?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The effectiveness of 3-nitrobenzoic acid towards the inhibition of the corrosion of mild steel and aluminium in solution of HCl was investigated using theoretical and experimental methods (weight loss, thermometric, polarization, FTIR and SEM techniques). Inhibition efficiency of 3-nitrobenzoic acid, evaluated from weight loss technique ranged from 71% to 90% and from 71% to 82% for mild steel and aluminium, respectively. Results from linear polarization and potentiodynamic studies were comparable to weight loss results. Calculated kinetic (activation energy), thermodynamic (changes in entropy and enthalpy) and adsorption parameters indicated that the adsorption of the inhibitor on the surface of the respective metal is accompanied by molecular association and is endothermic, spontaneous and favoured the mechanism of physical adsorption. Best-fitted adsorption isotherms were Langmuir and Frumkin models, which gave evidences for the existence of interaction, characterized by attractive behaviour of the inhibitor on both mild steel and aluminium surfaces. Scanning electron micrographs of the metal before and after inhibition clearly revealed that the inhibitor prevented crevice and pitting corrosion by forming adsorbed protective layer on the respective metal surface. FTIR spectra of the inhibitor and the corrosion products indicated the formation of new bond, existence of interaction between the inhibitor molecules and the involvement of some functional groups in the adsorption and inhibition processes. Quantum chemical study revealed that the inhibitor is adsorbed on the metal surface through the nitro functional group in the ring. Calculated semi empirical parameters were comparable to those reported for excellent corrosion inhibitors.
1. Introduction
Efforts in tackling degradation of metals and their alloys through extension of lifespan have shown that it is difficult to produce or design metallic structures that are completely resistant to corrosion attack. Corrosion is an electrochemical process that returns metals to their natural state. Although corrosion cannot be terminated, anodic/cathodic protection, galvanizing, painting, oiling and the use of corrosion inhibitors are some measures that have proven to be successful in retarding the rate of corrosion of metals [Citation1–4]. Of significant interest is the use of corrosion inhibitors, which are compounds that retard the rate of corrosion, when added in minute concentration [Citation5]. Progressive trends in the application of corrosion inhibitors to protect industrial fabrications exposed to aggressive medium have led to the development on the use of green corrosion inhibitors, which are less toxic, eco-friendly, cost-effective and biodegradable [Citation6]. Some organic compounds have been investigated as possible green corrosion inhibitors for the corrosion of metals and the outstanding characteristics that favour their corrosion inhibition efficiency are the possession of hetero atom (i.e. N, S, O or P) in their aromatic or long carbon chain, availability of π-electron and suitable functional groups, among others [Citation7].
In spite of the excessive number of organic inhibitors that have been investigated, researches in materials chemistry and chemical metallurgy are widely open to contain newer or novel corrosion inhibitors, especially those with high inhibition efficiency and less toxicity. Consequently, the present study is aimed at investigating the corrosion inhibition potential of 3-nitrobenzoic acid for aluminium and mild steel in 0.1 M HCl. The metals chosen are commonly used industrial metals while the expected inhibitor is established to exhibit less toxicity (LD50 = 640 mg/kg (iv, mouse); besides, it is aromatic and has some functional groups that could present it as an efficient corrosion inhibitor. Literature is scanty on the use of 3-nitrobenzoic acid as a corrosion inhibitor for metal, but some derivative of this compound have been reported as good corrosion inhibitors. For example, Paandey et al. [Citation8] recently found that benzamide-2-chloro-4-nitrobenzoic acid and anthranilic acid-2-chloro-4-nitrobenzoic acid are efficient corrosion inhibitors for mild steel in acidic medium using polarization and electrochemical impedance spectroscopy. Their corrosion inhibition efficiencies were favoured by increase in concentration while the inhibitors acted as mixed type. Muralidharan et al. [Citation9] investigated the corrosion inhibition properties of salicylic acid, ortho-nitrobenzoic acid and anthranilic acid using weight loss and small amplitude voltammetric methods and obtained maximum inhibition efficiency ranging from 44% to 82%. The compounds protected the corrosion of the metal through chemisorption mechanism while their adsorption characteristics favours the Flory-Huggins isotherm. In a related development, the work of Dinnappa and Mayanna [Citation10] investigated the corrosion inhibition capacities of benzoic acid, p-toluic acid, p-nitrobenzoic acid, phthalic acid and terephthalic acid at 303 K using weight loss and polarization methods. The inhibition efficiency of these compounds was found to increase with concentration while their adsorption behaviour suited the Bockris-Swinkels adsorption isotherm within n = 5, configurational functions. In this study, inhibitory potential of 3-nitrobenzoic acid for mild steel and aluminium in solution of HCl was investigated and compared using weight loss, polarization, scanning electron microscopy and Fourier-transformed infrared spectroscopy techniques. Quantum chemical calculations were carried out to find out the local and global selectivity functions of 3-nitrobenzoic acid as a corrosion inhibitor.
2. Materials and methods
2.1. Materials
Chemicals (including 3-nitrobenzoic acid) and reagents used for the studied were supplied by Sigma Aldrich Chemical Company through their sales representative. Jeobros metals supplied the mild steel and aluminium metal sheets, which were further reduced to various coupons of 5 × 4 × 0.05 cm for weight loss experiments. Each coupon was polished with different grades of emery paper, washed in ethanol, and rinsed in acetone and allowed to dry in the air. Metal specimens used for electrochemical experiments were constructed to 1 cm2 area, which served as the working electrode. Concentration of HCl used for the study was 0.1 M while the concentration range for the 3-nitrobenzoic acid was 0.002–0.100 M.
2.2. Weight loss experiment
Weight loss experiment was performed by ensuring that the test solution (i.e. inhibitor or blank solution) completely covered the metal coupon (of known weight), placed in a 250 ml beaker. Each coupon was withdrawn from the solution after every 24 hours. The withdrawn metal coupon was washed dried and weighed. From the difference in weight, weight loss, corrosion rate and inhibition efficiency were calculated using the following equations [Citation11]:
(1)
(1)
(2)
(2)
(3)
(3)
where w1 is the initial weight of the metal before immersion, w2 is the weight of the metal after 24-hour immersion, CR is the corrosion rate of the metal, A is the area of the metal coupon and t is the period of immersion in hours while %IE is the inhibition efficiency in percentage.
2.3. Polarization experiment
Linear polarization resistance (LP) experiments were carried using a potentiostat (model: AuT71791 and PGSTAT 30) operating under potential range of −1000 to 2000 mV and a scan rate of 0.33 mV/s at 303 K. Triplicate analyses were carried out in order to obtain the average. The instrument has an autolab frequency response analyser that is coupled to a potentiostat and linked to a computer system. The working electrode (which was immersed in the test solution) was the metal, the counter electrode was a platinum electrode in a glass corrosion cell kit, while the reference electrode was an Ag/Ag system.
The corrosion rate of the metal from potentiodynamic polarization measurement was calculated through corrosion current density lcorr, obtained by extrapolating the linear Tafel segments of the anodic and cathodic curves produced by the machine while the inhibition efficiency (l%) was calculated using Equation (4) [Citation12]:
(4)
(4)
From the plots of over potential versus current, values of resistant polarization were evaluated through the slope of the plots. Consequently, the inhibition efficiency was calculated using Equation (5) [Citation13]:
(5)
(5)
where Rp and Rp(Inh) are the polarization resistance for the uninhibited and inhibited systems, respectively
2.4. Scanning electron microscopy
Scanning electron microscope, model number JSM-5600 LV, produced by JEOL, TOKYO, Japan, was used to produce micrographs of the metal coupon before and after inhibition. Selected coupons were retrieved from the test solution (containing 0.01 M of NBA at room temperature) after 168 hours of immersion. Each sample was mounted on a metal stub and sputtered with gold in order to make it conductive. Scanned micrographs were taken at an accelerating voltage of 2.00 and 15.00 kV.
2.5. Fourier-transformed infrared spectroscopy
SCHIMADZU FTIR 8400S Fourier transformed infrared spectroscopy was used to produce FTIR spectra of the inhibitor and the corrosion product of the metal (in the absence and presence of the inhibitor). Each sample was prepared with KBr powder and the selected scanned range was 400–4000 cm−1.
3. Results and discussions
3.1. Weight loss
Patterns obtained for the variation of weight loss with time at different temperatures for the corrosion of mild steel and aluminium in 0.1 M HCl are shown graphically in Figures and . The plots generally reveal that weight loss increases with increasing period of immersion and with increase in temperature, but decreases with an increase in the concentration of the NBA. This implies that the corrosion rates of mild steel and aluminium increase with an increase in the period of contact and with an increase in temperature, but decrease with an increase in the concentration of NBA. Hence, NBA inhibited the corrosion of mild steel and aluminium in solution of HCl and its inhibition efficiency increases with an increase in concentration, but decreases with increasing temperature as shown in .
Figure 1. Variation of weight loss with time for the corrosion of mild steel in 0.1 M HCl containing various concentrations of NBA at 303, 313, 323 and 333 K.
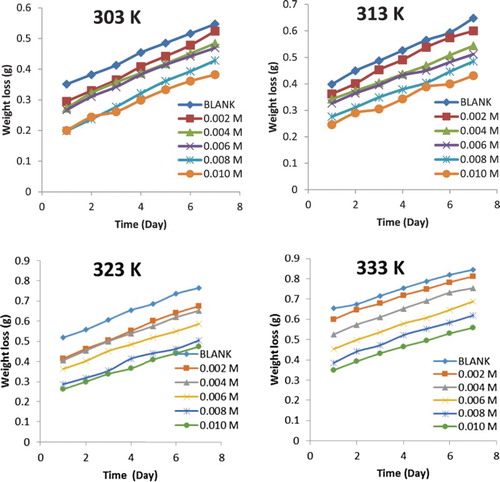
Figure 2. Variation of weight loss with time for the corrosion of aluminium in 0.1 M HCl containing various concentrations of NBA at 303, 313, 323 and 333 K.
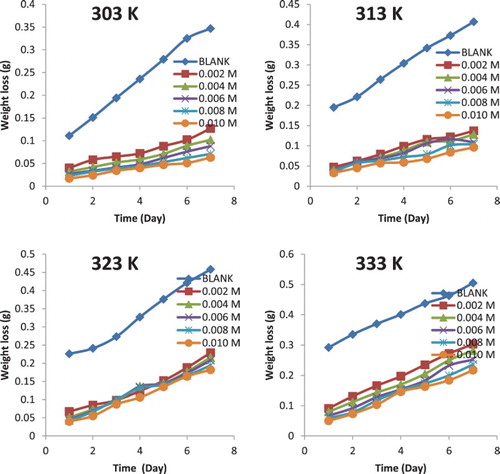
Table 1. Inhibition efficiency of NBA (from weight loss and thermometric measurements) and corrosion rate of mild steel and aluminium metals.
It can also be deduced from the results presented in that NBA is an adsorption inhibitor and functioned through the initial mechanism of physical adsorption because the inhibition efficiency increases with an increase in the concentration while the extent of adsorption decreases with an increase in the temperature, a trend that favours physisorption mechanism [Citation14]. At all temperatures, the inhibition efficiency of NBA for mild steel is better than that of aluminium, indicating that NBA is a better inhibitor for mild steel than aluminium. In the presence of hydrochloric acid, aluminium and mild steel can react to liberate hydrogen gas. However, the reactions maybe slowed down by the formation of hydroxide or oxide (in the case of aluminium) or chloride (in the case of mild steel). The formation of aluminium oxide or hydroxide may retard corrosion attack on the metal as shown in the following equations:
Similarly, mild steel is capable of forming a protective layer of iron according to the following equations [Citation15]:
From the above mechanism, it is evident that before the corrosion of aluminium in solution of HCl can proceed, the oxide or hydroxide protective layer must be dissolved. On the other hand, the chloride ion produced by ionization of hydrochloric acid is capable of acting as an adsorbed layer on the surface of mild steel, thereby protecting the metal to some extent. Hence, HCl seems to play some minor protective role to mild steel while in aluminium, it catalyses corrosion. This explains why the rate of corrosion of aluminium in HCl is higher than the rate of corrosion of mild steel in similar medium [Citation16]. Hence, NBA is a better inhibitor for mild steel in solution of HCl than for aluminium because chloride ion resulting from ionization of HCl partly complements its inhibition action.
3.2. Polarization methods
Results obtained from the linear plot of potential against current and the associated inhibition efficiencies are presented in . It is evident from the presented data that values of polarization resistant increase with an increase in the concentration of NBA, which indicates that NBA retarded the corrosion of mild steel and aluminium in solution of HCl [Citation17]. However, higher Rp values were obtained for mild steel than aluminium, indicating better inhibition efficiency for mild steel, a trend similar to those obtained from gravimetric and gasometric methods.
Table 2. Linear and potentiodynamic polarization parameters for the corrosion of aluminium and mild steel in the absence and presence of NBA at 303 K.
On the other hand, the bases for potentiodynamic polarization study is connected with the Tafel plot arising from the linear relationship between electrode potential and logarithm of the current for a sufficiently polarized electrode in both cathodic and anodic directions. The region in which this relationship exist is called the Tafel region. Potentiodyanmic plots for the corrosion of aluminium and mild steel in solution of HCl are shown in , while data deduced from the plots (corrosion current, corrosion potential and Tafel constants) are recorded in . The results reveal a decrease in corrosion current, Tafel anodic and cathodic constants (for both mild steel and aluminium corrosion) with increasing inhibitor concentration, which suggests that NBA inhibited the corrosion of mild steel and aluminium in solution of HCl. These trends responded favourably to an increase in the inhibition efficiency with increasing concentration of NBA. Comparison of results obtained from linear polarization and potentiodynamic polarization methods yielded correlations coefficients that approximated maximum (i.e. R2≈ 1).
Figure 3. Potentiodynamic polarization curves for the corrosion of (a) mild steel (b) aluminium in 0.1 MHCl in the absence and presence of different concentrations of NBA.
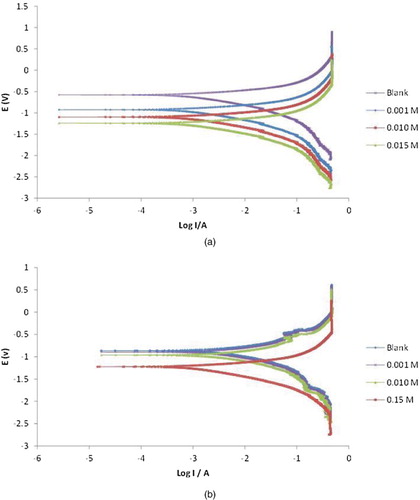
It is also evident from the results presented in that the shift in corrosion potential is less than the threshold value of ± 85 mV, which implies that NBA acted as a mixed type inhibitor, suppressing both anodic dissolution and cathodic hydrogen gas evolution for both aluminium and mild steel [Citation18].
3.3. Effect of temperature
Arrhenius equation is a useful model for evaluating the effect of temperature on the corrosion rate of mild steel and aluminium in HCl solution. The equation relates the corrosion rate (CR) with temperature as follows [Citation19]:
(6)
(6)
where Ea is the corrosion activation energy, A is the pre-exponential constant, R is the gas constant and T is the temperature. Equation (6) is a linear model; therefore, a plot of lnCR against 1/T is a straight line as shown in . Activation parameters deduced from the slope and intercept of the plots are presented in . It is seen from the plots that the activation energy progressively increases as the concentration of the inhibitor increases. The activation energy for the blank was 44.73 J/mol (and 30.97 J/mol for aluminium), but in the presence of the inhibitor, the activation energies ranged from 63.70 to 75.69 J/mol and from 17.77 to 35.69 J/mol for mild steel and aluminium, respectively. Values of activation energy obtained for aluminium in the presence of the inhibitor are seen to be slightly lower than those obtained for mild steel under similar environment. Higher activation energy points towards better resistant to corrosion and vice versa. Therefore, NBA inhibited the corrosion of mild steel in solution of HCl better than aluminium. However, in both cases, the activation energies were lower than 80 kJ/mol, which supports the mechanism of physical adsorption.
Figure 4. Arrhenius plots for the corrosion of mild steel and aluminium in 0.1 M HCl containing various concentrations of NBA.
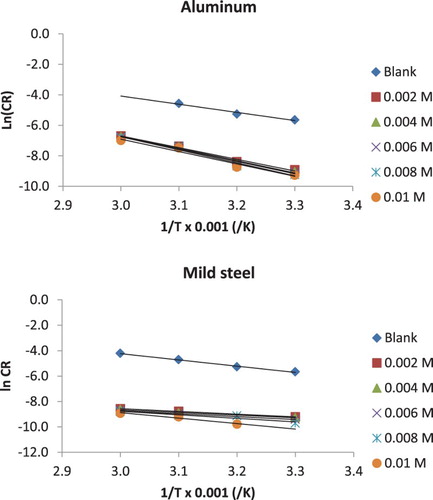
Table 3. Arrhenius and Transition state parameters for the adsorption of NBA on aluminium and mild steel surfaces.
3.4. Thermodynamic/adsorption study
The initial mechanism in any corrosion inhibition process is the adsorption of the inhibitor on the surface of the metal. The adsorption may be facilitated by charge or electron transfer from the inhibitor to the metal surface. Thermodynamic parameters are unique in predicting the direction and spontaneity of corrosion reactions. The cardinal equation in estimating the standard changes in adsorption entropy and enthalpy is the Transition state equation, expressed in Equation (7) [Citation20]:
(7)
(7)
where N is the Avogadro’s number, h is the Plank constant,
is the change in entropy of adsorption,
is the change in enthalpy of adsorption, R is the gas constant and T is the enthalpy. The application of the transition state equation to the inhibition of the corrosion of mild steel and aluminium in solution of HCl by NBA is confirmed by the high degree of linearity displayed by plots of
versus 1/T as shown in . contains values of
and
that were calculated from slopes and intercepts of the plots. It is seen from the presented results that the change in adsorption entropy is generally positive; hence, the corrosion of mild steel and aluminium in the absence and presence of NBA is endothermic. Negative values obtained for changes in entropy confirm that there is association of the inhibitor’s molecules.
Figure 5. Transition state plots for the corrosion of mild steel and aluminium in 0.1 M HCl containing various concentrations of NBA.
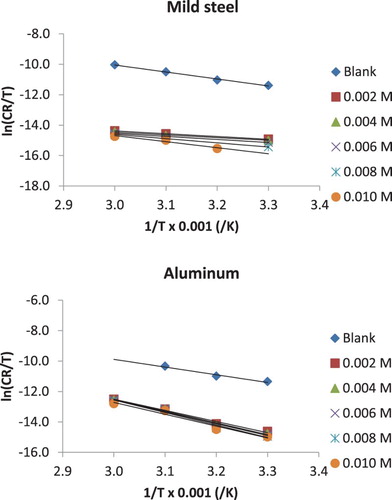
Table 4. Langmuir and Frumkin parameters for the adsorption of NBA on mild steel and aluminium surfaces.
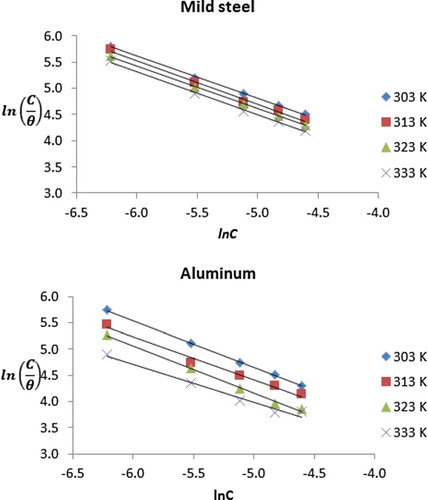
The adsorption characteristics of NBA for the inhibition of the corrosion of mild steel and aluminium were examined through adsorption isotherms, which are models that relate the degree of surface coverage of an inhibitor to its concentration at constant temperature. The best models that fitted the adsorption behaviour of NBA on mild steel and aluminium surfaces were the Langmuir and Frumkin adsorption isotherms. The Langmuir isotherm is documented to be an ideal isotherm for adsorption involving the non-existence of interaction between the adsorbed molecules and can be expressed according to Equation (8) [Citation21]:
(8)
(8)
where θ is the degree of surface coverage, C is the concentration of the inhibitor in the bulk electrolyte, kads is the adsorption desorption constant. presents linear plots of
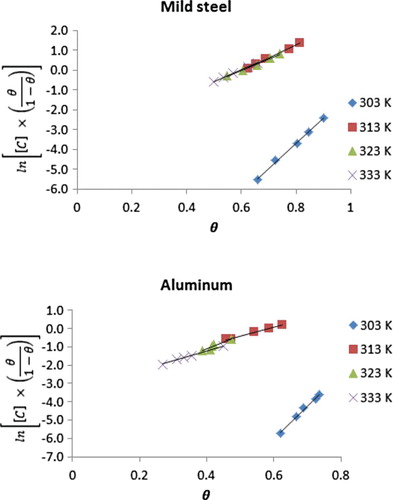
versus lnC according to Equation (8). The degree of linearity and other Lagmuir adsorption parameters are recorded in . R2 values ranged from .9945 to 0.992 and from .9775 to .9998 (for mild steel and aluminium, respectively), which point towards excellent degree of linearity. However, the corresponding slope values ranged from 0.8080 to 0.8368 and from 0.7239 to 0.9018, which deviate from the expected value of unity for an ideal Langmuir isotherm (Equation (7)). Therefore, there is interaction between the inhibitor’s molecules, suggesting the need for an adsorption isotherm that will explain the interaction. Frumkin isotherm (Equation (9)) is a modified type of Langmuir model for situation where molecular interaction exists. According to Murthy and Vijayaragavan [Citation22], the linear form of the Frumkin adsorption equation can be expressed as follows:
(9)
(9)
where Kads is the adsorption–desorption constant, α is the lateral interaction parameter, which describes the adsorption of the inhibitor on the adsorbed layer. Since the above equation is linear, a plot of
versus θ is expected to be linear with slope and intercept equal to
and
, respectively. Frumkin isotherms for the adsorption of NBA on mild steel and aluminium are illustrated in . Adsorption parameters deduced from the Frumkin plots are recorded in . Positive values of calculated lateral interaction parameters reveal that there is attractive behaviour of the inhibitor (NBA) on mild steel and aluminium surfaces.
Table 5. Frequencies and peak of FTIR absorption by NBA and the corrosion product of mild steel and aluminium in the presence of NBA.
Standard free energy of adsorption of NBA on mild steel and aluminium surfaces was calculated from the Gibb Helmholtz equation (Equation (10)) which relates the equilibrium constant of adsorption (i.e. 55.5kads) to the free energy change [Citation23].
(10)
(10)
Calculated free energy values (which were recorded in ) are negative and are within the range of values that support the mechanism of physical adsorption which involves charge transfer from charged inhibitor to charged metal surface [Citation24].
values up to −20 kJ/mol or less are consistent with physisorption mechanism, while
up to −40 kJ/mol and more are consistent with chemisorption mechanism [Citation25].
3.5. FTIR study
presents peaks and frequencies of FTIR absorption by NBA and the corrosion products of mild steel and aluminium inhibited by NBA, respectively. Prominent functional groups identified in the FTIR spectrum of NBA included OH stretch at 3516 cm−1, C–H aromatic stretch at 2952 cm−1, C–H stretch at 2858 cm−1, carbonyl vibration at 1955 cm−1, C = O stretch at 1746 cm−1, C–C stretches in ring at 1589 and 1454 cm−1, C–H rocking vibration at 1372 cm−1, N–O symmetric stretch at 1289 cm−1, C–N stretch at 1128 cm−1 and C–O stretch at 1072 cm−1. However, when NBA was used for the inhibition of the corrosion of mild steel and aluminium in solution of HCl, some functional groups were shifted to the right or left, some were missing while some new functional groups were formed as shown in . Shift in frequency of IR absorption suggests the existent of interaction between the inhibitor and the respective metal surface while missing functional groups represent those trapped in the adsorption of the inhibitor onto the metal surface. Of significant interest is the disappearance of several missing functional groups in the corrosion product of mild steel than that of aluminium. This suggest that adsorption of NBA unto mild steel surface is more oriented and stronger than its adsorption unto aluminium surface. Hence NBA is a better corrosion inhibitor for mild steel than aluminium as observed in this work.
Table 6. Condensed Fukui indices of NBA calculated from DFT and Ab initio levels of theory.
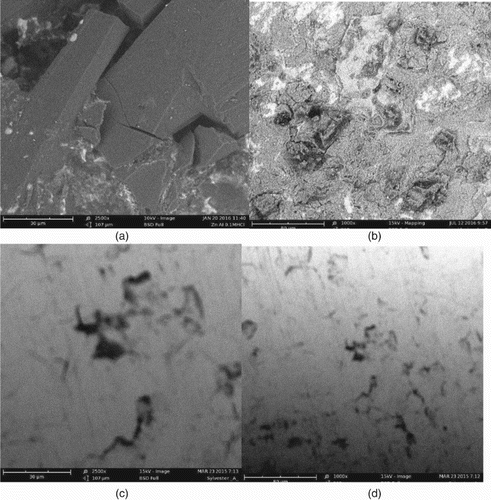
3.6. Scanning electron microscopy
Scanning electron micrographs of mild steel and aluminium surfaces before and after inhibition are shown in . It is clearly seen from the micrographs that in the absence of NBA as an inhibitor, crevices and pitting corrosion had set into the respective surfaces of the metal. However, the use of NBA greatly improves the surfaces of the metals through the formation of protective film that prevented corrosion.
3.7. Computational chemistry study
Quantum chemical descriptors calculated for NBA molecule using PM7 Hamiltonian in the MOPAC computational software included the total energy of the molecule (−2291.54 eV), electronic energy (−10569.73 eV), core–core repulsion energy (8278.19 eV), cosmo area (177.26 Å2), cosmo volume (173.43 Å3), energy of the highest occupied molecular orbital (−11.350 eV) and energy of the lowest unoccupied molecular orbital (−1.875 eV). These values are within the range of values reported for some excellent corrosion inhibitors [Citation26–29]. These descriptors are of fundamental significance in describing the readiness of a molecular specie towards reactivity/adsorption and hence corrosion inhibition. The frontier molecular orbital energies (i.e. energy of the highest occupied molecular orbital, EHOMO and the energy of the lowest unoccupied molecular orbital, ELUMO) are useful indices for predicting the direction of corrosion inhibition through their ability to predict the tendency towards the donation (EHOMO) and acceptance (ELUMO) of electron. The difference between ELUMO and EHOMO represents the energy gap of a molecule, a quantum index which describes the energy barrier that must be overcome before an electron moves from the HOMO level to the LUMO level. Generally, increasing value of EHOMO and decreasing ELUMO and ΔE point towards better reactivity, adsorption and inhibition [Citation30]. Total energy and electronic energy are also quantum parameters that are useful in predicting molecular reactivity. The more negative the value of the electronic energy, the more reactive the molecule is expected. On the other hand, corrosion inhibition efficiency often shows positive correlation with the total molecule energy of the molecule [Citation31].
Unlike global selectivity which approaches molecular reactivity based on the contribution of the entire molecule, local selectivity projects that the reactivity of a molecular specie lies in the reactive centre or atom in the molecule. Therefore, the tendency for a molecule to donate or accept electron is of essence in local selectivity analysis. The fundamental model in analysing local selectivity is the Fukui function. The Fukui function is based on the fact that if an N-electron system gains electron, it becomes N + 1-electron system, but N-1-electron system if it loses electron. Based on this concept, three types of Fukui functions are known. These are the electrophilic Fukui function (f−) when it loses electron, neuclophilic Fukui function (f+) when it gains electron and the Fukui function for the radical (fo) [Citation32]. According to Eddy and Ita [Citation33], the finite difference approximation defines condensed Fukui functions as follows:
(11)
(11)
(12)
(12)
where
,
and
are the Mulliken or Heirsfield charges in the N, N-1 and N + 1 electron systems, respectively. Calculated values of Mulliken charge and condensed electrophilic and neuclophilic Fukui functions (from DFT and Ab initio methods) are recorded in . The site with the highest positive value of
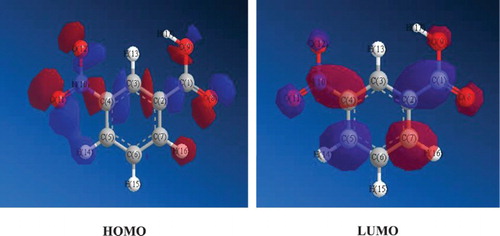
is the preferred site for electrophilic attack and this is resident in the nitro group of the NBA molecule while the preferred site for neuclophilic attack is in alkene carbon (C2) of the NBA aromatic ring. In , the HOMO and LUMO diagrams of NBA are shown. The electrophilic Fukui function corresponds to the HOMO while the neuclophilic Fukui function corresponds to the LUMO. Electron is expected to move from the HOMO to the LUMO during adsorption or bond formation. The diagrams evidently reveal that the density of the positive charges (red) concentrates more on the two nitro oxygen atoms of the NBA molecule, which is in agreement with the Fukui function analysis.
3.8. Mechanism of inhibition
The initial mechanism in any corrosion inhibition is the adsorption of the inhibitor on the surface of the metal. From experimental results, NBA is adsorbed on mild steel and aluminium surfaces by physical adsorption mechanism, which involves charge transfer from charged inhibitor to the charged metal surface. Interestingly, the inhibitor is a charged molecule with strong inductive and extremely strong resonance effect because of the position the carboxylic and nitro functional groups occupy in the benzene ring. The presence of nitro group in the ring strongly increases delocalization of electron and makes it to be more reactive. Therefore, although a lone pair of electron resides in the carbonyl oxygen atom (O9), the inhibitor is preferentially adsorbed onto the metal surface through the nitro functional group. However, the participation of the carbonyl group cannot be completely ruled out because the engagement of multiple adsorption layer (consequence of physical adsorption) is evident in the FTIR spectra of the corrosion products.
4. Conclusions
The results and findings of this study lead to the following conclusions:
NBA is a good adsorption inhibitor for mild steel and aluminium corrosion, having better efficiency for mild steel than aluminium.
The adsorption of the inhibitor on the respective metal surface is exothermic because enthalpy changes are negative. The adsorption is also spontaneous and proceeds through physisorption mechanism because it is characterized by negative values of Gibb free energy change which are also negatively less than the threshold value of – 40 kJ/mol.
Lateral interaction exists between the adsorbed inhibitor molecules and is reflected by the attractive behaviour of the inhibitor.
NBA is adsorbed to mild steel and aluminium surfaces through some of its functional groups, notably the nitro group.
Computational chemistry calculations reveal results that provided a linkage between microscopic and macroscopic behaviour of the inhibitor, i.e. linking experimental and theoretical result.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Nsikak Bassey Essien http://orcid.org/0000-0002-6664-3274
References
- Prasanna BM, Praven BM, Hebbar N, et al. Electrochemical study effect of asprin on mild steel in 1 M hydrochloric acid. J Assoc Arab Univ Basic Appl Sci. 2017;22:62–69.
- Karthik G, Sundaravadivelu M. Studies on the inhibition of mild steel corrosion in hydrochloric acid solution by atenolol drug. Egypt J Petr. 2016;25(2):183–191. doi: 10.1016/j.ejpe.2015.04.003
- Karthikaiselvi R, Subhasini S. Study of adsorption properties and inhibition of mild steel corrosion in hydrochloric acid media by water soluble composite polyvinyl alcohol-o-methoxy aniline). J Assoc Arab Univ Basic Appl Sci. 2014;16:74–82.
- AdejoroF IA, OjoS K, Obafemi K. Corrosion inhibition potentials of ampicillin for mild steel in hydrochloric acid solution. J Tabah Univ Sci. 2015;9:196–202. doi: 10.1016/j.jtusci.2014.10.002
- Thirumalairaj B, Jaganathan M. Corrosion protection of mild steel by new binary inhibitor system in hydrochloric acid solution. Egypt J Petr. 2016;25:423–432. doi: 10.1016/j.ejpe.2015.09.002
- Haque J, Srivastava V, Verma C, et al. Experimental and quantum chemical analysis of 2-amino-3-((4-((S)-2-amino-2-carboxyethyl)-1H imidazole-2-yl)thio) propionic acid as new and green corrosion inhibitor for mild steel in 1 M hydrochloric acid solution. J Mol Liq. 2017;225:848–855. doi: 10.1016/j.molliq.2016.11.011
- Gupta RK, Malviya M, Verma C, et al. Aminoazobenzene and diaminoazobenzene funtionalized grapheme oxides as novel class of corrosion inhibitors for mild steel: experimental and DFT studies. Mat Chem Phys. 2017;198:360–373. doi: 10.1016/j.matchemphys.2017.06.030
- Pandey A, Verma C, Singh B, et al. Synthesis, characterization and corrosion inhibition properties of benzamide-2-chloro-4-nitrobenzoic acid and anthranilic acid-2-chloro-4-nitrobenzoic acid for mild steel corrosion in acidic medium. J Mol Struct. 2018;1155:110–122. doi: 10.1016/j.molstruc.2017.10.114
- Muralidharan S, Krishnamoorthy S. Benzoic acids as corrosion inhibitors for pure ion in sulphuric acid. Corr Sci Eng. 1988;4(8):705–710.
- Dinnappa RK, Mayanna SM. Benzoic acid and substituted benzoic acids as interfacial corrosion inhibitors for copper HClO4. J Appl Electrochem. 1981;11(1):111–116. doi: 10.1007/BF00615329
- Ameh PO, Eddy NO. Theoretical and experimental studies on the corrosion inhibition potentials of 3-nitrobenzoic acid for mild steel in 0.1 M H2SO4. Cogent Chem. 2016;2(2016):1253904.
- Ituen E, Akaranta O, James A. Electrochemical and anticorrosion properties of 5-hydroxytryptophan on mild steel in a simulated well-acidizing fluid. J Talibah Univ Sci. 2017;11(5):788–800. doi: 10.1016/j.jtusci.2017.01.005
- Vengatesh G, Karthik G, Sundaravadivelu M. A comprehensive study of ondansetron hydrochloride drug as a green corrosion inhibitor for mild steel in 1 M HCl medium. Eqypt J Petr. 2017;26:705–719. doi: 10.1016/j.ejpe.2016.10.011
- Loto RT, Loto CA, Joseph O, et al. Adsorption and corrosion inhibition properties of thiocarbanilide on the electrochemical behavior of high carbon steel in dilute acid solutions. Results Phys. 2016;6:305–314. doi: 10.1016/j.rinp.2016.05.013
- Noor EA, Moubaraki AU. Corrosion behavior of mild steel in HCl solution. Int J Electrochem Sci. 2008;3:806–816.
- Choe H, Lee HS. Experimental study on the electrochemical anti-corrosion properties of steel structures applying the arc thermal metal spraying method. Materials. 2014;7(12):7722–7736. doi: 10.3390/ma7127722
- Eddy NO, Momoh-Yahaya H, Oguzie EE. Theoretical and experimental studies on the corrosion inhibition potentials of some purines for aluminum in 0.1 M HCl. J Adv Res. 2015;6:203–216. doi: 10.1016/j.jare.2014.01.004
- Kumari PP, Shetty P, Rao SA. Electrochemical measurements for the corrosion inhibition of mild steel in 1 M hydrochloric acid by using aromatic hydrazide derivative. Arab J Chem. 2017;10:653–663. doi: 10.1016/j.arabjc.2014.09.005
- Abd El Rehim SS, Sayyah SM, El-Deeb MM, et al. Adsorption and corrosion inhibitive properties of P(2-aminobenzothiazole) on mild steel in hydrochloric acid media. Int J Ind Chem. 2016;7:39–52. doi: 10.1007/s40090-015-0065-5
- Yadav DK, Maiti B, Quraishi MA. Electrochemical and quantum chemical studies of 3,4-dihydropyrimidin-2(1H)-ones as corrosion inhibitors for mild steel in hydrochloric acid solution. Corr Sci. 2010;52(11):3586–3598. doi: 10.1016/j.corsci.2010.06.030
- Obot IB, Madhankumar A. Enhanced corrosion inhibition effect of tannic acid in the presence of gallic acid at mild steel/HCl acid solution. J Ind Eng Chem. 2016;26:106–111.
- Murthy ZVP, Vijayaragavan K. Mild steel corrosion inhibition by acid extract of leaves of hibiscus sabdariffa as a green corrosion inhibitor and sorption behavior. Green Chem Lett Rev. 2004;7(3):209–219. doi: 10.1080/17518253.2014.924592
- Negm NA, Elkholy YM, Zahran MK, et al. Corrosion inhibition efficiency and surface activity of benzothiazol-3-ium cationic schiff base derivatives in hydrochloric acid. Corr Sci. 2010;52(10):3623–3536. doi: 10.1016/j.corsci.2010.07.001
- Benabid S, Douadi T, Issadi S, et al. Electrochemical and DFT studies of a new synthesized schiff base as corrosion inhibitor in 1 M HCl. Measurement (Mahwah N J). 2017;99:53–63.
- Jacob KS, Parameswaran G. Corrosion inhibition of mild steel in hydrochloric acid solution by scjiff base furionthiosemicarbazone. Corr. Sci. 2010;52(1):224–228. doi: 10.1016/j.corsci.2009.09.007
- Finsgar M, Lesar A, Kokalj A, et al. A comparative electrochemical and quantum chemical calculations study of BTAH and BTAOH as copper corrosion inhibitors in near neutral chloride solution. Electrochim Acta. 2008;53:8287–8297. doi: 10.1016/j.electacta.2008.06.061
- Issa RM, Awad MK, Atlam FM. Quantum chemical studies on the inhibition of the corrosion of copper surface by substituted uracils. Appl. Surf. Sci. 2008;5(1):2433–2441. doi: 10.1016/j.apsusc.2008.07.155
- Mashuga ME, Olasunkanmi LO, Ebenso EE. Experimental and theoretical investigation of the inhibitory effect of new pyridazine derivatives for the corrosion of mild steel in 1 M HCl. J. Mol. Struct. 2017;1136:127–139. doi: 10.1016/j.molstruc.2017.02.002
- Sun S, Geng Y, Tian L, et al. Density functional theory study of imidazole, benzimidazole and 2-mercaptobenzimidazole adsorption onto clean Cu(III) surface. Corr. Sci. 2012;53:140–147. doi: 10.1016/j.corsci.2012.05.024
- Eddy NO. Theoretical study on some amino acids and their potential activity as corrosion inhibitors for mild steel in HCl. Mol. Simul. 2010;35(5):354–363. doi: 10.1080/08927020903483270
- Belfilali AC, Hammouti B, Aouniti A, et al. Quantum chemical study of inhibition of the corrosion of mild steel in 1 M hydrochloric acid solution by newly synthesized benzamide derivatives. Res Chem Interm. 2014;40(3):1069–1088. doi: 10.1007/s11164-013-1022-6
- Abdel-Azim AA, Milad R, El-Ghazawy R, et al. Corrosion inhibition efficiency of water soluble ethoylatedrimethyl propane by gravimetric analysis. Egypt J Petr. 2014;23(1):15–20. doi: 10.1016/j.ejpe.2014.02.003
- Eddy NO, Ita BI. Experimental and theoretical studies on the inhibition potentials of some derivatives of cyclopenta-1,3-diene. Int J Quant Chem. 2011;111(14):3456–3473.