?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In this study, poly(vinyl alcohol) (PVA)-modified Saudi bentonite nanocomposite films were prepared using the solution method. Two surfactants, cetylpyridinium chloride and tetrabutyl phosphonium bromide, were used to modify the clay after the ion-exchange process by Na+ ions. The effect of the addition of modified Saudi bentonite (2.5%) on the thermal and flammability properties of the nanocomposite films was investigated. The prepared samples were characterized using Fourier-transform infrared spectroscopy, X-ray diffraction, and scanning electron microscopy. Thermogravimetric analysis and differential scanning calorimetry were carried out to observe the thermal behaviour of the PVA-based nanocomposite films. Vertical flammability tests (UL94 VB) were also carried out to study the flame retardancy behaviour of the nanocomposite films. The nanocomposite films showed better flammability than pure PVA, wherein the ignition time of the nanocomposites increased and flame propagation rate decreased. The thermal stability of the nanocomposites increased by approximately 10% for the onset and endset temperature of decomposition. The type of surfactant played an important role in dispersion of the clay into the polymeric matrix and enhancing the flammability properties of the films wherein the tetrabutyl phosphonium bromide was better flame retardant than cetylpyridinium chloride.
1. Introduction
Over the past few decades, the polymer industry has developed rapidly owing to the extensive use of polymers for a wide range of applications. As a result, the development of novel polymeric materials such as polymer nanocomposites with unique properties has gained immense attention. Such composites are prepared by incorporating nanoparticle fillers into polymer matrices. An important feature of polymer nanocomposites is heat resistance. Hence, in order to realize their practical applications, it is imperative to investigate their thermal stability and flammability. Polymers are flammable, i.e. they can be easily ignited by external heat. Their combustion produces smoke and toxic gases. Hence, many efforts have been made to reduce the flammability of polymers and decrease the emission of toxic gases during combustion.
Poly(vinyl alcohol) (PVA) is one of the most widely used polymers. It is water soluble, nontoxic, hydrophilic, and biocompatible [Citation1], and hence is widely used as a matrix material. PVA/clay composites have been extensively studied for various applications [Citation1–5]. Therefore, the use of PVA as a polymeric matrix in this study is quite attractive. The Kingdom of Saudi Arabia has various types of clay mineral deposits distributed across regions of the country [Citation6]. Bentonite clay deposits are found in the western region of the kingdom [Citation6]. This kind of clay has a wide range of applications in various technology areas including nanocomposite. Bentonite is used in polymer nanocomposites as a filler. It has a layered structure, which makes it suitable for preparing polymer nanocomposites [Citation7]. It can be expanded to intercalate polymer chains, thus is useful in fabricating intercalated nanocomposites [Citation7]. In addition, the layers can even be separated completely and dispersed randomly into a polymer matrix to prepare exfoliation nanocomposites [Citation7]. Therefore, bentonite can be an important economic resource for Saudi Arabia.
Although several studies have been published on polymer/bentonite nanocomposites, the Saudi bentonite needs to be studied further to gain more information about its performance in the applied field. Since the Saudi bentonite is found to be Ca-bentonite, it is not suitable because of its low expansion potential [Citation8]. Therefore, the Na+-modification process of the clay surface is a necessary step. This research aims at Na+-modification of the surface of the Saudi bentonite by the ion-exchange reaction, followed by its further modification by two types of surfactants to improve the dispersion of Saudi bentonite into a polymeric matrix. Afterwards, these modified forms of bentonite can be used as a polymer reinforcement filler to prepare and characterize PVA/bentonite nanocomposite. Studying the thermal and flammability properties of the prepared nanocomposites to gain further information as to the effect of the 2.5 wt.% modified Saudi bentonite on the final properties of the polymer is a second objective. Finally, a comparison of the results of the prepared nanocomposites is required to show how surfactant materials could affect the performance of Saudi bentonite clay as a filler.
2. Materials and methods
2.1. Materials
PVA was purchased from LOBA Chemie and used as a polymeric matrix. Two different cationic surfactants were used to modify the surface of the filler: cetylpyridinium chloride (C21H38ClN; molecular weight = 358.01 g mol−1; 99% pure; BDH Co.) and tetrabutyl phosphonium bromide (C16H36BrP; molecular weight = 339.33 g mol−1; 99% pure). They were labelled as S1 and S2, respectively.
The filler was bentonite. It was locally collected from Khulais, Saudi Arabia, and had the following composition [Citation9]: 35.22% montmorillonite 35.22%, 13.33% kaolinite, 22.8% mica, 8.57% quartz, 6.66% feldspar, 5.714% ilmenite, 3.81% dolomite, and 3.81% gypsum and calcite. Other chemicals were used for the process: sodium chloride extra pure (NaCl; 58.44 g mol−1; purity of 97.5%; BDH Co.), and silver nitrate (AgNO3; 169.87 g mol−1; BDH Co.). Table summarizes all the materials and prepared samples with their codes.
Table 1. Definition of the used materials and the prepared sample cods.
2.2. Na+-modification
The separation process was carried out by dispersing 100 g of bentonite in 1 L of freshly deionized water. The resulting mixture was sonicated at room temperature and then saturated via cationic exchange by dispersing in 500 mL of a 0.5 mol L−1 NaCl solution. This mixture was shaken for 24 h and the clear supernatant was discarded. This procedure was repeated at least five times. The product so obtained was then washed with distilled water by centrifuging until the AgNO3 test for chloride showed negative. The Na+-saturated bentonite was then dried at 120°C for 1 h and pulverized using a pestle mortar. It was labelled as NaB.
2.3. Organo modification
The surface of NaB was modified using two different cationic surfactants: S1 and S2.
The surfactant aqueous solutions were prepared by dissolving 1.24 g of S1 and 1.69 g of S2 in 100 mL of distilled water.
Surface modification of bentonite was carried out by first magnetically stirring 5 g of bentonite in 500 mL of distilled water for 24 h at room temperature. The surfactant solution was then added to this mixture and the resulting mixture was stirred for 12 h. Then, the clay was filtered washed with distilled water until no chloride or bromide ion was detected with 0.1 N AgNO3. The modified clay was then dried at 60°C for 24 h under vacuum and pulverized using a pestle mortar to obtain a fine powder. The clay samples modified with S1 and S2 were labelled as SB1 and SB2, respectively.
2.4. Preparation of nanocomposite films
To prepare the PVA nanocomposite, first, 2 g of PVA was dissolved in 100 mL of distilled water and the mixture was magnetically stirred at 90°C for 24 h until a homogenous solution was obtained. To this solution, 2.5 wt.% bentonite (modified (SB1 and SB2) and Na+-saturated (NaB)) was added and the reaction mixture was stirred for another 3 h at 90°C. The mixture obtained after stirring was transferred to a 100 mm × 20 mm Petri dish and was left for three days to dry (evaporate the solvent completely) under ambient conditions. The samples so obtained were labelled PVA/SB1, PVA/SB2, and PVA/NaB.
2.5. Characterization
Fourier-transform infrared (FTIR) spectroscopy (1000, Perkin Elmer) over the frequency range of 4000–400 cm−1 to observe the effect of surface modification on the structure of bentonite. The crystal structure of the samples was examined by X-ray diffraction (XRD) using a (PRO X-ray diffractometer) made in Holland. A wavelength of 1.54 Å was used and the source was operated at a generator tension of 40 kV and a generator current of 40 mA. Scanning electron microscopy (SEM) (JEOL JSM-6360LV) was used to examine the morphology of the samples. The thermal properties of the samples were investigated by differential scanning calorimetery (DSC, Q2000 V24.4 Build 116) to determine the glass transition temperature (Tg) of the samples. The DSC experiments were carried out in a nitrogen atmosphere. The samples (about 5 mg) were pressed in aluminium pans and were heated from ambient temperature to 200°C at a heating rate of 10°C min−1 under the flow of nitrogen. The Tg of the samples were determined from their second heating runs. Thermogravimetric analysis (TGA, TA Instrument Auto (TGA) 2950 V5.4A) was also carried out to evaluate the thermal stability of the samples. The samples (∼10–15 mg) were heated in an open platinum crucible from room temperature to 650°C under a high-purity nitrogen atmosphere at a heating rate of 10°C min−1. In order to investigate the flammability properties of the samples, vertical flammability test standardized as UL94 VB [Citation10] was applied. The samples (79 mm × 15 mm × 2 mm) were burned vertically. The flammability values reported here are the averages of five samples. The ignition time was recorded as the time between the initial flame supply from the heat source and the appearance of flame on the sample. The samples were placed on a retort stand over the heat source at a fixed distance. The rate of fire spread was recorded as the flame propagation rate (FPR). The test samples were marked with “X” at a distance of 3 cm from the end just above the flame source. The flame propagation time (FPT) was recorded as the time between the initial flame supply and the combustion of the X mark. The FPR was calculated as the ratio of the distance between the “X” mark on the samples and the flame source and the FPT.
(1)
(1)
3. Results and discussion
3.1. Characterization
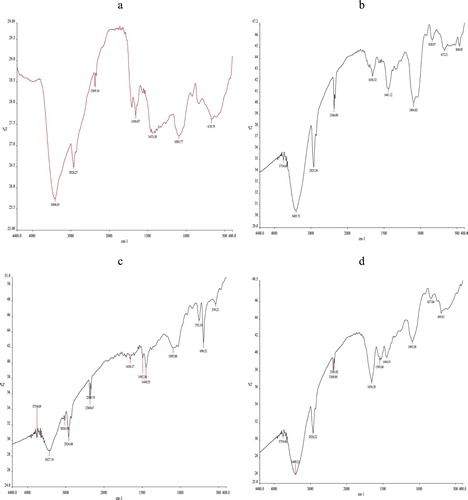
Figure shows the FTIR spectra of PVA and the nanocomposites. PVA showed peaks corresponding to hydroxyl groups (–OH) (at about 3414 cm−1), (–CH2–) asymmetric and symmetric stretching (2926 and 2859 cm−1, respectively), C=O stretching (1656 cm−1), (C–H) bending (1433 cm−1), (C–O) stretching (1095cm−1), C–H rocking (838 cm−1), and angular deformation outside the plane of the (C–H) bond (670 cm−1) [Citation11,Citation12]. The nanocomposites showed shifted peaks towards higher wave number, suggesting change the bonds in PVA chains probably because of the bonding between the PVA chains and bentonite. Moreover, they showed important changes at 3750 cm−1 – a small peak in structural hydroxyl stretching (–OH) region – and at 900–400 cm−1. The former change probably is assigned to the OH in clay. The latter change is attributed to Si–O–Al and Si–O–Si bending in bentonite [Citation13] (Table and Figure ).
Figure 2. Schematic presentation of the interaction between the polymer and clay ([Citation26] cited in [Citation14]).
![Figure 2. Schematic presentation of the interaction between the polymer and clay ([Citation26] cited in [Citation14]).](/cms/asset/de3a8fbb-281b-4675-976c-a4d6af3b2883/tusc_a_1579295_f0002_ob.jpg)
Table 2. Spectral data of PVA and its nanocomposites.
Figure shows the XRD patterns of clay (Figure (a–c)), pristine PVA (Figure (d)), and PVA/clay nanocomposites (Figure (e–g)). The bentonite sample saturated by Na ions (Figure (a)) showed an interlayer spacing (d001) of 13.9 Å (2θ = 7.5°). After surface modification, its interlayer spacing increased to 29.9 Å (2θ = 2.5°) and 38.5 Å (2θ = 1.5°) for SB1 and SB2 (Figure (b,c)), respectively. SB2 sample showed a larger interlayer spacing than SB1. This can be attributed to the presence of butyl groups, which stretch the stacks of bentonite. Because to its large interlayer spacing, of SB2 may formed an exfoliated-intercalated nanocomposite. In contrast, SB1 formed aggregates because of its small interlayer spacing. Hence, the intercalation process of SB2 was better than that of SB1. This suggests that the modification process with S2 is more efficient than that with S1 for bentonite.
Figure 3. XRD patterns of bentonite and the nanocomposites; (a) NaB, (b) SB1, (c) SB2, (d) PVA, (e) PVA/NaB, (f) PVA/SB1, and (g) PVA/SB2.
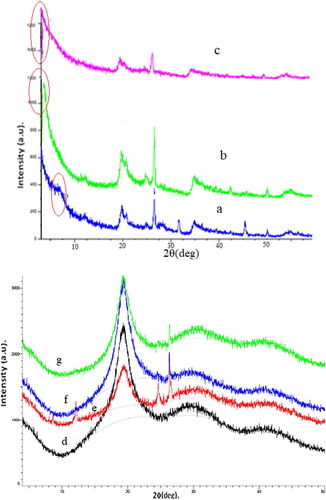
PVA is a semi-crystalline polymer and its crystallinity is affected by the synthesis conditions ([Citation15,Citation16] cited in [Citation17]). Pure PVA exhibited amorphous features and a broad peak at around 2θ = 20°, owing to its semi-crystalline nature (Figure (d)) ([Citation12], [Citation18,Citation19] cited in [Citation12]). Significant changes were observed in the XRD patterns of the nanocomposite samples. PVA/NaB showed a small sharp peak at 2θ = 7.5°, owing to the interlayer spacing of bentonite (Figure (e)). In this nanocomposite, the clusters of bentonite layers were not separated by the PVA chain. This peak was not observed in the PVA/SB1 and PVA/SB2 nanocomposites (Figure (f,g)), indicating that the PVA chains were successfully intercalated between the layers of modified bentonite.
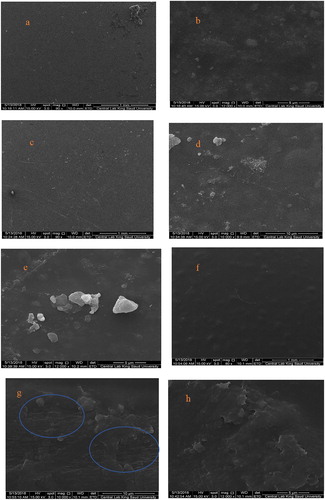
Figure shows the SEM images of the samples. The SEM images were captured at low and high magnifications to examine the surface and morphology of the samples. At small magnifications (Figure (a,c,f), the surface of the samples appeared rough because of the incorporation of bentonite particles. At large magnifications, aggregations of bentonite particles are clearly observed in the PVA/NaB (Figure (b)) and PVA/SB1 nanocomposites (Figure (d,e)). Meanwhile, these aggregations disappeared in PVA/SB2, and tortuous lines were observed (Figure (g,h); blue circles), which were probably caused by intercalation of PVA into the layers of bentonite, indicating that S2 is an efficient surface modifier to prepare exfoliated-intercalated nanostructures. This suggests that the S2 as a modifier contributes to better dispersion of bentonite particles throughout the polymeric matrix and retards their coalescence, thus improving the interfacial adhesion between the clay and PVA chains. This is consistent with the XRD results, which indicate better intercalation for PVA/SB2 samples compared with others.
3.2. Analysis of thermal and flammability properties
Figure shows the DSC curves of the samples over the temperature range of 20–200°C. These curves were used to determine the glass transition (Tg) of PVA, PVA/NaB and PVA/SB1 samples.
Figure 5. DSC curves of PVA and its nanocomposites; (a) PVA, (b) PVA/NaB, (c) PVA/SB1 and (d) PVA/SB2.
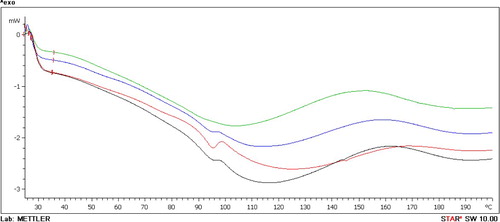
The curves showed two endothermic peaks. The first peak corresponded to Tg followed by the second broad peak, which corresponds to the evaporation of bound water [Citation20].
The Tg of PVA is 96°C, and this is consistent with the previously reported results (it was recorded at 96.3°C) ([Citation21] cited in [Citation22]), and it shifted after the addition of bentonite to 95°C for PVA/NaB with a clear peak and to approximately 94°C with a weak peak of PVA/SB, whereas the peak of Tg was not observed in the DSC curve of PVA/SB2. Strawhecker and Manias [Citation23] reported that “the fully intercalated PVA hybrids [i.e. all the polymer is intercalated in MMT galleries] DSC does not detect any traces of thermal transitions between 35 and 250°C” and for “‘neatly intercalated’ nanocomposites, both the Tg and Tm are too weak and/or too broad to measure, or they are suppressed due to the polymer confinement” [Citation23]. This result agrees with the XRD result, which showed better intercalation of PVA into BS2 layers compared with other samples.
Figure shows the TGA and DrTG curves of PVA and its nanocomposites. TGA was carried out to investigate the thermal stability of the nanocomposites. The thermal decomposition (attributing to the decomposition of the polymer structure) of all the samples occurred in three stages (Table ). The first stage correlated to evaporation of water. It occurs between about 100°C and 240°C, and the weight loss was about 5%.
Figure 6. TGA and DrTG thermograms of PVA and its nanocomposites; (a) PVA, (b) PVA/NaB, (c) PVA/SB1 and (d) PVA/SB2.
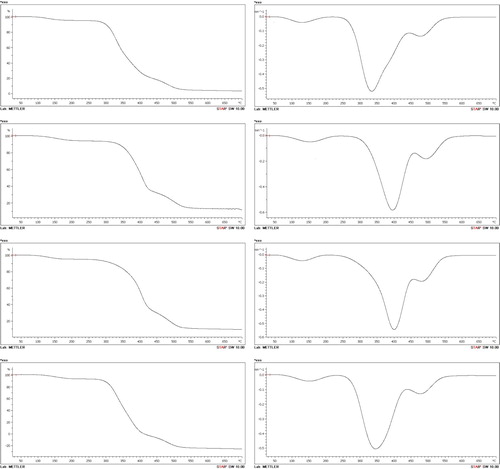
Table 3. TGA data of PVA and its composites.
In the case of PVA, the second stage began at 280°C and ended at 380°C. The third stage initiated at 380°C and ended at 500°C. The second stage can be attributed to the decomposition of the side chain (–OH) of PVA [Citation12,Citation22], and the weight loss was about 75% with a strong peak in the DrTG curve. On the other hand, the third stage can be attributed to the decomposition of the main chain (C–C backbone) of PVA [Citation12,Citation22], and the weight loss was about 15%. The nanocomposite samples showed higher onset temperatures (by 10–20°C) for both the second and third stages, as shown in Table . This suggests that the addition of modified clay improved the thermal stability of the nanocomposites. In addition, the weight loss corresponding to the second step was significantly lower in the nanocomposite samples than that in pure PVA. The improved thermal stability of the nanocomposite samples can be attributed to the barrier effect of the bentonite nanolayers, which improved the heat resistance of the nanocomposites and reduced the permeability of the products of volatile degradation [Citation24]. Chang et al. reported that the incorporation of clay improves the thermal stability of polymer/clay composites [Citation24]. However, the performance of PVA/SB2 seemed to be better than that of the other samples, and this, according to Xie et al. was “because the thermal stability of exchanged MMT is related to the thermal stability of the parent salts, the much higher initial decomposition temperature of the parent phosphonium salts compared to the ammonium salts compensates for the loss due to the influence of the aluminosilicate” [Citation25], where MMT is montmorillonite. The results suggest the efficient intercalation of PVA into the layers of clay, and this seems to agree with the XRD and SEM results, which showed better intercalation and dispersion of BS2 particles throughout the polymeric matrix in the PVA/SB2 sample compared with the others.
Figure shows a photograph of the flammability test of pure PVA and one of its nanocomposites (PVA/SB1) after 10 s of initial burning. The flammability test results revealed that pure PVA (Figure (a)) a continuous drip of the film, higher smoke emission, and a larger flame height than the PVA nanocomposites (Figure (b)), meanwhile, no dripping melt or smoking was notable for all the nanocomposites samples (Table ).
Figure 7. Photographs of (A) the PVA and (B) PVA/SB1 nanocomposite after 10 s from initial burning of the flammability test.
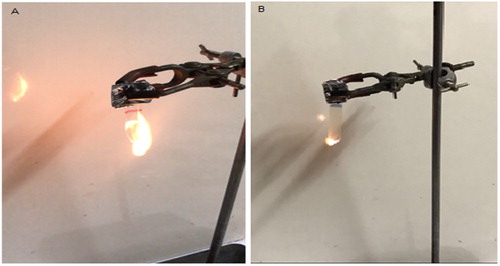
Table 4. Flammability test observations of PVA and its nanocmposites.
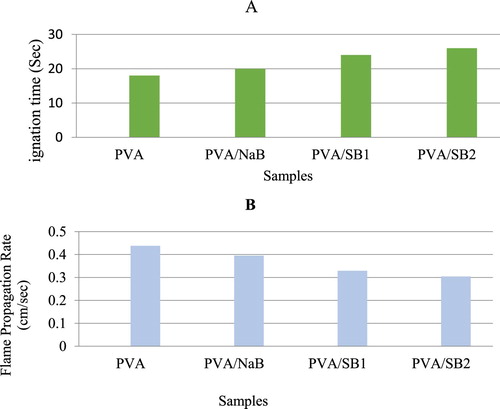
Figure shows the ignition time (a) and FPR (b) of pure PVA and its nanocomposites. The results showed that the ignition time increased after the addition of clay. The ignition time of the samples increased in the order: PVA/SB2 > PVA/SB1 > PVA/NaB. In contrast, the FPR of the samples decreased after the addition of clay: PVA/SB2 > PVA/SB1 > PVA/NaB. This is because the surface modification of clay by the surfactants facilitated the separation of the layers of clay, thus leading to a high diffusion of PVA between the bentonite layers. The bentonite layers formed an effective diffusion barrier, thus hindering the diffusion of the gases formed during the thermal decomposition of the polymer by creating a tortuous path that retards the diffusion of gas through the PVA matrix and reduction of FPR, and the increase in the tortuosity degree increases the degree of flame retardancy and reduction of FPR. The increase in the tortuosity degree depends on the dispersion degree of bentonite platelets through the polymeric matrix. The higher the increase of clay dispersion, the higher the increase in tortuosity; subsequently, the shielding effect of the bentonite layers wherein the platelets of clay can block gas molecule diffusion through a long, tortuous path increases compared with stacking platelets that have a short, straight path. Therefore, the ignition time of PVA/SB2 was higher than those of PVA/SB1 and PVA/NaB, and its FPR was lower than that of the others.
From the results discussed above, how the modification by surfactants affects the performance of Saudi bentonite clay as a filler can be summarized. Overall, they help in the expansion of the distance between the bentonite layers to facilitate the intercalation of PVA chains into the layers. The modification process of bentonite by surfactants was carried out under the same conditions with a difference between the performance of BS1 and BS2f as fillers, and this indicates that the type of surfactant is an effective factor. In S2 surfactant, the presence of four butyl groups of tetrabutyl phosphonium ions helps in stretching the bentonite stacks; subsequently, the intercalation process of PVA chains is better. In addition,
“because of the greater steric tolerance of the phosphorus atom and the participation of its low-lying d-orbitals in the processes of making and breaking chemical bonds, phosphonium salts are generally capable of undergoing a wider range of reactions and behave differently than their ammonium counterparts toward an external base (B)” [Citation24].
4. Conclusion
PVA was used as a matrix to prepare nanocomposite films with modified Saudi bentonite. Two surfactants, cetylpyridinium chloride and tetrabutyl phosphonium bromide, were used to modify the clay after the ion-exchange process by Na+ ions. The effect of the addition of 2.5% modified Saudi bentonite on the thermal and flammability properties of the PVA/bentonite composites was investigated. The structure and morphology of the prepared samples were analysed using FTIR, XRD, and SEM. The results of XRD showed that the intercalation process of PVA was more successful for PVA with bentonite modified by tetrabutyl phosphonium bromide, while the SEM results revealed a good level of dispersion of its particles through the PVA matrix compared with the other nanocomposite samples.The thermal stability and flammability of the prepared nanocomposites improved – particularly for the sample of PVA with bentonite modified by tetrabutyl phosphonium bromide. The type of surfactant also affected these properties of the nanocomposites, wherein the bentonite modified with tetrabutyl phosphonium bromide gave better performance compared with another surfactant. It can be concluded that the Saudi organo-modified bentonite can be a good natural flame retardant for polymer – particularly ones modified by tetrabutyl phosphonium bromide.
Acknowledgements
Mashael Alshabanat designed, performed some parts of experiments, analysed the results, wrote the paper and revised it. Mrefah AL-Anazy performed the other parts of experiments, wrote some parts of paper and revised it.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Mashael Alshabanat http://orcid.org/0000-0001-5378-824X
Mrefah AL-Anazy http://orcid.org/0000-0001-7386-5820
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- Ferrández-Rives M, Aurora Beltrán-Osuna Á, Gómez-Tejedor JA, et al. Electrospun PVA/bentonite nanocomposites mats for drug delivery. Materials (Basel). 2017;10:1–17. doi: 10.3390/ma10121448
- Sirait M, Gea S, Siregar N, et al. Fabrication of poly(vinyl alcohol)/bentonite nanocomposites using sol-gel method. Asian J Chem. 2018;30(10):2210–2214. doi: 10.14233/ajchem.2018.21388
- Manjula B, Babul Reddy A, Sadiku ER, et al. Design and characterization of bio-nanocomposites for 5-fluorouracil drug delivery and antimicrobial study. Int J Adv Sci Eng Technol. 2017;5:88–93.
- Johansson C, Clegg F. Hydrophobically modified poly(vinyl alcohol) and bentonite nanocomposites thereof: barrier, mechanical, and aesthetic properties. J Appl Polym Sci. 2015;132(13):1–13. doi: 10.1002/app.41737
- Belozerov AG, Karasev NS, Ovchinnikov NL, et al. The effect of preliminary delamination of silicate filler on its exfoliation degree in polyvinyl alcohol/montmorillonite nanocomposites. Nanotechnol Russ. 2014;9:410–415. doi: 10.1134/S1995078014040053
- Sheta AS, Al-Omran AM, Falatah AM, et al. Characteristics of natural clay deposits in Saudi Arabia and their potential use for nutrients and water conservation. J King Saud Univ Agric Sci. 2006;19(1): 25–38.
- Ray SS, Okamoto M. Polymer-layered silicate nanocomposite: a review from preparation to processing. Prog Polym Sci. 2003;28:1539–1641. doi: 10.1016/j.progpolymsci.2003.08.002
- Alves JL, Zanini AE, de Souza ME, et al. Study of selection and purification of Brazilin bentonite clay by elutriation: a XRF, SEM and Rietveld. Cerâmica. 2016;62:01–08. doi: 10.1590/0366-691320
- Mekhamer WK. The colloidal stability of raw bentonite deformed mechanically by ultrasound. J Saudi Chem Soc. 2010;14:301–306. doi: 10.1016/j.jscs.2010.04.013
- UL 94. Standard for tests for flammability of plastic materials for parts in devices and appliances. Northbrook (IL): Underwriters Laboratories; 1996.
- Ghabboun J, Husseini GA, Faroun M, et al. Mapping of embedded functionalized carbon nanotubes in poly(vinyl alcohol)/nanotube composite using electrostatic force microscopy. Int J Polym Anal Ch. 2012;17(4):268–277. doi: 10.1080/1023666X.2012.658636
- Ahad N, Saion E, Gharibshahi E. Structural, thermal, and electrical properties of PVA-sodium salicylate solid composite polymer electrolyte. J Nanomater. 2012; Article ID 857569, 8 pages.
- Motawie AM, Madany MM, El-Dakrory AZ, et al. Physico-chemical characteristics of nano-organo bentonite prepared using different organo-modifiers. Egypt J Petrol. 2014;23:331–338. doi: 10.1016/j.ejpe.2014.08.009
- Mohomane SM. Preparation and characterization of polychloroprene/modified clay nanocomposites. Master thesis submitted at University of The Free State (Qwaqwa Campus); 2010. Supervisor: Prof. A.S. Luyt.
- Prichard GJ. Poly(vinyl alcohol): basis principles and uses. New York: Gordon and Breach; 1970.
- Brunelli DD, Barboza VC, Joekes I, et al. Mapping phases of poly(vinyl alcohol) and poly(vinyl acetate) blends by Ft-IR microspectroscopy and optical fluorescence microscopy. J Appl Polym Sci. 1998;69:645–655. doi: 10.1002/(SICI)1097-4628(19980725)69:4<645::AID-APP3>3.0.CO;2-J
- Guirguis OW, Moselhey MTH. Thermal and structural studies of poly(vinyl alcohol) and hydroxypropyl cellulose blends. Nat Sci (Irvine). 2012;4:57–67.
- Nanda P, De SK, Manna S, et al. Effect of gamma irradiation on a polymer electrolyte: variation in crystallinity, viscosity and ion-conductivity with dose. Nucl Instrum Methods Phys Res B. 2010;268(1):73–78. doi: 10.1016/j.nimb.2009.09.063
- Bhargav PB, Mohan VM, Sharma AK, et al. Investigations on electrical properties of (PVA:NaF) polymer electrolytes for electrochemical cell applications. Curr Appl Phys. 2009;9(1):165–171. doi: 10.1016/j.cap.2008.01.006
- Prajapati GK, Gupta PN. Comparative study of the electrical and dielectric properties of PVA–PEG–Al2O3–MI (M = Na, K, Ag) complex polymer electrolytes. Physica B. 2011;406:3108–3113. doi: 10.1016/j.physb.2011.05.019
- Chen N, Zhang J. The role of hydrogen-bonding interaction in poly(vinyl alcohol)/poly(acrylic acid) blending solutions and their films. Chinese J Polym Sci. 2010;28(6):903–911. doi: 10.1007/s10118-010-9167-x
- Siddaiah T, Ojha P, Ramesh Kumar NOGV, et al. Structural, optical and thermal characterizations of PVA/MAA:EA polyblend films. Mater Res. 2018;21(5):e20170987. doi: 10.1590/1980-5373-mr-2017-0987
- Strawhecker KE, Manias E. Structure and properties of poly(vinyl alcohol)/Na+ montmorillonite nanocomposites. Chem Mater. 2000;12:2943–2949. doi: 10.1021/cm000506g
- Chang J-H, Jang T-G, Ihn KJ, et al. Poly(vinyl alcohol) nanocomposites with different clays: pristine clays and organoclays. J Appl Polym Sci. 2003;90:3208–3214. doi: 10.1002/app.12996
- Xie W, Xie R, Pan W-P, et al. Thermal stability of quaternary phosphonium modified montmorillonites. Chem Mater. 2002;14:4837–4845. doi: 10.1021/cm020705v
- C Kornmann. 2000. Synthesis and Characterization of Thermoset-Clay Nanocomposites [Ph.D. thesis]. Lulea University of Technology, Sweden, p.55.