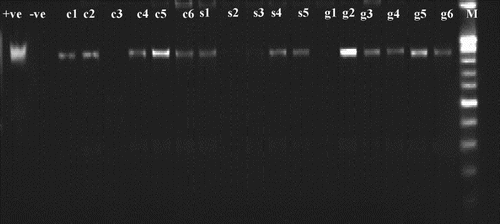
Figure 3. Gel electrophoresis of nested PCR of primers fU5/rU3. Positive nPCR product of 880 bp is shown. +ve: positive control, −ve: healthy control, c1 to c6: randomly selected samples collected from city farms, s1 to s5: randomly selected samples collected from suburb farms, g 1 to g6: randomly selected samples collected from governorate farms, M: 1Kb DNA marker (Invitrogen™, USA)
Statistical analysis were performed on samples by calculating the percentage of infection from total number of samples and/or samples of specific location or farm, where percentage represents the number of samples that shows positive result divided on the total number of samples and multiplied by 100%.
3. Results
3.1. Occurrence and identification of date palm phytoplasma
The collected date palm leaf samples in this survey showed a range of symptom severity, where yellow streaks, overall stunting of new leaves () and reduced fruit size were observed. Farm’s border palms showed more symptom severity than inner ones, with respect to the cultivated varieties.
Representative DNA samples obtained from different locations in this study showed positive nPCR results using fU5/rU3 primers (), confirming the presence of phytoplasma infection.
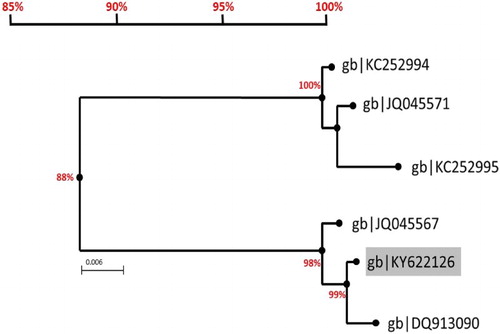
Sequencing data of nPCR products, in this study, showed 99–100% identity among analysed samples. While blasting sequences were identified as 16SrI rDNA (gene bank: KY622126), sequence alignment revealed the relationship to other phytoplasma species infecting date palms in Saudi Arabia ().
Figure 4. Phylogenic tree of related date palm phytoplasma in Saudi Arabia. The sequenced nPCR product, submitted to genebank (KY622126) was blasted against all available sequences through the online “blastn” tool on http://ncbi.nlm.gov website for identification. The sequence is aligned with the five reported phytoplasma sequences found in Saudi Arabia. Acholeplasma palmae was used as the outgroups to root the phylogenetic tree. The current 16S rDNA sequence (KY622126) is 98 and 99% similar to 16SrI group found in the eastern provenance and 88% similar to the 16SrII date palm phytoplasmas found in Alkharj, Rhiyadh and Qassim regions.
3.2. Disease distribution
Dot-blot hybridization of the collected samples showed that date palm phytoplasma is present in all surveyed locations (), reaching up to 13.4% in Al-Madinah city, 0.76% in suburbs and 3.23% in the governorate, with an overall 17.4% infection in all collected samples from different locations ().
Figure 5. Dot-blot hybridization using AlkPhos direct labelling. Eight to ten blotted sample DNA, on Nylone membranes, from each farm (containing the date palm cultivars) are hybridized with the phytoplasma 16S rDNA probe. Developed films (1–7) were scanned and analysed according to their respective order of blotting.
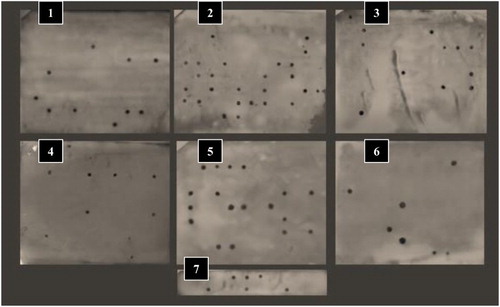
Table 1. Distribution of infection.
Cultivated date palms showed differences in their severity of visualized symptoms and percentage of infection (). While uncategorized male palms showed 6% infection, Helalya males were 100% infected. Cultivars that did not show any infection included Afandia, Baid, Baid-Aswad, Barhi, Burni, Burni-Al-Ula, Helia, Jozana, Lobana, Mabroom, Maktomy, Nahal, Sokari-Yanbu, Shalbi, Swedah and Teamer.
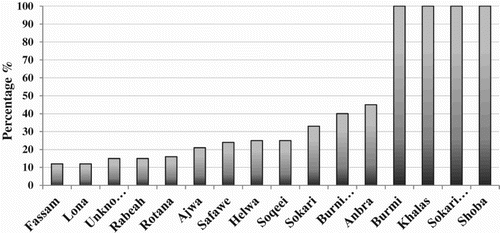
Figure 6. Percentage of infection in cultivated date palm cultivars. Al-Madinah date palm cultivars that showed positive hybridization to phytoplasma 16S rDNA probe, the percentage is calculated by the number of hybridization-positive samples over total number of cultivar samples from all locations.
4. Discussion
The Wijam is a new invading disease in Al-Madinah provenance of Saudi Arabia, infecting the most important economical crop in the region. A recent study has confirmed that date palm phytoplasma is found in Alhasa, Alkharj, Riyadh and Qassim but not in Al-Madinah [Citation15]. According to the agricultural statistics of Al-Madinah, the annual production of dates has dramatically dropped, where 128,807 tons were recorded in 2013 and only 73,900 tons were produced in 2015 [Citation20,Citation21] as shown in supporting information (Figure A). While the estimated average production per palm tree is 48.0 kg of dates [Citation27], local cultivars has reached an average of 41.82 kg till 2013 and 20.38 kg per palm tree in 2016 (Supporting information, Figure B).
The recent development of yellow streaking, stunting of new growths and fruit size reduction symptoms on cultivated date palms of Al-Madinah region, was reported for the first time during the summer of 2014 by few local farmers who believed it was nutrient deficiency or water irrigation problem. Investigating such observations revealed a phytoplasma-like infection in the area. Representative samples were immediately collected and sent for analysis at the plant protection department of King Faisal University. Preliminary results confirmed the presence of 16Sr DNA phytoplasma (Alhudaib, Supporting information Figure C), which motivated the initiation of this study.
PCR and sequencing analysis was employed in this study to identify the phytoplasma species responsible for the observed symptoms on date palms of Al-Madinah region. The amplified sequences from different locations were identical among each other and matching the previously reported 16S ribosomal RNA gene from Ihsa region (DQ913090; JQ045567) and different from other reported species infecting date palms in Saudi Arabia [Citation13,Citation15] as illustrated in . Al-Madinah date palms were reported earlier to be phytoplasma-free [Citation15], suggesting that the emergence of date palm phytoplasma in this region is recent.
Dot-blot hybridization results from the 525 collected samples () demonstrate the percentage and distribution of date palm phytoplasma in Al-Madinah provenance; the overall infection percentage reached 17.4%. The infection was more concentrated in the city farms, particularly in the eastern side, while the governorate farms came next and suburbs had the least infection percentage (). The absence of infection in the western side of the city and south, east and west of suburbs is due to the topographical nature of the region, where no farms are found in the mountain areas. On the other hand, phytoplasma infection was obvious in Ula (north), Yanbu and Badr (West) and Mahd (south) parts the governorate area.
The 34 date palm cultivars, collected in this study, showed differences in their infectivity. Although phytoplasma infection was more obvious on farm’s border grown palms, cultivars showed tolerance or susceptibility to the infection regardless to their position in the farm. Out of the 34 cultivars, 16 showed no symptoms and were negative to hybridization with 16S rDNA phytoplasma probe. Symptomless date palms were further investigated by PCR analysis and showed no amplification, suggesting that these cultivars are possibly more tolerant or less susceptible to phytoplasma infection. The other 18 cultivars differed in their symptom severity and hybridization results in all surveyed locations (), noting that the two most important date varieties in Al-Madinah (Ajwa and Anbara) scored 21% and 45%, respectively. Moreover, four cultivars (Burmi, Khalas, Sokari-Madini and Shoba) were 100% positively infected, Helalya Males were also 100% infected.
Planting phytoplasma-tolerating date palm cultivars, such as Burni or Mabroom, at the borders of the farm might be a preliminary solution for the newly established farms to dilute the phytoplasma from the transmitting insect vectors. Furthermore, oxytetracycline treatment, or alternative antibiotics, of infected palms should be implemented, as suggested by previous reports [Citation28], to reduce the symptoms, slow the spread of phytoplasma and give more time for future studies and disease management programmes. Monitoring the spread of Wijam disease in Al-Madinah area is a necessity in future surveys and additional molecular characterization studies of 16S rDNA phytoplasma species are required in both the infected palms and transmitting vector.
5. Conclusions
The results of this study demonstrates, for the first time, the occurrence of Date Palm phytoplasma in Al-Madinah provenance, KSA and shows the percentage and distribution of the disease in the studied area; an alarm call to start searching for a treatment and introduce management programmes. The study also shows that date palm cultivars differ in their susceptibility/tolerance to phytoplasma infection and suggests using tolerant cultivars to grow at the edges and borders of date palm farms to dilute the disease before spreading. Physiological studies and additional molecular data are required to understand the transmission of the disease and its interactions in vectors and host plants to find a solution and stop the spread of the date palm phytoplasma disease in the region.
Acknowledgements
Our appreciation to Dr Khalid Alhudaib and Dr Adel Rezk, from the plant protection department at King Faisal University, for their kind assistant in obtaining preliminary results and for their advice.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Omar A. Al-Baity http://orcid.org/0000-0002-5516-215X
Additional information
Funding
References
- Bertaccini A, Duduk B, Paltrinieri S, et al. Phytoplasmas and phytoplasma diseases: a severe threat to agriculture. American J Plant Sci. 2014;5:1763–1788. doi: 10.4236/ajps.2014.512191
- Lee IM, Davis RE. Mycoplasmas which infect plant and insects. In: Maniloff J, McElhansey RN, Finch LR, Baseman JB, editors. Molecular biology and pathogenesis. Washington (DC): American Society of Microbiology; 1992. p. 379–390.
- Lee IM, Davis R, Gundersen-Rindal D. Phytoplasmas: phytopathogenic Mollicutes. Ann Rev Microbiol. 2000;54:221–255. doi: 10.1146/annurev.micro.54.1.221
- Maramorosch K. Historical reminiscences of phytoplasma discovery. Bull Insect. 2011;64:S5–S8.
- Ntushelo K, Elliott ML, Harrison NA. Palm yellows phytoplasmas and their genetic classification. A J Biotech. 2013;12(22):3376–3382.
- Doi Y, Teranaka M, Yora K, et al. Mycoplasma or PLT grouplike microrganisms found in the phloem elements of plants infected with mulberry dwarf, potato witches’ broom, aster yellows or pawlownia witches’ broom. Jap J Phytopathol. 1967;33:259–266. doi: 10.3186/jjphytopath.33.259
- Hoshi A, Ishii Y, Kakizawa S, et al. Host parasite interaction of phytoplasmas from a molecular biological perspective. Bull Insect. 2007;60:105–107.
- Bertaccini A, Duduk B. Phytoplasma and phytoplasma diseases: a review of recent research. Phytopathol Mediterr. 2009;48:355–378.
- Namba S, Oshima K, Gibb K. Phytoplasma genomics. In: Blanchard A, Browning G, editors. Mycoplasmas: molecular biology, pathogenicity and strategies for control. Norfolk: Horizon Bioscience; 2005. p. 97–133.
- Harrison NA, Gundersen-Rindal D, Davis RE. Genus I. “Candidatus Phytoplasma” gen. nov. In: Krieg NR, Staley JT, Brown DR, Hedlund BP, Paster BJ, Ward NL, Ludwig W, Whitman WB, editors. Bergey’s manual of systematic bacteriology. 2nd ed., Vol. 4. New York (NY): Springer-Verlag; 2011. p. 696–719.
- Elarosi H. Studies on plant diseases affecting date palm trees at the Eastern Province of Saudi Arabia. King Abdulaziz City for Science and Technology, Riyadh, KSA, Book No. (26) (1989).
- El-Zayat MM, Shamloul AM, Abdulsalam KS, et al. Molecular detection and identification of a prokaryotic pathogen associated with Al-Wijam declining disease of date palms in Saudi Arabia. Arab J Biotechnol. 2002;5:193–206.
- Alhudaib K, Arocha Y, Wilson M, et al. Identification and molecular characterization of a phytoplasma associated with Alwijam disease of date palm in Saudi Arabia. Arab J Plant Pathol. 2007;25:116–122.
- Alhudaib K, Arocha Y, Wilson M, et al. First report of a 16SrI, Candidatus Phytoplasma asteris group phytoplasma associated with a date palm disease in Saudi Arabia. Plant Pathol. 2008;57:366. doi: 10.1111/j.1365-3059.2007.01667.x
- Alhudaib K, Rezk A, Alsalah M. Phytoplasma disease in date palm in Saudi Arabia. Proceedings of the 5th international date palm conference. United Arab Emirates: Publisher Khalifa International Date Palm Award; 2014. p. 311–318.
- Yasin BR, El-Fawal HA, Mousa SA. Date (Phoenix dactylifera) polyphenolics and other bioactive compounds: a traditional Islamic remedy’s potential in prevention of cell damage, cancer therapeutics and beyond. Int J MolSci. 2015;16:30075–30090. doi: 10.3390/ijms161226210
- Jaradat AA. Biodiversity of date palm, In: Encyclopedia of Life Support Systems: Land Use, Land Cover and Soil Sciences (EOLSS). Developed under the Auspices of the UNESCO, EOLSS Publishers, Oxford, UK 2011 Available from: http://www.eolss.net.
- El-Habba MS, Al-Mulhim F. The competitiveness of the Saudi Arabian date palm: an analytical study. A J Agri. Res. 2013;8(43):5260–5267.
- Al-Wusaibai N, Abdallah AB, Al-Husseini M, et al. Morphological characterization of Saudi Arabian date palm cultivars based on vegetative and reproductive traits. Proceedings of the 5th international date palm conference. Publisher Khalifa International Date Palm Award, United Arab Emirates 2014;57–64.
- Anonymous. 27th Agricultural statistical year book. Ministry of Agricultural. Saudi Arabia; 2014. ISBN: 1319-0644.
- Anonymous. Annual statistical book. General Authority of statistics. Saudi Arabia. Issue 51 (2016) (Chapter 13). On-line Available from: https://www.stats.gov.sa/ar/443-0
- Al-Obaid AA. Economies of dates in Saudi Arabia. In: The Extension Bulletin for Date Palm Trees, Extension Centre, Faculty of Agriculture, King Saud University, Saudi Arabia; 1996. p. 1–15.
- El-Juhany LI. Degradation of date palm trees and date production in Arab countries: causes and potential rehabilitation. Aust J Basic Appl Sci 2010;4:3998–4010.
- Dellaporta SL, Wood J, Hicks JB. A plant DNA minipreparation: version II. Plant Mol Biol Rep. 1983;1:19–21. doi: 10.1007/BF02712670
- Gundersen D, Lee I-M. Ultrasensitive detection of phytoplasmas by nested-PCR assay using two universal primer pairs. Phytopathol Mediterr. 1996;35:144–151.
- Lorenz K-H, Schneider B, Ahrens U, et al. Detection of the apple proliferation and pear decline phytoplasmas by PCR amplification of ribosomal and nonribosomal DNA. Phytopathol. 1995;85:771–776. doi: 10.1094/Phyto-85-771
- Al-Abbad A, Al-Jamal M, Al-Elaiw Z, et al. A study on the economic feasibility of date palm cultivation in the AlHassa oasis of Saudi Arabia. J Dev Agric Econ. 2011;3(39):463–468.
- Abdusalam K, Abdel-Megeed M, Rezk M, et al. The influence of oxytetracycline on Wijamed date palm tree. Ann Agri Sci. (Ain Shams University, Egypt). 1993;38:301–309.
