?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Spinel type manganese oxide (Mn3O4) nanoparticles were synthesized by ultrasonic irradiation assisted co-precipitation method using PEG and NaOH. The formation of tetragonal hausmannite phase (Mn3O4) with spinel structure was confirmed from XRD and FTIR studies. The average crystallite size of Mn3O4 nanoparticles was determined from Scherrer formula (39.9 nm) and Williamson–Hall relation (36.5 nm). The presence of elements, the overall oxidation state of manganese (Mn2+, Mn3+ and Mn4+) and chemical composition of these nanoparticles were determined from Mn 2p, Mn 3s and O 1s spectra using XPS. The spherically shaped morphology of these nanoparticles was analysed by SEM. The expected chemical composition of Mn3O4 nanoparticles was analysed by EDS. The electrochemical performance of these Mn3O4 nanoparticles was studied by CV, CP and AC impedance analysis. The maximum specific capacitance of Mn3O4 nanoparticles was found to be 296 F/g at a current density of 1 A/g2. Nyquist plot shows lower value of resistance that indicates the good electrical conductivity of the Mn3O4 electrode, which accounts for the improved electrochemical performance of the supercapacitors. Hence, the use of surfactant and the presence of ultrasonic irradiation in the synthesis process plays a dominant role in the electrochemical performance of Mn3O4 supercapacitors.
1. Introduction
In recent years, high performance electrical energy storage materials are needed in electrical vehicles, batteries and power electronics. Supercapacitor is one of the energy storage devices; it can store the energy by diffusion/surface redox process in the electrode materials. Mn3O4 is a promising electrode material for supercapacitor due to the availability of multiple oxidation states. It is also applied in a variety of fields such as solid oxide fuel cells (SOFCs), sensors, supercapacitor and lithium-ion batteries [Citation1,Citation2]. Mn3O4 can also be used as an active catalyst for the oxidation of carbon monoxide and methane or the selective reduction of nitrobenzene [Citation3,Citation4]. Among the various types of spinel system, Mn3O4 material belongs to normal spinel having the general formula of AB2O4 with divalent manganese (Mn2+) occupying in the tetrahedral (A) sites and trivalent manganese (Mn3+) occupying in the octahedral (B) sites i.e. Mn2+[Mn3+2]O2−4. Bulk Mn3O4 exhibits a tetragonal Jahn–Teller distortion at the trivalent manganese (Mn3+) site at high temperatures with I41/amd space group [Citation5].
Based on the practical applications; Mn3O4 can be synthesized by different methods. Chen et al. have synthesized three different morphologies of Mn3O4 (nanoparticles, nanorods and nanofractals) through chemical liquid homogeneous precipitation method by controlling the dripping speed of NaOH solution [Citation6]. WS Seo et al. [Citation7] have synthesized Mn3O4 nanoparticles by thermal decomposition of a single precursor [Mn(acac)2] in oleylamine under an inert atmosphere. The average size of 10 nm Mn3O4 was synthesized through esterification process by Li et al. using alcohol and Mn(Ac)2,4 H2O [Citation8]. Mn3O4 hexagonal nanoplates and nanoparticles were synthesized by Ahmed et al. via hydrothermal oxidation process at low temperature and a solvothermal oxidation method [Citation9]. Ganesh Kumar et al. have synthesized pure single-phase spinel-tetragonal-Mn3O4 nanoparticles under open-air conditions using manganese salts [Citation10]. Mn3O4 nanowires were obtained via solvothermal treatment of c-MnOOH in polypropylene glycol for 24 h [Citation11]. By polyol method, Mn3O4 nanoparticles were obtained using Mn(CH3COO)2 as precursors and diethylene glycol [Citation12]. Also, high intensity ultrasonic irradiation method was reported for the synthesis of Mn3O4 nanoparticles with an average size of 4 and 15 nm by Rohani et al. [Citation13,Citation14]. Some researchers are also focused on preparing 1-Dimensional nanomaterials through ultrasonic irradiation method for their unique properties [Citation15]. 1-D Mn3O4 nanorods were obtained by Yang et al. using high temperature calcinations of c-MnOOH [Citation16].
Recently, researchers are paying much attention towards simple, effective and low temperature solution method for the preparation of pure phase nanomaterials. Among the wet-chemical method, ultrasonic irradiation assisted co-precipitation method is facile and attractive method for the synthesis of nanomaterial in low temperature medium due to the efficient mixing and faster mass transfer of the reactants under ultrasound [Citation17]. In addition to this method, the good morphology of nanomaterials is obtained. Ultrasonic assistance synthesized nanomaterials are more active in photocatalysis due to the high surface area and particle size [Citation18].
Stabilization has gained special attention and is achieved using surfactants as protective molecules, which prevent the agglomeration on the surface of the nanoparticles [Citation19]. Among these molecules, polyethylene glycol (PEG) has been widely used as both size controller and capping agent to protect nanoparticles from agglomeration. The stabilization of metal colloids, the size and shape of nanomaterials depend strongly on the solution concentration of PEG.
In this present investigation, PEG works both as size controller and capping agent for the synthesis of spinel type manganese oxide (Mn3O4) by ultrasonic-assisted co-precipitation method using metal nitrate as a starting precursor. The novelty of present work is to understand that the preparation conditions of this spinel type Mn3O4 material have potential impact on the electrode parameters such as phase, morphology and cation distribution thereby controlling the electrochemical performance of Mn3O4 supercapacitors. Hence, we made an attempt to prepare Mn3O4 spinel material via ultrasonic-assisted co-precipitation method. The phase identification of these manganese oxide nanoparticles was characterized by X-ray diffraction (XRD) and Fourier transfer infrared spectroscopy (FTIR) studies. The X-ray photoelectron spectroscopy (XPS) was used to analyse the presence of elements, oxidation states and chemical composition of the synthesized nanoparticles. Morphological nature and elemental analysis of the samples were studied using scanning electron microscopy (SEM) and energy dispersive X-ray spectrometry (EDS). For supercapacitor application, the samples were characterized by cyclic voltammetry (CV), chronopotentiometry (CP) and AC impedance analysis. To the best of our knowledge, there is no report about the ultrasonic-assisted co-precipitation synthesis of spherically shaped Mn3O4 nanoparticles under low temperature for supercapacitor application.
2. Experimental
2.1. Synthesis
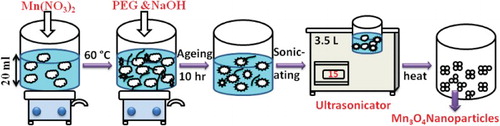
Pure spinel type manganese oxide nanoparticles were prepared by ultrasonic irradiation assisted co-precipitation method. Stoichiometric amounts of manganese nitrate (Mn(NO3)2·4H2O) (Alfa Aesar 98% purity), was dissolved in about 20 ml of distilled water. Then, few grams of polyethylene glycol (PEG) and NaOH were added dropwise into a nitrate solution. The aqueous solution was maintained at pH = 10 while stirring for 4 h at a temperature of 60°C. The mixed solutions were aged for 10 h before sonication. The prepared solution was transferred into an Erlenmeyer as a reaction flask and placed in an ultrasonic water bath. Then, the solutions were sonicated for one hour at a power of 5 W/cm2. The obtained precipitates were washed with de-ionized water and acetone several times; dried at 120°C. The resulted dried powder was grounded to form fine powder. All experiments were carried out in ambient conditions under air atmosphere. The schematic diagram represents the synthesized Mn3O4 nanoparticles are shown in .
2.2. Characterization and instrument
X-ray diffraction measurements for the as-synthesized powders were carried out by Bruker D2 Phaser Powder X-ray diffractometer with a CuKα source (λ = 1.5418 Å). X-ray diffraction profiles were obtained for 2θ ranging from 10° to 80° with a scanning rate of 0.02/min. FTIR measurements were analysed by BRUKER ALPHA spectrophotometer using opus 6.5 (version) software. For recording FTIR spectra, the powder was mixed with KBr and then pressed into a disc of 1 mm thickness. FTIR measurements were carried out at room temperature in the range of 400–1400 cm−1. The chemical states of elements present in the synthesized powders were analysed by X-ray photoelectron spectroscopy (XPS) using Kratos Analytical Axis Ultra DLD with Al Kα1 radiation. For wide spectrum, the energy of an X-ray photon of 1.486 keV with pass energy of 160 eV was used and narrow scan spectra of Mn 2p, Mn 3s and O 1s were recorded in the binding energy range from 0 to 1200 eV using pass energy of 40 eV. All XPS spectra were recorded in vacuum below 5 × 10−10 mbar. The XPS spectra were collected using the combination of electrostatic and magnetic lens for an analysed area of (700 × 300 µm). Surface charging effects were minimized using a charge balance operating at 3.6 and 1.8 V maintained as filament bias. During XPS analysis, sample charging was neutralized using an electron flood gun. The C 1s signal from adventitious hydrocarbon at 284.8 eV was used as an energy reference to correct for charging. All data processing was carried out using CasaXPS software package (version 2.2.14). A Shirley-type background subtraction was used as a baseline for all peaks, and curve fitting using an 80% Gaussian/20% Lorentzian function. The surface morphology and elemental analysis were done using scanning electron microscope (SEM) Quanta FEG 250 operated at 20 kV and Energy Dispersive X-ray Spectrometry (EDS) EDAX-TSL. The ultrasonic equipment used for the synthesis of Mn3O4 nanomaterials was a PCI81 Analytics ultrasonic bath with tank dimension of 300 × 150 × 100; overall dimension of 330 × 175 × 250, operating voltage of 230 V. The electrochemical performance test was carried out by three-compartment cell employing as-prepared working electrode, Ag/AgCl as reference electrode and platinum wire as counter electrode. The 3 M KOH solution was used as an electrolyte for electrochemical measurement using CHI 661 C electrochemical workstation with DELL personal computer for data acquisition and potential control. The Cyclic voltammetry (CV), galvanostatic charge–discharge and electrochemical impedance studies were carried out.
2.3. Preparation of working electrodes
In the typical procedure, 85 wt.% of electroactive material (Mn3O4) was mixed together with 5 wt.% of conducting graphite and 10 wt.% of polyvinylidene fluoride until it becomes a homogeneous powder. To this mixture, a few drops of 1-methyl-2-pyrrolidinone were added to make the paste. The resulting paste was coated onto nickel foil (1 × 1 cm) collector, then dried at 80°C for 4 h. The mass of the electroactive material was approximately taken to be 0.2 mg measured in Shimadzu analytical balance.
3. Results and discussion
3.1. X-ray diffraction (XRD) studies
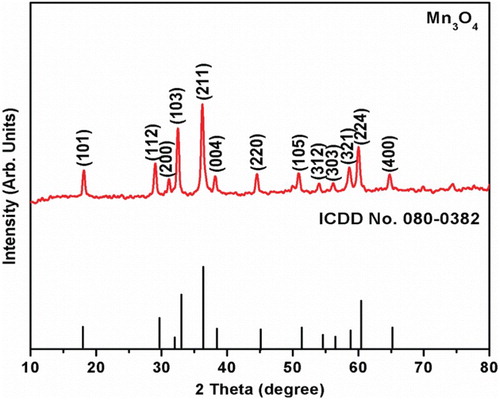
The phase identification of the synthesized powder was examined by X-ray diffractometer. shows the XRD pattern of synthesized Mn3O4 nanoparticles obtained by co-precipitation method. It shows that all the diffraction peaks of (101), (112), (200), (103), (211), (004), (220), (105), (312), (303), (321), (224) and (400) planes were well matched with ICDD no.080-0382 and it can be attributed to the tetragonal hausmannite phase of Mn3O4 spinel structure. No other extra/impurity diffraction peaks were detected in this XRD pattern. This implies the purity of the synthesized powders which is further confirmed from FTIR and XPS studies. The broadening of diffraction peaks indicates the small size of the crystallites within the resulting powders. The average crystallite size (d) of the synthesized Mn3O4 powders was calculated from Scherrer formula using the equation [Citation20],(1)
(1) where d is the average crystallite size, θ is the diffraction angle, λ (0.154 nm) is the wave length of X-ray beam, k is the constant (0.9 assuming nanoparticles are spherical in shape) and β is the full-width half maximum (FWHM). It is observed that the average crystallite (d) size of the as-synthesized Mn3O4 nanoparticles is 39.9 nm. However, in comparison with the literatures, this value is in good agreement with the reported value of 25 nm by thermal decomposition method [Citation21], 30 nm by simple precipitation method [Citation22], 22 nm by sonochemical synthesis respectively [Citation23].
The average crystallite size (d) and the micro strain in the synthesized Mn3O4 nanoparticles were estimated by using Williamson–Hall relation [Citation24],(2)
(2) Here ε is the micro strain developed due to imperfection in crystals. The Williamson–Hall (W–H) equation incorporates the Scherrer formula of average crystallite size and the micro strain terms. Figure S1 shows the W–H graph of Mn3O4 nanoparticles by plotting βcosθ versus 4sinθ for all the diffraction peaks. The W–H plot is a straight line in which the reciprocal of an intercept gives the average crystallite size and the slope gives the micro strain. The average crystallite size and micro strain obtained from W–H plot are mentioned in . From the table, it is evident that the average crystallite sizes obtained from both results are in good agreement with each other. The micro strain value obtained from the slope of W–H plot is positive, it gives the relaxation strain. This clearly indicates that surfactant and the presence of ultrasonic irradiation induces strain which leads to the formation of spherically shaped nanoparticles as observed in SEM [Citation25].
Table 1. XRD parameters of synthesized Mn3O4 nanomaterials.
The dislocation density (δ), defined as the length of dislocation lines per unit volume, gives the amount of defects in a crystal. The dislocation density (δ) was calculated using the formula [Citation26](3)
(3) The dislocation density (δ) value obtained from the above equation is noted in . The table shows the lower value of dislocation density. This means that use of surfactant and the presence of ultrasonic irradiation in the synthesis of Mn3O4 nanoparticles, reduces the dislocation density (δ). This clearly indicates that surfactant and the presence of ultrasonic irradiation enhances the crystallinity of Mn3O4 nanomaterials, making it a potential candidate for optoelectronic devices [Citation27].
3.2. Fourier transfer infrared spectroscopy (FTIR) studies
FTIR analysis was carried out to examine the presence of phase of Mn3O4 nanoparticles. Figure S2 shows the FTIR spectra of the prepared Mn3O4 spinel nanoparticles in the wavelength region of 400–4000 cm−1. It is known that spinel type Mn3O4 comes under the normal cubic structure in which Mn2+ ion occupies in tetrahedral (A) site and Mn3+ ion occupies in octahedral (B) sites of Mn2+[Mn3+2]O2−4. The FTIR spectra band at 462.5 cm−1 corresponds to the vibration of trivalent manganese ions in the octahedral coordination [Citation28]. The vibration band at 507.4 cm−1 was attributed to the distortion of Mn–O in an octahedral (B) site [Citation29]. The strong band at 622.3 cm−1 was assigned to the stretching vibration mode of Mn2+–O at tetrahedral (A) site [Citation30,Citation31]. The absorption band at 1300–1600 cm−1 is due to C–H bending vibration and adsorbed water molecules on the nanoparticles. The broad band observed in the range of 3000–3500 cm−1 indicates the stretching vibrations of H2O molecules on the surface of samples. These FTIR observations confirm that no additional/impurity phase was formed which further supports the XRD and XPS results.
3.3. X-ray photoelectron spectroscopy (XPS) studies
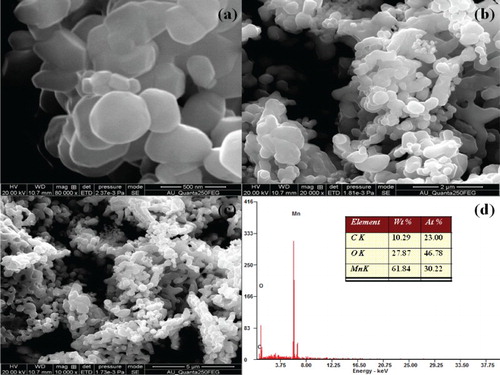
The overall oxidation state of Mn ions and chemical composition of the synthesized Mn3O4 nanoparticles was examined by X-ray photoelectron spectroscopy (XPS). shows the XPS survey spectrum of manganese oxide nanoparticles, the presence of Mn, O and C elements were detected. No other elements were identified; it indicates the purity of the samples which is further supported from XRD and FTIR results. The data of atomic ratio obtained from survey spectrum of Mn3O4 nanoparticles are nearly close to 3/4 agreeing with the expected chemical composition of normal spinel structure of synthesized Mn3O4 nanoparticles.
Figure 3. XPS (a) Survey spectrum, (b) deconvolution spectra of Mn 2p, (c) deconvolution spectra of Mn 3s and (d) deconvolution spectra of O 1s for Mn3O4 nanoparticles.
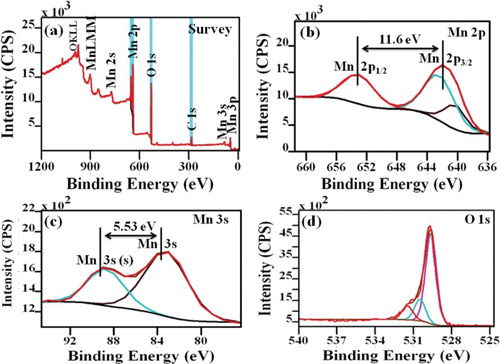
(b) shows the high-resolution XPS spectra of Mn 2p peak. Due to the occurrence of spin orbit splitting, Mn 2p peak gives rise to a doublet with the two possible states 3/2 and 1/2 having different binding energies at 641.84 and 653.4 eV with energy splitting of 11.6 eV, which is similar with the previous report on XPS spectrum of Mn3O4 [Citation32]. Generally, Mn3O4 has normal spinel structure with Mn having different oxidation states which can be present in the tetrahedral sites (Mn2+) and octahedral sites (Mn3+) with the chemical composition of Mn2+[Mn3+2]O2−4. In order to determine the different oxidation states of Mn ions, we can deconvolute the Mn 2p peak by Gaussian–Lorentzian function. The Mn 2p3/2 peak consists of two components with binding energies values of 641.5 and 643.1 eV, which corresponds to binding energies of Mn2+ and Mn4+ species [Citation33]. Meanwhile, this species at binding energies of 641.5 and 643.1 eV are attributed to MnO and MnO2. Theoretical calculation represents 40 wt% of MnO2 is in Mn3O4 and remaining 60 wt% is in Mn2+. The area ratio (i.e. molar ratio) of Mn4+ versus Mn2+ is almost 1:2 which agree well with theoretical oxide compositions of MnO2–2MnO.
The formation of manganese oxide (Mn3O4) was further confirmed from splitting of Mn 3s spectra. This splitting of spectra is proportional to the remaining electron in the 3s orbital and the other unpaired electrons, which all have parallel spins [Citation32]. (c) shows the XPS Mn 3s spectra of Mn3O4 nanoparticles. In the Mn 3s spectra, a 2p3/2-2p1/2 doublet at 83.7 and 89.23 eV is observed, and the splitting width (5.53 eV) agrees with an earlier report on Mn3O4 [Citation34].
The change in the oxidation state of manganese during redox reaction was also confirmed by analysing the O 1s core level XPS spectra. (d) shows the high-resolution O 1s XPS spectra for Mn3O4 nanoparticles. It shows that the O 1s spectra can be fitted into three components at 529.63, 530.49 and 531.47 eV, which are related to the Mn–O–Mn, Mn–OH and H–O–H bonds respectively. The variation in the relative intensity of the Mn–O–Mn and Mn–OH signals indicates the change in the oxidation state of manganese. The formula used to determine manganese oxidation state from the intensities of the Mn–O–Mn and Mn–OH signals is given by the equation [Citation35],where S is the bond corresponding to the signals from the O 1s spectra. From the equation, the obtained mean manganese oxidation state is found to be 2.99 (∼3) for Mn3O4 nanoparticles. Therefore, the overall oxidation states of manganese are Mn2+, Mn3+ and Mn4+. The growth mechanism of Mn3O4 nanoparticles can be represented as follows;

 3.4. Morphology studies
3.4. Morphology studies
Field emission scanning electron microscope (FE-SEM) is used to decipher the surface morphology of the synthesized Mn3O4 nanoparticles. The SEM micrographs of Mn3O4 nanoparticles are shown in (a–c). It is observed that the particles are nanosized have spherical morphology and are slightly agglomerated. The reason behind the formation of spherical shape and agglomeration of nanoparticles in the presence of ultrasonic irradiation can be explained by three reasons:
Use of surfactant in the synthesis process induces strain which leads to the formation of spherically shaped nanoparticles as discussed in XRD studies.
PEG acting as a capping agent has strong adsorbed on the surface of Mn3O4 particles with coordination bonds. This strong capping agent on the surface of nanoparticles hinders the particle growth. By applying the ultrasonic waves can led to an irreversible desorption of capping agents (PEG) from the surface of nanoparticles by cavitational collapse [Citation36,Citation37]. Keeping them from excessive growth and leading to the formation of nanoparticles. For this reason, the spherical shape of particles can be observed in the presence of ultrasonic irradiation.
Aggregation of Mn3O4 nanoparticles results in particle size in the range of <100 nm and this is the cause for agglomeration.
3.5. Energy dispersive X-ray spectroscopy (EDS) analysis
Energy dispersive X-ray spectroscopy is a useful analytical technique to analyse the chemical elements and compositions in the sample. EDS spectra for the synthesized spinel manganese oxide nanoparticles is shown in (d). Form the EDS result confirms the presence of Mn and O as elementary components in the synthesized Mn3O4 nanopowders. The data of atomic ratio obtained from EDS spectrum of the synthesized nanoparticles are listed as an insert (table) of (d). The obtained values are nearly close to 3/4 agreeing with the expected chemical composition of normal spinel structure of synthesized Mn3O4 nanoparticles. Based on the EDS observations, it is confirmed that no impurity or additional elements were present which further supports the XRD and XPS results.
3.6. Electrochemical properties
3.6.1 Cyclic voltammogram analysis
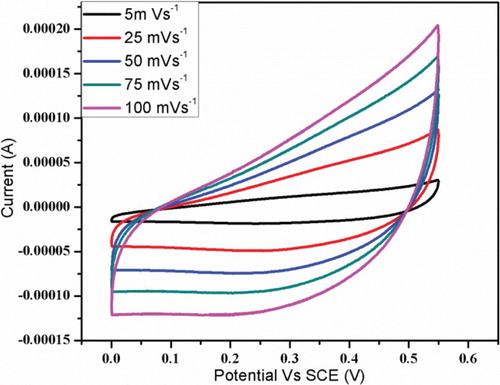
The supercapacitor behaviour of synthesized Mn3O4 nanoparticles at room temperature was studied by cyclic voltammetry (CV). The CV curve of Mn3O4 nanoparticles electrode in 1M aqueous Na2SO4 electrolyte solution at different scan rate of 5, 25, 50, 75 and 100 mV s-1are shown in . It could be clearly observed that all the curves show no redox peaks in the range of 0–0.55 V (vs. SCE). Furthermore, a CV curve shows rectangular under various scanning rates. This indicates the synthesized Mn3O4 electrode has excellent ideal capacitive behaviour at room temperature. This agrees with the previous reports [Citation9,Citation35,Citation38].
3.6.2 Galvanostatic charge–discharge analysis
The electrochemical properties of synthesized Mn3O4 nanoparticles at room temperature were also studied by galvanostatic charge–discharge tests. shows the cyclic performance and corresponding charge–discharge curves of Mn3O4 nanoparticles at current density between 1 and 10 A/g2. The specific capacitance was calculated from the charge–discharge curves using the relation [Citation39](4)
(4) where SC is the specific capacitance, I is the current, Δt is the discharging time, ΔV is the potential drop during discharge and m is the mass of the active electrode material.
Figure 6. Charge-discharge curves of the prepared electrode materials at different current densities of 1–10 A g−2.
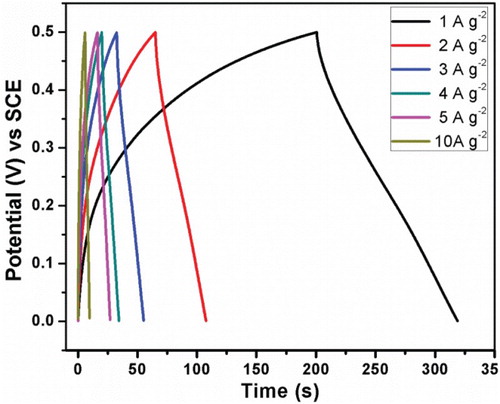
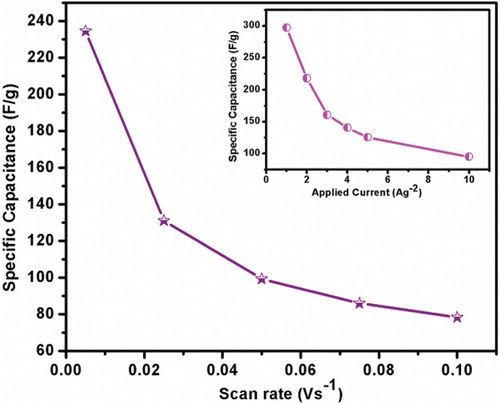
An SC of 296 F/g for the prepared Mn3O4 electrode material is obtained at a current density of 1 A/g2. The obtained SC value is in good agreement with those reported by Hao Jiang et al. for Mn3O4 nano-octahedrons prepared via hydrothermal route using EDTA (322 F/g) [Citation35], Mn3O4 nanoparticles (diameter 10 nm) synthesized by Lu Wang et al. through ultrasonic-assisted route of whose SC of 262.1 F/g at a current rate of 0.4 Ag−1 [Citation40] and microwave-assisted reflux-synthesized Mn3O4 nanoparticles by Kalimuthu Vijaya Sankar et al. of SC 94 F/g in 6 M KOH electrolyte solution [Citation41].
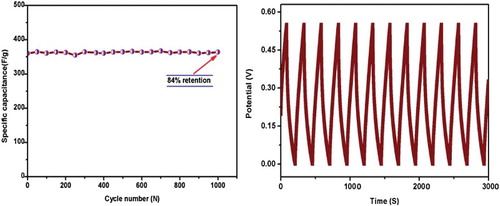
shows the specific capacitance versus scan rate for prepared Mn3O4 electrode materials at a different scan rate of 5–100 mV s−1. Insert figure shows the specific capacitance versus applied current. From (insert), specific capacitances calculated from cyclic voltammetry (CV) are in agreement with those calculated from galvanostatic charge–discharge technique. Moreover, from both studies, specific capacitance values are found to decrease in the range of 234–78 F/g from CV and 296–95 F/g from galvanostatic charge–discharge. This decreasing behaviour of SC may be due to low scan rates as there is sufficient time for diffusion of ions into the host Mn3O4 material and maximum utilization of active species available but at high scan rates only surface ions participate in the redox reaction and agglomeration is observed from FESEM images. Generally, the specific capacitance of the materials depends on high crystallinity, as it enhances the ionic mobility of the charge carriers [Citation42]. To define the material capability for long and high-power deliverance, a long charge–discharge characteristic has been carried out. (a) shows the long cycling profile of the electrode Mn3O4 material carried out for over 1000 cycles at 3 A/g in in 3 M KOH solution. The Columbic efficiency (η) of the electrode Mn3O4 material was calculated using the formula [Citation39](5)
(5) where td is the discharge time and tc is charging time from charge–discharge curves. (a, b) shows the electrode Mn3O4 material realizes the maximum columbic efficiency of 84% as expected from its nearly symmetrical charge–discharge curves and strong cyclic stability during long cycle periods, resulting in reasonable reversibility characteristics.
3.6.3. Electrochemical impedance spectral analysis
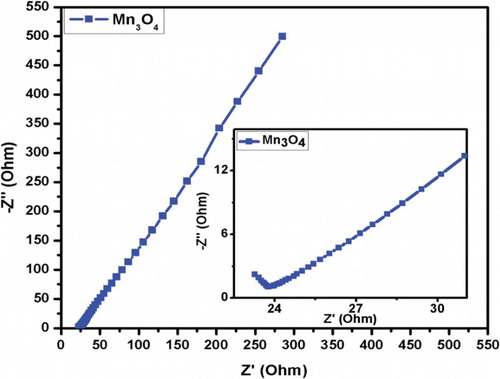
The chemical kinetics of electron and ions at the electrode in the electrode–electrolyte interface was examined by electrochemical impedance spectroscopy. shows the electrochemical impedance plot or Nyquist plot drawn real part Z′ against the imaginary part Z″ for Mn3O4 electrode materials in the frequency range of 1–105 Hz. Incomplete semicircle near high frequency region depicts the lower resistance of Mn3O4 electrode materials and diffusion controlled rate kinetics of the redox process. The diameter of the semicircle gives the measure of charge transfer resistance (Rct). Negligible diameter implies the lower Rct resistance of the material. Hence, lower resistance i.e. high conductivity of Mn3O4 electrode material makes it a better candidate of supercapacitor application as less energy will be wasted during the charge–discharge process.
4. Conclusion
In summary, ultrasonic irradiation assisted co-precipitation method has been used to successfully synthesize Mn3O4 nanoparticles using PEG and NaOH. The XRD studies show that the as-synthesized nanoparticles possess hausmannite phase Mn3O4 with spinel structure. The average crystallite size obtained from Scherrer formula (39.9 nm) and Williamson-Hall relation (36.5 nm) are in good agreement with each other. The lower value of microstrain and dislocation density is due to the use of surfactant and the presence of ultrasonic irradiation in the synthesis process and hence increases the crystallinity of Mn3O4 nanoparticles. The formation of spinel Mn3O4 nanoparticles was also confirmed from FTIR analysis in the band region of 450–650 cm−1. The presence of elements (Mn and O), chemical composition and oxidation states of manganese (Mn2+, Mn3+ and Mn4+) were determined from Mn 2p, Mn 3s and O 1s spectra using X-ray photoelectron spectroscopy studies. The surface morphology of the sample shows spherical with slightly agglomerated assembly of nanoparticles and the elemental analysis reveals that the sample contains Mn and O elements, respectively. The investigation of the electrochemical performance of spinel Mn3O4 nanoparticles was performed by cyclic voltammetry (CV), chronopotentiometry (CP) and AC impedance analysis. The maximum SC of Mn3O4 nanoparticles was found to be 296 F/g at a current density of 1 A/g2. Nyquist plot shows lower value of Rct which indicates the good electrical conductivity of the Mn3O4 electrode, which accounts for the improved electrochemical performance of the supercapacitors.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
R. Tholkappiyan http://orcid.org/0000-0001-6729-5227
References
- Bernrad MC, Goff HL, Thi BB. Electrochromic reactions in manganese oxides. J Electrochem Soc. 1993;140:3065. doi: 10.1149/1.2220986
- De Vries AH, Hozoi L, Broer R. Importance of interatomic hole screening in core-level spectroscopy of transition metal oxides: Mn 3s hole states in MnO. Phys Rev B. 2002;66:1397. doi: 10.1103/PhysRevB.66.035108
- Stobbe ER, de Boer BA, Geus JW. The reduction and oxidation behaviour of manganese oxides. Catal Today. 1999;47:161–167. doi: 10.1016/S0920-5861(98)00296-X
- Grootendorst EJ, Verbeek Y, Ponce V. The role of the Mars and Van Krevelen mechanism in the selective oxidation of nitrosobenzene and the deoxygenation of nitrobenzene on oxidic catalysts. J Catal. 1995;157:706–712. doi: 10.1006/jcat.1995.1336
- Regmi R, Tackett R, Lawes G, et al. Suppression of low-temperature magnetic states in Mn3O4 nanoparticles. Magn Mater. 2009;321:2296–2299. doi: 10.1016/j.jmmm.2009.01.041
- Chen ZW, Lai JKL, Shek CH. Shape-controlled synthesis and nanostructure evolution of single-crystal Mn3O4 nanocrystals. Scr Mater. 2006;55:735–738. doi: 10.1016/j.scriptamat.2006.05.041
- Seo WS, Jo HH, Lee K, et al. Size-dependent magnetic properties of colloidal Mn3O4 and MnO nanoparticles. Angew Chem. 2004;116:1135–1137. doi: 10.1002/ange.200352400
- Li X, Zhou L, Gao J, et al. Synthesis of Mn3O4 nanoparticles and their catalytic applications in hydrocarbon oxidation. Powder Technol. 2009;190:324–326. doi: 10.1016/j.powtec.2008.08.010
- Abdelazez Mohamed AK, Qiumei Z, Kangbing W, et al. Mn3O4 nanoplates and nanoparticles: synthesis, characterization, electrochemical and catalytic properties. J. Solid State Chem. 2010;183:744–751. doi: 10.1016/j.jssc.2010.01.015
- Kumar VG, Aurbuch D, Gedanken A. A comparison between hot-hydrolysis and sonolysis of various Mn(II) salts. Ultrason Sonochem. 2003;10:17–23. doi: 10.1016/S1350-4177(02)00091-3
- Li F, Wu J, Qin Q, et al. Facile synthesis of γ-MnOOH micro/nanorods and their conversion to β-MnO2, Mn3O4. J. Alloys Compd. 2010;492:339–346. doi: 10.1016/j.jallcom.2009.11.089
- Sicard L, LeMeins JM, Methivier C, et al. Polyol synthesis and magnetic study of Mn3O4 nanocrystals of tunable size. Magn Mater. 2010;322:2634–2640. doi: 10.1016/j.jmmm.2010.03.016
- Rohani Bastami T, Entezari MH. Sono-synthesis of Mn3O4 nanoparticles in different media without additives. Chem Eng J. 2010;164:261–266. doi: 10.1016/j.cej.2010.08.030
- Gopalakrishnan IK, Bagkar N, Ganguly R, et al. Synthesis of superparamagnetic Mn3O4 nanocrystallites by ultrasonic irradiation. J Cryst Growth. 2005;280:436–441. doi: 10.1016/j.jcrysgro.2005.03.060
- Li B, Zhao Y, Xu X, et al. A simple method for the preparation of containing Sb nano- and microcrystallines via an ultrasound agitation. Ultrason Sonochem. 2007;14:557–562. doi: 10.1016/j.ultsonch.2006.09.007
- Yang Z, Zhang Y, Zhang W, et al. Nanorods of manganese oxides: synthesis, characterization and catalytic application. J Solid State Chem. 2006;179:679–684. doi: 10.1016/j.jssc.2005.11.028
- Pradhan A, Jones RC, Caruntu D, et al. Gold–magnetite nanocomposite materials formed via sonochemical methods. Ultrason Sonochem. 2008;15:891–897. doi: 10.1016/j.ultsonch.2008.01.004
- Kesavan V, Sivanand PS, Chandrasekaran S, et al. Catalytic aerobic oxidation of cycloalkanes with nanostructured amorphous metals and alloys. Angew Chem Int Ed. 1999;38:3521–3523. doi: 10.1002/(SICI)1521-3773(19991203)38:23<3521::AID-ANIE3521>3.0.CO;2-S
- Cheng X, Zhang X, Yin H, et al. Modifier effects on chemical reduction synthesis of nanostructured copper. Appl Surf Sci. 2006;253:2727–2732. doi: 10.1016/j.apsusc.2006.05.125
- Salavati-Niasari M, Davar F, Mazaheri M. Synthesis of Mn3O4 nanoparticles by thermal decomposition of a [bis(salicylidiminato)manganese(II)] complex. Polyhedron. 2008;27:3467–3471. doi: 10.1016/j.poly.2008.04.015
- Gao J, Lowe MA, Abruna HD. Spongelike nanosized Mn3O4 as a high-capacity anode material for rechargeable lithium batteries. Chem Mater. 2011;23:3223–3227. doi: 10.1021/cm201039w
- Baykala A, Kavasb H, Durmuşa Z, et al. Cent Eur J Chem. 2010;8:633.
- Askarinejad A, Morsali A. Direct ultrasonic-assisted synthesis of sphere-like nanocrystals of spinel Co3O4 and Mn3O4. Ultrason Sonochem. 2009;16:124–131. doi: 10.1016/j.ultsonch.2008.05.015
- Williamson GK, Hall WH. X-ray line broadening from filed aluminium and wolfram. Acta Metall. 1953;1:22–31. doi: 10.1016/0001-6160(53)90006-6
- Tu KN. Interdiffusion and reaction in bimetallic Cu-Sn thin films. Acta Met. 1973;21:347–354. doi: 10.1016/0001-6160(73)90190-9
- Williamson GB, Smallman RC. III. Dislocation densities in some annealed and cold-worked metals from measurements on the X-ray Debye-Scherrer spectrum. Philos Mag. 1956;1:34–46. doi: 10.1080/14786435608238074
- Guneri E, Gode F, Ulutas C, et al. Chalcogenide Lett. 2010;7:685.
- Gandhi S, Gopinathan nair MR, Anbarasan R. Sonochemical synthesis and characterization of nanostructured Mn 3 O4 and its surface catalytic effect on poly (vinyl alcohol). Int J Nanosci. 2012;11:1250004. doi: 10.1142/S0219581X12500044
- Bin Asif SA, Khan SB, Asiri AM. Visible light functioning photocatalyst based on Al2O3 doped Mn3O4 nanomaterial for the degradation of organic toxin. Nanoscale Res Lett. 2015;10:482. doi: 10.1186/s11671-015-1174-y
- Mansournia M, Azizi F, Rakhshan N. A novel ammonia-assisted method for the direct synthesis of Mn3O4 nanoparticles at room temperature and their catalytic activity during the rapid degradation of azo dyes. J Phys Chem Solids. 2015;80:91–97. doi: 10.1016/j.jpcs.2015.01.001
- Atique Ullah AKM, Fazle Kibria AKM, Akter M, et al. Synthesis of Mn3O4 nanoparticles via a facile gel formation route and study of their phase and structural transformation with distinct surface morphology upon heat treatment. J Saudi Chem Soc. 2017. doi: 10.1016/j.jscs.2017.03.008.
- Moses Ezhil Raj A, Grace Victoria S, Bena Jothy V, et al. XRD and XPS characterization of mixed valence Mn3O4 hausmannite thin films prepared by chemical spray pyrolysis technique. Appl Surf Sci. 2010;256:2920–2926. doi: 10.1016/j.apsusc.2009.11.051
- Ramírez A, Hillebrand P, Stellmach D, et al. Evaluation of MnOx, Mn2O3, and Mn3O4 electrodeposited films for the oxygen evolution reaction of water. J Phys Chem C. 2014;118(26):14073–14081. doi: 10.1021/jp500939d
- Geng Z, Wang Y, Liu J, et al. δ-MnO2–Mn3 O4 nanocomposite for photochemical water oxidation: active structure stabilized in the interface. ACS Appl Mater Interfaces. 2016;8:27825–27831. doi: 10.1021/acsami.6b09984
- Jiang H, Zhao T, Yan C, et al. Hydrothermal synthesis of novel Mn3O4 nano-octahedrons with enhanced supercapacitors performances. Nanoscale. 2010;2:2195. doi: 10.1039/c0nr00257g
- Wang Y, Zhang J, Yang Y, et al. NaOH concentration effect on the oriented attachment growth kinetics of ZnS. J Phys Chem B. 2007;111:5290–5294. doi: 10.1021/jp0688613
- Zhang J, Lin Z, Lan Y, et al. A multistep oriented attachment kinetics: coarsening of ZnS nanoparticle in concentrated NaOH. J Am Chem Soc. 2006;128:12981–12987. doi: 10.1021/ja062572a
- Dubal DP, Dhawale DS, Salunkhe RR, et al. Chemical synthesis and characterization of Mn3O4 thin films for supercapacitor application. J Alloys Compd. 2010;497:166–170. doi: 10.1016/j.jallcom.2010.02.182
- Nirmalesh Naveen A, Selladurai S. Investigation on physiochemical properties of Mn substituted spinel cobalt oxide for supercapacitor applications. Electrochim Acta. 2014;125:404–414. doi: 10.1016/j.electacta.2014.01.161
- Wang L, Chen L, Li Y, et al. Preparation of Mn3O4 nanoparticles at room condition for supercapacitor application. Powder Technol. 2013;235:76–81. doi: 10.1016/j.powtec.2012.10.010
- Sankar KV, Kalpana D, Selvan RK. Electrochemical properties of microwave-assisted reflux-synthesized Mn3O4 nanoparticles in different electrolytes for supercapacitor applications. J Appl Electrochem. 2012;42:463–470. doi: 10.1007/s10800-012-0424-2
- Sharma RK, Oh H-S, Shul Y-G, et al. Growth and characterization of carbon-supported MnO2 nanorods for supercapacitor electrode. Phys B. 2008;403:1763–1769. doi: 10.1016/j.physb.2007.10.007