ABSTRACT
The neuroprotective effects of Tualang honey in stress-induced young and aged rats were investigated. Tualang honey (200 mg/kg body weight) was administered for 28 days. Stress-induced rats were subjected to loud noise 100 dB(A) 4 hours daily for 14 days. Stress exposure significantly induced memory impairment, increased brain oxidation indices, and decreased antioxidant enzymes activities and neuronal density in mPFC, CA2 and CA3 hippocampal areas in both young and aged rats. The stressed rats treated with Tualang honey showed a significant decrease in stress hormone levels and brain oxidation indices, and increase in memory, antioxidant enzymes activities, and neuronal density in mPFC and hippocampus compared to the vehicle-treated stressed rats. The protective effect of Tualang honey was more prominent in young than aged rats. These results suggest the neuroprotective effects of Tualang honey against oxidative stress and memory decline due to stress exposure and/or ageing through its antioxidant property.
KEYWORDS:
1. Introduction
Stress exposure may induce deleterious effects on brain structure and cognition [Citation1,Citation2] and increase the risks of developing neuropsychiatric disorders [Citation3]. Noise exposure exceeds 90 dB has been reported to be a source of stressor [Citation4]. The adverse impact of noise on learning, memory and hippocampal neurogenesis has been reported by previous studies [Citation5–9]. The mechanisms underlying the decline of memory after noise exposure are not entirely clear. Oxidative reaction initiated by noise exposure has been implicated in the memory impairment. Noise exposure may induce the generation of free radicals and deplete the activities of antioxidant enzymes in plasma and tissues [Citation10,Citation11]. Previous studies have shown that increased oxidative stress is the cause of neuronal degeneration in auditory nuclei as well as the brain regions critical for cognitive functions [Citation6,Citation12,Citation13].
Accumulation of oxidative damage and reduction of the antioxidant defence system also play a key role in organismal ageing and functional senescence. Ageing in humans, as well as in experimental animals, is associated with a slow deterioration of cognitive performance and, in particular, of learning and memory [Citation14,Citation15]. Oxidative stress has long been considered to play an important role in age-associated neurodegenerative diseases such as Alzheimer’s disease [Citation16–18]. The central nervous system is particularly susceptible to oxidative stress because of its high oxygen consumption and relative insufficiency in both reactive oxygen species (ROS) scavenging enzymes and endogenous antioxidants compared with other organs [Citation19–21]. Numerous studies have indeed reported increases in protein oxidation and lipid peroxidation in various regions of aged mammalian brains [Citation22–24]. These findings have led to the notion that antioxidant defence mechanisms in the brain are not sufficient to prevent age-related increase in oxidative damage and that dietary intake of antioxidants might be beneficial for preserving brain function.
Malaysia Tualang honey is a wild pure multifloral honey produced by Asian rock bee species (Apis dorsata), which builds hives on the branches of Tualang tree (Kompassia excelsa) located mainly in the rainforest of northern Peninsular Malaysia. Honey contains significant antioxidant activities as well as choline and acetylcholine which are essential for brain function and as neurotransmitters [Citation25–29]. Recent studies reported that honey is one of the natural preventive therapies of both cognitive decline and dementia, as it possesses antioxidant properties and it enhances the brain’s cholinergic system [Citation30]. Correspondingly, another study reported that consumptions of honey may improve spatial memory in middle-aged rats compared to those sucrose-fed or sugar-free diet [Citation31]. In closely related studies, it was demonstrated that Tualang honey was able to improve memory performance in stressed ovariectomized rats [Citation32] and postmenopausal women [Citation33]. Therefore, it is hypothesized that Tualang honey could revert oxidative stress by removing the ROS that were produced during noise stress and thus prevent oxidative damage in memory-related brain areas, particularly in aged rats.
2. Material and methods
2.1. Experimental animals
Young (2 months old; n = 48) male Sprague-Dawley rats weighing 250–300 g were obtained from Animal Research and Service Centre (ARASC), Universiti Sains Malaysia. Aged (16 months old; n = 48) male Sprague-Dawley rats weighing 550–700 g were purchased from Sterling Ascent Sdn. Bhd., Malaysia. The rats were maintained in standard polypropylene cages (40 × 25 × 16 cm) under a reversed 12-h light/dark cycle (lights off at 08:00 h) at a consistent room temperature of 27 ± 1°C in the laboratory of ARASC, Universiti Sains Malaysia. The rats received commercial rat chow food pellets (Gold Coin Ltd., Malaysia) and water ad libitum. Rats were allowed to acclimatize to the holding room for 24 h before the behavioural procedures. The procedures in this study were approved by Animal Ethics Committee of Universiti Sains Malaysia (USM/Animal Ethics Approval/2013(85)(444), in accordance with the internationally accepted principles for laboratory animal use and care.
2.2. Honey supplementation
The Tualang honey used was from a single batch honey supplied by Federal Agricultural Marketing Authorities (FAMA), Malaysia. The honey was filtered by FAMA to remove solid particles, concentrated in an oven at 40°C and evaporated to achieve a water content of about 20%. It was then subjected to γ irradiation at 25 kGy at Steril Gamma (M) Sdn. Bhd. (Selangor, Malaysia) for sterilization and bottled 230 g per jar. The final concentration of the bottled Tualang honey was 1.3 g/mL. Tualang honey at 200 mg/kg body weight/day [Citation32,Citation34] was administered via oral gavage 14 days prior to stress procedure and the treatments were continued throughout the 14 days of stress procedure. The Tualang honey was freshly dissolved in 1 mL of distilled water prior administration. Control groups received an identical volume of distilled water as a placebo for the same period of time.
2.3. Experimental design
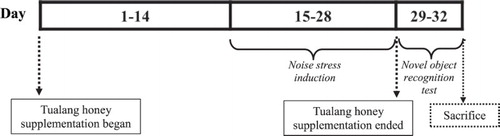
The animals were randomly assigned to the following groups (n = 12): (i) no stress with placebo, (ii) no stress with Tualang honey, (iii) stress with placebo and (iv) stress with Tualang honey, according to their respective age category. As illustrated in , honey supplementation was started on day 1 and continued for a period of 28 days. On day 15 until 28, noise stress procedure was implemented. All rats were subjected to novel object recognition test following the final day of stress procedure and killed by decapitation upon completion of the test. Individual bodyweight was recorded weekly using electrical balance. Blood samples (5 ml) were collected immediately upon decapitation. All blood samples were left to clot for 2 h prior to centrifugation for 15 min at 4000 rpm. Approximately 3 ml of serum was collected and stored at −20°C until assay. The brain of each animal was quickly harvested and weighed. The right brain hemisphere was homogenated (10% w/v) in ice-cold 0.1 M phosphate-buffered saline at pH 7.4. The homogenate was then centrifuged at 10,000× g for 10 min and kept at −80°C until analysed. The left brain hemisphere was immediately fixed in 10% formalin for histopathological study.
2.4. Noise stress exposure
The animals of the test groups (iii and iv) were exposed to white noise for 4 h (09:00–13:00 h) daily for 14 days. Noise was recorded from the generator and amplified by speakers in a separate room. Speakers were located 30 cm above the cages. The noise level was set at 100 dB(A) and intensity was measured by a sound level meter CENTER 325 (Range: 80–130 dB(A), Accuracy: +1.5 dB(A), made in Taiwan). Sound levels were verified in the centre of the cage before each exposure and varied by less than 1 dB(A) in the space the cage occupied. The control groups (i and ii) were kept in the same room for the same period of time without switching on the noise.
2.5. Novel object recognition test
The test was carried out in a separate room that was ventilated, soundproofed and maintained at a constant temperature (27 ± 1°C). The animals were brought into the test room 1 h before the test commenced to minimize the arousal caused by the transference. The test was performed during the active period of the animals (dark phase) between the hours of 09:00–14:00. All the animals were tested in a random order. The trained observer remained blind to the treatment group of the rats until scoring was completed.
The test employed was similar to that described elsewhere [Citation35]. The test uses the natural preference for a novel object displayed by rats and normally used to assess cognitive alterations associated with ageing, genetic manipulations or drug treatments. The chamber was an open field apparatus (60 × 60 × 30 cm). Training sessions were conducted on two successive days during which they were allowed to explore the arena for 10 min each day. In the training session, two identical sample objects were placed in the field in a symmetrical position about 10 cm away from the wall. After the two successive training sessions, testing/retention sessions were conducted. The retentions sessions consisted of two sessions, i.e. short-term memory and long-term memory in which the retention interval for short-term memory and long-term memory were 2 h and 24 h after the last training session, respectively. In the retention session, rats were placed back in the same field, wherein one of the familiar objects used in the training session was replaced by a novel object and the rat was allowed to explore for 5 min.
All objects consisted of plastic toys and had a height of about 5 cm. Objects presented similar textures, colours and sizes, but distinctive shapes. The location of objects was alternated with each new animal; it was approximately placed in 50% trials in the right side and 50% in the left side of the field. Between tests, the objects were cleaned with 10% ethanol solution to mask any olfactory cues. Exploration was defined as sniffing or touching the object with the nose. Sitting on the object was not considered as exploration.
Total exploration times of the familiar and novel objects were recorded and used to calculate a discrimination index [time spent with novel object−time spent with familiar object]/[total time exploring both objects]. The discrimination index can range from −1 to 1 wherein −1 indicating complete preference for the familiar object, 0 signifying no preference for either object and 1 indicating complete preference for the novel object. Increased preference to novel object was interpreted as successful memory retention for the familiar object. An absence of any difference in the exploration of the two objects was interpreted as memory deficit.
2.6. Serum corticosterone and adrenocorticotropic hormone (ACTH) levels
Serum levels of corticosterone and ACTH were measured by enzyme-linked immunosorbent assay (ELISA) kits using a polyclonal antibody specific for corticosterone (LDN Labor Diagnostika Nord GmbH & Co. KG, Nordhorn, Germany) and a monoclonal antibody specific for ACTH (Cloud-Clone Corp., Houston, United States).
2.7. Brain oxidative stress indices
The evaluation of oxidative stress in the brain homogenates was performed by measuring the levels of plasma malondialdehyde (MDA) and protein carbonyl (PCO). The concentration of MDA was analysed using commercially available kits from Northwest Life Sciences Specialties, Washington, United States whereas the level of PCO was determined by commercially available kits from Cayman Chemical, Michigan, United States.
2.8. Brain antioxidant enzymes activities
The activities of superoxide dismutase (SOD), glutathione peroxidase (GPx), and glutathione reductase (GR) in the brain homogenates were measured using commercially available kits from Northwest Life Sciences Specialties, Washington, United States. Commercially available kits from Bioassay Systems, California, United States and Oxford Biomedical Research, Michigan, United States were used to determine the activities of catalase (CAT) and the total antioxidant capacity, respectively.
2.9. Protein concentration
Following homogenization, an aliquot was removed from each brain sample to determine its protein concentration using commercially available kits from Bioassay Systems, California, United States. Briefly, protein concentration was quantified by comparing the colorimetric intensity of the reaction product from each sample with a series of protein standards. All the data of antioxidant enzymes activities and oxidative indices levels were normalized to their total protein concentration in the sample in order to account for possible differences in protein concentrations between samples.
2.10. Histopathological analysis
The left brain hemispheres were embedded in paraffin wax, cut into 5 µm-thick coronal sections using a rotary microtome, mounted on slides and followed by Nissl staining, which was performed according to the standard procedure. The slides were observed under a light microscope and images were captured to visualize the arrangement of pyramidal neurons in the medial prefrontal cortex (mPFC) and each hippocampal region: CA1, CA2, CA3 and DG. The Nissl-positive cells were counted at different magnifications using High Definition Medical Image Analysis Program (analySIS docu 5.0, Münster, Germany). The mean of two fields was taken as the number of Nissl-positive cells for each section and the mean of four sections was taken as the Nissl-positive cells of each group. Cells which had a shrunken or unclear body with surrounding empty spaces were excluded.
2.11. Statistical analysis
All analyses were performed using IBM SPSS Statistics Campus Edition V24.0 for Win/Mac. Statistical data are expressed as mean ± S.E.M, and a result was deemed to be statistically significant if P < .05. Factorial analyses of variance (ANOVA) was utilized to examine the main effects of age (young vs. aged), stress (nonstressed vs. stressed) and honey treatment (placebo vs. honey) on the memory, antioxidant enzymes activities, oxidative stress markers and stress hormone levels, and the number of Nissl-positive cells in the mPFC and hippocampal regions. After confirming the normality of data and the homogeneity of variance of data, the significance of the differences between the means of the test and control studies was established by one-way ANOVA coupled with post hoc Tukey HSD test.
3. Results
3.1. Effects of Tualang honey on body weight
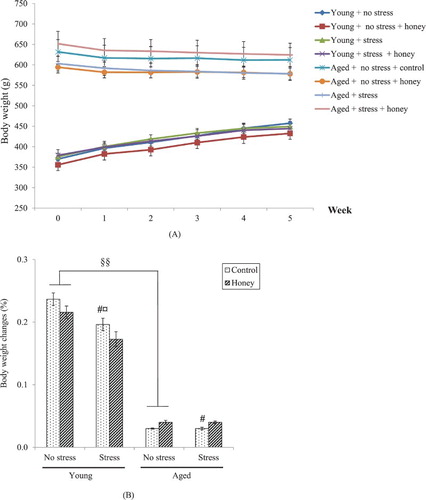
Mean body weights of all groups over five weeks experimental period was illustrated in (A,B) illustrates the percentage of body weight changes calculated as [(Final body weight–Initial body weight)/Initial body weight] × 100%. There were significant effects of age (P < .01) and stress (P < .05) on the percentage of body weight changes. Stress exposure significantly (P < .05) decreases body weight gain in young rats.
Figure 2. Effects of age, stress, and honey treatment on (A) body weight and (B) percentage of body weight changes. The values are expressed as mean ± S.E.M. Significant main effects of age (§§P < .01). Significant main effects of stress (#P < .05). Significant difference between no stress and stress control (¤P < .05).
3.2. Effects of Tualang honey on serum corticosterone and ACTH levels
Factorial ANOVA revealed significant (P < .01) effects of age on corticosterone and ACTH levels, whereby aged rats possessed significant higher corticosterone and ACTH levels compared to the young (). There were significant effects of stress on corticosterone (P < .05) and ACTH levels (P < .01), and significant effects of honey treatment on corticosterone (P < .01) and ACTH levels (P < .01). In young rats, stress exposure significantly (P < .05) increases both corticosterone and ACTH levels while honey treatment significantly (P < .05) decreases corticosterone and ACTH levels in the stressed rats. In aged rats, stress exposure significantly (P < .05) increases ACTH level only while honey treatment significantly (P < .05) decreases corticosterone and ACTH levels in the stressed rats.
Table 1. Effects of age, stress, and honey treatment on serum corticosterone and ACTH levels.
3.3. Effects of Tualang honey on object recognition memory
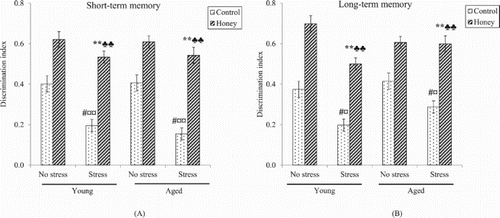
Factorial ANOVA revealed significant (P < .05) effects of stress on short- and long-term memory, indicating that stress was associated with memory deficit ((A,B)). One-way ANOVA confirms that stress exposure caused significant decrease in short- and long-term memory in both young (P < .01) and aged rats (P < .05). Interestingly, there were significant (P < .01) effects of honey treatment on short- and long-term memory. Stressed rats supplemented with honey showed significantly (P < .01) higher mean discrimination index in both short- and long-term memory compared to control stressed rats, indicating better memory performance.
Figure 3. Effects of stress and honey treatment on mean discrimination index ratio of (A) short-term memory and (B) long-term memory. The values are expressed as mean ± S.E.M. Significant main effects of stress (#P < .05). Significant main effects of honey treatment (**P < .01). Significant difference between no stress and stress control (¤P < .05, ¤¤P < .01). Significant difference between stress control and stress treated with honey (♣♣P < .01).
3.4. Effects of Tualang honey on brain oxidation indices
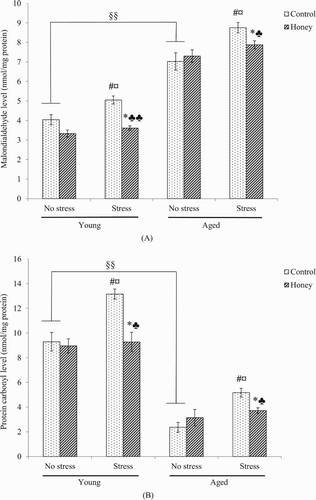
There was significant (P < .05) effect of stress on MDA and PCO levels, whereby stress exposure caused significant (P < .05) increase in the indices in both young and aged rats ((B)). Interestingly, there was significant (P < .05) effect of honey treatment on the MDA and PCO levels, whereby stressed rats treated with honey possessed significantly lower MDA (young; P < .01, aged; P < .05) and PCO (P < .05) levels compared to control stressed rats.
Figure 4. Effects of age, stress, and honey treatment on (A) MDA and (B) PCO levels. The values are expressed as mean ± S.E.M. Significant main effects of age (§§P < .01). Significant main effects of stress (#P < .05). Significant main effects of honey treatment (*P < .05). Significant difference between no stress and stress control (¤P < .05). Significant difference between stress control and stress treated with honey (♣P < .05, ♣♣P < .01).
3.5. Effects of Tualang honey on brain antioxidant enzymes
Significant effects of age and stress were observed on the activities of SOD (P < .01), GPx (P < .01), GR (P < .01) and CAT enzymes (P < .01, P < .05), and total antioxidant status (P < .01) (). Stress exposure decreases the activity of antioxidant enzymes and the total antioxidant status. There was significant effect of honey treatment on SOD (P < .01) and GR (P < .05) enzymes activities, and total antioxidant status (P < .01). Young stressed rats treated with honey possessed significantly higher SOD (P < .01), GPx (P < .05) and GR (P < .05) enzymes activities, and total antioxidant status (P < .05) compared to control rats. Meanwhile, in aged stressed rats, only SOD activity was significantly (P < .01) affected by honey treatment, suggesting that the antioxidant actions of honey was more effective in young than aged rats.
Table 2. Effects of age, stress, and honey treatment on brain antioxidant enzymes.
3.6. Effect of Tualang honey on neuronal density in mPFC and hippocampus
Factorial ANOVA revealed significant (P < .01) effect of age on the neuronal density in mPFC and all the hippocampal regions, in which aged rats showed marked reductions of neuronal number compared to the young (). Significant effect of stress was observed on the neuronal density in mPFC (P < .05), CA2 (P < .01) and CA3 (P < .05) hippocampal regions. Stress exposure caused significant (P < .05) reductions in the neuronal number in mPFC, CA1, CA2 and CA3 hippocampal regions in young rats whereas only CA2 hippocampal region was affected in aged rats. Interestingly, there was significant (P < .01) effect of honey treatment on the number of neurons in mPFC and all the hippocampal regions. Honey treatment was able to cause significant (P < .05) increase in the number of neurons in the mPFC and all the hippocampal regions in young stressed rats, while in aged stressed rats, only mPFC and CA2 hippocampal region were affected.
Table 3. Effects of age, stress, and honey treatment on neuronal densities in mPFC and hippocampal regions.
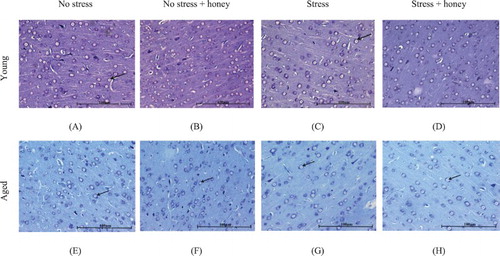
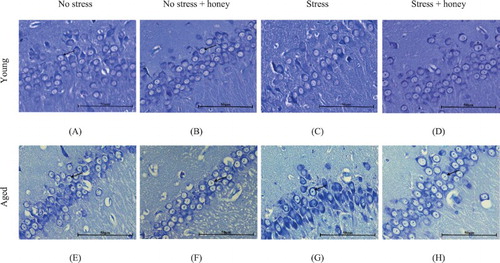
3.7. Effects of Tualang honey on histology of mPFC and hippocampus
Aged rats appeared to have lower neuronal count compared to the young ( and ). Stressed rats exhibited moderately shrunken neuronal cell bodies with cytoplasmic vacuolation. The arrangement of pyramidal neurons was sparse and the Nissl substance was decreasing or dissolving. In contrast, stressed rats treated with honey exhibited abundant pyramidal neurons, the architecture of these neurons was preserved and Nissl substances in the cytoplasm were clearly visible. Arrangement of pyramidal neurons of the stressed rats treated with honey appeared quite similar to the nonstressed rats.
4. Discussion
One of the mechanisms underlying the ageing process is proposed to be the oxidative damage caused by free radicals [Citation36]. Accordingly, results of the present study demonstrated that the aged rats exhibited significant higher brain MDA level than the young. Increased levels of MDA have also been found in the aged canine brain [Citation37] as well as in the hippocampus and cerebellum of aged rodents [Citation38]. In order to protect cells from oxidative damage, aerobic metabolism generally depends on a stringent control of ROS by antioxidants. Decline in antioxidant enzymes activities with ageing has been well documented [Citation39–41]. Similarly, in the present study, significant lower activities of these enzymes were recorded in the aged rats. A reduction in the activity of antioxidant enzymes is associated with accumulation of highly reactive free radicals, leading to deleterious effects such as loss of integrity and function of cell membranes [Citation42]. Accordingly, the present study demonstrated that the aged rats possessed significant lower number of neurons in mPFC and all the hippocampal regions than that of the young. There are abundant evidences that ageing is associated with loss of hippocampal neurons, in which it is clearly most pronounced in the CA1 [Citation15,Citation43] and CA3 hippocampal regions [Citation43]. Some studies have also reported age-related neuronal loss in CA2 hippocampal region [Citation43,Citation44] as well as in PFC [Citation45,Citation46].
It is widely accepted that noise is a stressful environmental stimulus, and stress has been shown to impair cognition, such as the acquisition of memory, consolidation and recall [Citation47,Citation48]. The present study demonstrated that stressed rats exhibited significant higher serum corticosterone and ACTH levels, suggesting that the hypothalamic–pituitary–adrenal (HPA) axis had been implicated from the stress exposure. Glucocorticoids released from the adrenal gland in response to stress-induced activation of the HPA axis may trigger the cellular reduction–oxidation (redox) system, followed by oxidative stress [Citation49]. Accordingly, we demonstrated that the stressed rats exhibited significant higher lipid and protein oxidation indices compared to the nonstressed. It was also observed that the stressed rats possessed significant lower antioxidant enzymes activities and total antioxidant status. The decrease in antioxidant enzymes activities accompanied by increased oxidative indices confirm the fact that noise exposure induced the generation of free radicals and depleted the activities of antioxidant enzymes in plasma and tissues. Furthermore, both glucocorticoid exposure and oxidative stress may induce neuronal oxidative stress through enhanced mitochondrial respiration and oxidative phosphorylation [Citation50], and promote gliogenesis over neurogenesis in hippocampal neural stem cell progenitors [Citation51,Citation52]. Correspondingly, we demonstrated that the stressed rats possessed a lower number of pyramidal neurons in the hippocampus as well as in the mPFC compared to the nonstressed rats. This neuronal loss may have manifested their effects as memory impairment, as clearly indicated in our study whereby the stressed rats showed a significant decrease in short- and long-term memory compared to the nonstressed.
Interestingly, our data demonstrated that supplementation of Tualang honey was able to improve both short- and long-term memory in the stressed rats. The histopathological results revealed an increase in the number of neuronal cells in mPFC and hippocampal regions in the stressed rats treated with honey, which could be interpreted as tissue preservation and maintenance of the ability to retain information. We have also found that honey was able to reverse the increase of corticosterone and ACTH levels in the stressed rats. These findings suggest that honey reduced the adverse effects of noise stress and may be beneficial for the nervous system and vasculature, and protect the brain and body from stress-induced damage. Honey may modulate corticosterone and ACTH levels either by suppressing HPA mobilization in response to stress or by facilitating elevated plasma corticosterone and ACTH levels back to baseline following the termination of stress.
Honey has been used to cure a multitude of ailments since ancient times. Researchers are re-appraising its medicinal and nutritional values [Citation53,Citation54]. The present study revealed the potential of Tualang honey in improving the memory of young and aged rats. It is assumed that the improvement of memory by Tualang honey is due to its antioxidant capacity attributed to the flavonoids contents [Citation29,Citation55–57]. Other types of antioxidants present in honey include enzymatic (e.g. catalase, glucose oxidase, peroxidase) and non-enzymatic substances (e.g. ascorbic acid, α-tocopherol, carotenoids) [Citation28,Citation58,Citation59]. It is also worth mentioning that Tualang honey has been reported to have a higher level of antioxidant activity than other local Malaysian honeys, such as Gelam, Acacia, Indian forest and Pineapple honeys [Citation29,Citation60]. In addition, studies by Chepulis et al. [Citation31] reported that honey-fed rats exhibited better memory performance compared to sucrose-fed rats. Hence, this suggests that the better memory performance seen in the honey-fed rats was not due to the sugar content alone but may involve other honey components, possibly antioxidants.
Accordingly, the present study demonstrated that Tualang honey significantly improved brain oxidative status in young and aged rats as shown by elevated levels/activities of antioxidant enzymes and total antioxidant status, and reduced oxidative markers. Other studies have also demonstrated that honey is able to increase antioxidant enzymes levels and ameliorate oxidative stress in plasma and other tissues such as renal and pancreas [Citation61–68]. It is suggested that the aforementioned flavonoids contents and enzymatic and non-enzymatic substances in honey are responsible for its antioxidative effects. It was also noted that the antioxidative effect of Tualang honey was more prominent in young than aged rats. Young stressed rats treated with Tualang honey possessed significantly higher TAC, GPx and GR activities compared to young control stressed rats, whereas no significant difference was seen in those parameters between control and honey-treated aged stressed rats. This is probably due to the optimal antioxidant protection system in the young group which efficiently reduced oxidative stress. Antioxidant defence systems at old age may adapt less efficiently to defend against ROS generated as shown in other tissues such as lung, skeletal muscle and heart [Citation69].
The histological results revealed an increase in the number of neuronal cells in mPFC and hippocampal regions in the rats treated with Tualang honey. Therefore, it is suggested that the memory enhancing effects of Tualang honey could be due to the enhancement of neuronal proliferation in mPFC and hippocampus, a result paralleled to the previous study done in stressed ovariectomized rats [Citation32]. Another study demonstrated that honey effectively inhibited apoptosis or neuronal cell death in the hippocampus of streptozotocin-induced diabetic rats [Citation70]. As discussed earlier, exposure to stress increases oxidative stress in the hippocampus leading to the generation of ROS, which attacks and damages the nerve cell membrane and alters mitochondrial membrane permeability, resulting in neuronal loss. Honey has the ability to combat against damage to the cell membranes by neutralizing the free radicals, thus maintaining healthy neuronal cells in the mPFC and hippocampus.
5. Conclusion
It can be concluded that administration of Tualang honey improves memory deficits in young and aged stress-induced rats. These effects might be mediated by its antioxidant property and amelioration of neuronal damage. Further studies have to be conducted to determine the specific components of honey and their biological activity in order to support its potential use as an alternative therapy to protect against memory deterioration due to stress exposure and/or ageing.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- McEwen BS. Stress and the aging hippocampus. Front Neuroendocrinol. 1999;20:49–70. doi: 10.1006/frne.1998.0173
- McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105
- Heim C, Nemeroff CB. The impact of early adverse experiences on brain systems involved in the pathophysiology of anxiety and affective disorders. Biol Psychiatry. 1999;46:1509–1522. doi: 10.1016/S0006-3223(99)00224-3
- Ravindran R, Devi RS, Samson J, et al. Noise-stress-induced brain neurotransmitter changes and the effect of Ocimum sanctum (Linn) treatment in albino rats. J Pharmacol Sci. 2005;98:354–360. doi: 10.1254/jphs.FP0050127
- Basner M, Babisch W, Davis A, et al. Auditory and non-auditory effects of noise on health. Lancet. 2014;383:1325–1332. doi: 10.1016/S0140-6736(13)61613-X
- Chengzhi C, Yan T, Xuejun J, et al. Recovery of chronic noise exposure induced spatial learning and memory deficits in young male Sprague-Dawley rats. J Occup Health. 2011;53:157–163. doi: 10.1539/joh.L10125
- Cui B, Wu M, She X, et al. Impulse noise exposure in rats causes cognitive deficits and changes in hippocampal neurotransmitter signaling and tau phosphorylation. Brain Res. 2012;1427:35–43. doi: 10.1016/j.brainres.2011.08.035
- Haider S, Naqvi F, Batool Z, et al. Decreased hippocampal 5-HT and DA levels following sub-chronic exposure to noise stress: impairment in both spatial and recognition memory in male rats. Sci Pharm. 2012;80:1001–1011. doi: 10.3797/scipharm.1207-15
- Wright B, Peters E, Ettinger U, et al. Understanding noise stress-induced cognitive impairment in healthy adults and its implications for schizophrenia. Noise Health. 2014;16:166. doi: 10.4103/1463-1741.134917
- Ilhan A, Gurel A, Armutcu F, et al. Ginkgo biloba prevents mobile phone-induced oxidative stress in rat brain. Clin Chim Act. 2004;340:153–162. doi: 10.1016/j.cccn.2003.10.012
- Srikumar R, Parthasarathy NJ, Manikandan S, et al. Effect of Triphala on oxidative stress and on cell-mediated immune response against noise stress in rats. Mol Cell Biochem. 2006;283:67–74. doi: 10.1007/s11010-006-2271-0
- Cheng L, Wang S-H, Chen Q-C, et al. Moderate noise induced cognition impairment of mice and its underlying mechanisms. Physiol Behav. 2011;104:981–988. doi: 10.1016/j.physbeh.2011.06.018
- Cui B, Wu M, She X. Effects of chronic noise exposure on spatial learning and memory of rats in relation to neurotransmitters and NMDAR2B alteration in the hippocampus. J Occup Health. 2009;51:152–158. doi: 10.1539/joh.L8084
- Grady CL, Craik FI. Changes in memory processing with age. Curr Opin Neurobiol. 2000;10:224–231. doi: 10.1016/S0959-4388(00)00073-8
- Rosenzweig ES, Barnes CA. Impact of aging on hippocampal function: plasticity, network dynamics, and cognition. Prog Neurobiol. 2003;69:143–179. doi: 10.1016/S0301-0082(02)00126-0
- Floyd RA. Oxidative damage to behavior during aging. Science. 1991;254:1597–1598. doi: 10.1126/science.1684251
- Harman D. The aging process. Proc Natl Acad Sci. 1981;78:7124–7128. doi: 10.1073/pnas.78.11.7124
- Farr SA, Poon HF, Dogrukola-Ak D, et al. The antioxidants alpha-lipoic acid and N-acetylcysteine reverse memory impairment and brain oxidative stress in aged SAMP8 mice. J Neurochem. 2003;84:1173–1183. doi: 10.1046/j.1471-4159.2003.01580.x
- Jeong K, Shin Y-C, Park S, et al. Ethanol extract of Scutellaria baicalensis Georgi prevents oxidative damage and neuroinflammation and memorial impairments in artificial senescense mice. J Biomed Sci. 2011;18:14. doi: 10.1186/1423-0127-18-14
- Olanow C. An introduction to the free radical hypothesis in Parkinson’s disease. Ann Neurol. 1992;32:S2–S9. doi: 10.1002/ana.410320703
- Zhu J, Mu X, Zeng J, et al. Ginsenoside Rg1 prevents cognitive impairment and hippocampus senescence in a rat model of D-galactose-induced aging. PloS one. 2014;9:e101291. doi: 10.1371/journal.pone.0101291
- Leutner S, Eckert A, Muller W. ROS generation, lipid peroxidation and antioxidant enzyme activities in the aging brain. J Neural Transm. 2001;108:955–967. doi: 10.1007/s007020170015
- Liu J, Head E, Gharib AM, et al. Memory loss in old rats is associated with brain mitochondrial decay and RNA/DNA oxidation: partial reversal by feeding acetyl-L-carnitine and/or R-alpha-lipoic acid. Proc Nat Acad Sci USA. 2002;99:2356–2361. doi: 10.1073/pnas.261709299
- Liu R, Liu IY, Bi X, et al. Reversal of age-related learning deficits and brain oxidative stress in mice with superoxide dismutase/catalase mimetics. Proc Natl Acad Sci. 2003;100:8526–8531. doi: 10.1073/pnas.1332809100
- Aljadi A, Kamaruddin M. Evaluation of the phenolic contents and antioxidant capacities of two Malaysian floral honeys. Food Chem. 2004;85:513–518. doi: 10.1016/S0308-8146(02)00596-4
- Al-Mamary M, Al-Meeri A, Al-Habori M. Antioxidant activities and total phenolics of different types of honey. Nutr Res. 2002;22:1041–1047. doi: 10.1016/S0271-5317(02)00406-2
- Beretta G, Orioli M, Facino RM. Antioxidant and radical scavenging activity of honey in endothelial cell cultures (EA. hy926). Planta Med.. 2007;73:1182–1189. doi: 10.1055/s-2007-981598
- Gheldof N, Engeseth NJ. Antioxidant capacity of honeys from various floral sources based on the determination of oxygen radical absorbance capacity and inhibition of in vitro lipoprotein oxidation in human serum samples. J Agric Food Chem. 2002;50:3050–3055. doi: 10.1021/jf0114637
- Kishore RK, Halim AS, Syazana M, et al. Tualang honey has higher phenolic content and greater radical scavenging activity compared with other honey sources. Nutr Res. 2011;31:322–325. doi: 10.1016/j.nutres.2011.03.001
- Al-Himyari FA. The use of honey as a natural preventive therapy of cognitive decline and dementia in the Middle East. Alzheimers Dement. 2009;5:P247. doi: 10.1016/j.jalz.2009.04.248
- Chepulis LM, Starkey NJ, Waas JR, et al. The effects of long-term honey, sucrose or sugar-free diets on memory and anxiety in rats. Physiol Behav. 2009;97:359–368. doi: 10.1016/j.physbeh.2009.03.001
- Al-Rahbi B, Zakaria R, Othman Z, et al. Tualang honey supplement improves memory performance and hippocampal morphology in stressed ovariectomized rats. Acta Histochem. 2014;116:79–88. doi: 10.1016/j.acthis.2013.05.004
- Othman Z, Shafin N, Zakaria R, et al. Improvement in immediate memory after 16 weeks of Tualang honey (Agro Mas) supplement in healthy postmenopausal women. Menopause. 2011;18:1219–1224. doi: 10.1097/gme.0b013e31821e2044
- S.S. Zaid, S.A. Sulaiman, K.N. Sirajudeen, N.H. Othman. The effects of Tualang honey on female reproductive organs, tibia bone and hormonal profile in ovariectomised rats-animal model for menopause. BMC Complemen Altern Med. 2010;10:303. doi: 10.1186/1472-6882-10-82
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res.. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-X
- Harman D. Aging: overview. Ann N Y Acad Sci. 2001;928:1–21. doi: 10.1111/j.1749-6632.2001.tb05631.x
- Head E, Liu J, Hagen T, et al. Oxidative damage increases with age in a canine model of human brain aging. J. Neurochem. 2002;82:375–381. doi: 10.1046/j.1471-4159.2002.00969.x
- Gemma C, Mesches MH, Sepesi B, et al. Diets enriched in foods with high antioxidant activity reverse age-induced decreases in cerebellar beta-adrenergic function and increases in proinflammatory cytokines. J Neurosci. 2002;22:6114–6120. doi: 10.1523/JNEUROSCI.22-14-06114.2002
- Arivazhagan P, Thilakavathy T, Panneerselvam C. Antioxidant lipoate and tissue antioxidants in aged rats. J Nutr Biochem. 2000;11:122–127. doi: 10.1016/S0955-2863(99)00079-0
- Balu M, Sangeetha P, Haripriya D, et al. Rejuvenation of antioxidant system in central nervous system of aged rats by grape seed extract. Neurosci Lett. 2005;383:295–300. doi: 10.1016/j.neulet.2005.04.042
- Valls V, Peiro C, Muniz P, et al. Age-related changes in antioxidant status and oxidative damage to lipids and DNA in mitochondria of rat liver. Process Biochem. 2005;40:903–908. doi: 10.1016/j.procbio.2004.02.025
- Sheela C, Augusti K. Antiperoxide effects of S-allyl cysteine sulphoxide isolated from Allium sativum Linn and gugulipid in cholesterol diet fed rats. Ind J Exp Biol. 1995;33:337–341.
- Padurariu M, Ciobica A, Mavroudis I, et al. Hippocampal neuronal loss in the CA1 and CA3 areas of Alzheimer’s disease patients. Psychiatr Danub. 2012;24:152–158.
- Keuker JI, de Biurrun G, Luiten PG, et al. Preservation of hippocampal neuron numbers and hippocampal subfield volumes in behaviorally characterized aged tree shrews. J Comp Neurol. 2004;468:509–517. doi: 10.1002/cne.10996
- Burke SN, Barnes CA. Neural plasticity in the ageing brain. Nat Rev Neurosci. 2006;7:30–40. doi: 10.1038/nrn1809
- Stranahan AM, Jiam NT, Spiegel AM, et al. Aging reduces total neuron number in the dorsal component of the rodent prefrontal cortex. J Comp Neurol. 2012;520:1318–1326. doi: 10.1002/cne.22790
- Arnsten AF, Goldman-Rakic PS. Noise stress impairs prefrontal cortical cognitive function in monkeys: evidence for a hyperdopaminergic mechanism. Arch Gen Psychiatry. 1998;55:362. doi: 10.1001/archpsyc.55.4.362
- Lupien SJ, Wilkinson CW, Brière S, et al. The modulatory effects of corticosteroids on cognition: studies in young human populations. Psychoneuroendocrinol. 2002;27:401–416. doi: 10.1016/S0306-4530(01)00061-0
- Teague CR, Dhabhar FS, Barton RH, et al. Metabonomic studies on the physiological effects of acute and chronic psychological stress in Sprague-Dawley rats. J Proteome Res. 2007;6:2080–2093. doi: 10.1021/pr060412s
- Du J, Wang Y, Hunter R, et al. Dynamic regulation of mitochondrial function by glucocorticoids. Proc Natl Acad Sci. 2009;106:3543–3548. doi: 10.1073/pnas.0812671106
- Chetty S, Friedman AR, Taravosh-Lahn K, et al. Stress and glucocorticoids promote oligodendrogenesis in the adult hippocampus. Mol Psychiatry. 2014;19:1275–1283. doi: 10.1038/mp.2013.190
- Wang W, Esbensen Y, Kunke D, et al. Mitochondrial DNA damage level determines neural stem cell differentiation fate. J Neurosci. 2011;31:9746–9751. doi: 10.1523/JNEUROSCI.0852-11.2011
- Ahmed S, Othman NH. Review of the medicinal effects of Tualang honey and a comparison with Manuka honey. Mal J Med Sci. 2013;20:6–13.
- Allsop KA, Miller JB. Honey revisited: a reappraisal of honey in pre-industrial diets. Brit J Nutr. 1996;75:513–520. doi: 10.1079/BJN19960155
- Bogdanov S, Jurendic T, Sieber R, et al. Honey for nutrition and health: a review. J Am Coll Nutr. 2008;27:677–689. doi: 10.1080/07315724.2008.10719745
- Khalil M, Alam N, Moniruzzaman M, et al. Phenolic acid composition and antioxidant properties of Malaysian honeys. J Food Sci. 2011;76:C921–C928. doi: 10.1111/j.1750-3841.2011.02282.x
- Khalil MI, Mahaneem M, Jamalullail S, et al. Evaluation of radical scavenging activity and colour intensity of nine Malaysian honeys of different origin. J ApiProd ApiMed Sci. 2011;3:4–11. doi: 10.3896/IBRA.4.03.1.02
- Al ML, Daniel D, Moise A, et al. Physico-chemical and bioactive properties of different floral origin honeys from Romania. Food Chem. 2009;112:863–867. doi: 10.1016/j.foodchem.2008.06.055
- Ferreira IC, Aires E, Barreira J, et al. Antioxidant activity of Portuguese honey samples: different contributions of the entire honey and phenolic extract. Food Chem. 2009;114:1438–1443. doi: 10.1016/j.foodchem.2008.11.028
- Moniruzzaman M, Khalil MI, Sulaiman SA, et al. Physicochemical and antioxidant properties of Malaysian honeys produced by Apis cerana, Apis dorsata and Apis mellifera. BMC Complemen Altern Med. 2013;13:43. doi: 10.1186/1472-6882-13-43
- Al-Waili NS. Intrapulmonary administration of natural honey solution, hyperosmolar dextrose or hypoosmolar distill water to normal individuals and to patients with type-2 diabetes mellitus or hypertension: their effects on blood glucose level, plasma insulin and C-peptide, blood pressure and peaked expiratory flow rate. Eur J Med Res. 2003;8:295.
- Erejuwa OO, Sulaiman SA, Ab Wahab MS, et al. Honey supplementation in spontaneously hypertensive rats elicits antihypertensive effect via amelioration of renal oxidative stress. Oxid Med Cell Longev. 2012;2012:1–14. doi: 10.1155/2012/374037
- Erejuwa OO, Sulaiman SA, Wahab MS, et al. Antioxidant protection of Malaysian Tualang honey in pancreas of normal and streptozotocin-induced diabetic rats. Ann Endocrinol (Paris). 2010;71:291–296. doi: 10.1016/j.ando.2010.03.003
- Erejuwa OO, Sulaiman SA, Wahab MSA, et al. Comparison of antioxidant effects of honey, glibenclamide, metformin, and their combinations in the kidneys of streptozotocin-induced diabetic rats. Int J Mol Sci. 2011;12:829–843. doi: 10.3390/ijms12010829
- Erejuwa OO, Sulaiman SA, Wahab MSA, et al. Differential responses to blood pressure and oxidative stress in streptozotocin-induced diabetic wistar-kyoto rats and spontaneously hypertensive rats: effects of antioxidant (Honey) treatment. Int J Mol Sci. 2011;12:1888–1907. doi: 10.3390/ijms12031888
- Erejuwa OO, Sulaiman SA, Wahab MSA, et al. Antioxidant protective effect of glibenclamide and metformin in combination with honey in pancreas of streptozotocin-induced diabetic rats. Int J Mol Sci. 2010;11:2056–2066. doi: 10.3390/ijms11052056
- Gheldof N, Wang X-H, Engeseth NJ. Buckwheat honey increases serum antioxidant capacity in humans. J Agric Food Chem. 2003;51:1500–1505. doi: 10.1021/jf025897t
- Shafin N, Othman Z, Zakaria R, et al. Tualang honey supplementation reduces blood oxidative stress levels/activities in postmenopausal women. ISRN Oxid Med. 2014;2014:1–4. doi: 10.1155/2014/364836
- Hatao H, Oh-ishi S, Itoh M, et al. Effects of acute exercise on lung antioxidant enzymes in young and old rats. Mech Ageing Dev. 2006;127:384–390. doi: 10.1016/j.mad.2005.12.008
- Jafari Anarkooli I, Barzegar Ganji H, Pourheidar M. The protective effects of insulin and natural honey against hippocampal cell death in streptozotocin-induced diabetic rats. J Diabetes Res. 2014;2014:1–8. doi: 10.1155/2014/491571