?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The bioactive compounds in Vernonia amygdalina leaf were extracted using ethanol through Soxhlet and microwave-assisted extraction (MAE) techniques. The phytochemical analysis was carried out on the ethanolic extracts using the standard techniques. This analysis revealed the presence of flavonoids, alkaloids, steroids, terpenoids, glycosides, tannins, phenols, saponins, and the absence of anthraquinones. Furthermore, gas chromatography-mass spectroscopy (GC-MS) analysis results revealed the presence of more phytochemicals in the extract obtained through MAE compared to Soxhlet extraction technique, whereby phytol was present in a higher percentage. Fourier transform infrared spectroscopy analysis confirmed the presence of phytochemicals in the extracts. Moreover, the IC50 values of the extracts antioxidant activities were evaluated using DPPH and ABTS assays. There exists a clear correlation between total phenolic content, total flavonoid content and antioxidant activity of the extracts. Therefore, this study suggested that higher phenolic compounds responsible for natural antioxidant could be obtained from V. amygdalina leaf using the microwave-assisted extraction technique.
1. Introduction
The world is enriched with varieties of medicinal plants which have drawn the attention of researchers due to their myriad benefits to mankind, most especially their usage in pharmaceutical and food industries. Their medicinal and pharmacological properties were due to the presence of bioactive components that produce definite physiological action in the human body [Citation1]. Over 80% of the world's population had been reported using herbal products as alternative medicine, food supplements and nutraceutical products [Citation2]. Medicinal plants are associated with the presence of polyphenols, polysaccharides and hydrolyzable tannins [Citation3]. The extracts from the plants not only contain primary metabolites and minerals but also a diverse array of secondary metabolites which possess antioxidant property [Citation1].
Vernonia amygdalina is one of the promising medicinal plants that can be found in tropical Africa and Asia. It is commonly known to be “bitter leaf” in English due to its bitter taste. It was named after an English Botanist William Vernon [Citation4]. It is a soft-wooded shrub that can regenerate rapidly, the shrub typically grows to a height of 2–5 m. The elliptical leaves are green in colour, about 20 cm long, and 6 mm in diameter [Citation5]. V. amygdalina leaves are rich in fats, proteins, fibres, minerals, amino acids, carbohydrate, and vitamins [Citation6,Citation7]. Moreover, the extracts from V. amygdalina leaves possess several secondary metabolites which include flavonoids, polyphenols, saponins, tannins, and terpenoids [Citation7]. These phytochemicals might be responsible for its anti-diabetes, antimicrobial, anti-allergic, antibacterial, antimalarial, anti-fungi, anticancer, antileukemia, anti-inflammatory, analgesic, antipyretic, anti-oxidative, antihelminthic hepatoprotective, and hypolipidaemic physiological effects [Citation8,Citation9]. Thus, it is used in the formulation of traditional medicine for several ailments.
Over the years, different extraction methods which include Soxhlet, hydrodistillation, decoction, maceration, microwave-assisted extraction, ultrasound-assisted extraction, supercritical fluid extraction, and pressurized solvent extraction have been employed to isolate bioactive compounds from plant matrix [Citation10,Citation11]. Amongst these methods, microwave-assisted extraction (MAE) has gained significant research attention due to its shortened extraction time and reduced solvent consumption compared to conventional solvent liquid extraction [Citation12,Citation13]. Therefore, the aim of this study is to extract and characterize the bioactive compounds from ethanolic extracts from V. amygdalina leaf comparing Soxhlet and MAE techniques.
2. Materials and methods
2.1. Plant sample preparation
The fresh leaves of V. amygdalina were procured from a garden in Gambang (along Pahang capital), Malaysia. The fresh leaves were washed and shade-dried until constant weight was attained. The moisture content was recorded as 0.012 ± 0.15 g water/g dry sample. The sample was blended using a (Panasonic Blender PANA-MX-801S HG, Malaysia), sieved through standard 105 mm mesh size and stored in an airtight waterproof polyethene bag until further use.
2.2. Chemical procurement
The ethanol, methanol, ferric chloride (FeCl3), hydrochloric acid, Dragendorff's reagent, Mayer reagent, lead acetate, chloroform, sulphuric acid, ammonia, sodium nitroprusside, sodium hydroxide, acetate, sodium carbonate (Na2CO3), potassium persulfate salt, 2, 2-diphenyl-picrylhydrazyl (DPPH), 2,2′-Azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS+•), gallic acid, quercetin, aluminium chloride salt, and Folin–Ciocalteu phenol reagent were obtained from Sigma-Aldrich (M) Sdn Bhd, Selangor, Malaysia. All the chemical used were of analytical grade.
2.3. Extraction processes
The extraction of V. amygdalina leaves was carried out using two different extraction methods which include Soxhlet and microwave-assisted extraction techniques. An aqueous ethanol solution (60% v/v) was used as the extracting solvent.
2.3.1. Soxhlet extraction
One hundred grams (100 g) of powdered V. amygdalina leaves were placed in a Soxhlet apparatus. The extraction was carried out using 1000 mL of 60% v/v ethanol concentration for 4 h. After the extraction process, the suspension was filtered and concentrated to dryness using a rotary evaporator (Buchi Rotavapor R-200 coupled with Buchi Vac V-500 pump, Switzerland). The extractions were performed in triplicate and the results were expressed as mean ± SD.
2.3.2. Microwave-assisted extraction (MAE)
The MAE was conducted by adding a 10 g of powdered V. amygdalina leaf to 100 mL of ethanol in a 250 mL round bottom flask. The mixture was irradiated for 2 min at 600 W and 78°C in an enclosed ethos reflux microwave extractor (1000 W, Frequency 2450 MHz, Milestone, Italy) using 3-level of heating: 2 min of preheating to boiling temperature of the solvent, irradiation for 5 min at solvent boiling point, and 2 min of cooling to 30°C. The mixture was filtered and concentrated to dryness using a rotary evaporator (Buchi Rotavapor R-200 coupled with Buchi Vac V-500 pump, Switzerland). The extractions were performed in triplicate and the results were expressed as mean ± SD.
2.4. Preliminary phytochemical analysis
2.4.1. Flavonoid test
The presence of flavonoids in the extract was determined following the procedure described by Bargah [Citation14]. Formation of a yellow precipitate was taken as an indication for the presence of flavonoids.
2.4.2. Terpenoids test
Terpenoids were tested following the procedure described by Bargah [Citation14]. Formation of a greyish colour indicates the presence of terpenoids.
2.4.3. Alkaloids test
This test was carried out followed the procedure described by Bargah [Citation14].
2.4.4. Tannins test
This test was carried out followed the procedure described by Bargah [Citation14].
2.4.5. Saponin glycoside test
A 10 mL of distilled water was shaken vigorously for 30 s with 10 mL of the extract in a tube. The tube in a vertical position was observed for 30 min. The formation of honeycomb froth persisted for 10–15 min indicated the presence of saponins.
2.4.6. Glycosides test
Glycoside test was carried out followed the method explained by Bargah [Citation14].
2.4.7. Test for free anthraquinones derivatives
A 0.5 mg of the extract was shaken with 5 mL chloroform for about 5 min. The mixture was filtered and an equal volume of 100% ammonia solution was added. A pink violet or red colour in the ammoniacal layer (lower layer) indicates the presence of free anthraquinones (Bontrager's test).
2.4.8. Steroids screening
A 50 mg of V. amygdalina leaf extract was dissolved in 1 mL of chloroform. Sulphuric acid was carefully added to form a lower layer. A reddish brown colour at the interface showed the presence of steroidal ring [Citation14].
2.4.9. Phenols test
Phenol screening was carried out using the procedure described by Bargah [Citation14].
2.5. Total phenolic content determination
The total phenol content in the extract was evaluated using the procedure used by Saravanan and Parimelazhagan [Citation15] and Dahmoune et al. [Citation17]. The standard calibration curve was prepared, using a standard solution of gallic acid (50-500 mg/mL). TPC was expressed as mg gallic acid equivalents per gram of dried weight sample (mg GAE/g d.w.). The TPC in the extracts were calculated using Equation (1). Ethanol was used as the blank. The analyses were done in triplicate.
(1)
(1) where, c is the sample concentration from the calibration curve (mg/mL), V is the volume (mL) of the solvent used in the extraction, and m represents the weight (g) of the dried sample used.
2.6. Total flavonoid content determination
The total flavonoid content in the V. amygdalina leaf extract was determined following the method used by Oriakhi et al. [Citation18]. Quercetin (50-500 mg/mL) was used to prepare a standard calibration curve (y = 0.112x + 0.178, R2 = 0.9945), where y is the absorbance at 420 nm and x is the sample concentration in mg/mL. TFC was expressed as mg quercetin equivalents per gram of dried weight sample (mg QE/g d.w.). The TFC of the extracts were calculated using EquationEq. (2)(2)
(2) . Ethanol was used as the blank. The analyses were carried out in triplicate.
(2)
(2) where c is the sample concentration in from the calibration curve (mg/mL), V is the volume (mL) of the solvent used in the extraction, and m represents the weight (g) of the dried sample used.
2.7. Antioxidant activity
2.7.1. DPPH assay
The DPPH assay was carried out using the procedure used in the previous study [Citation19]. Briefly, 0.1 mM solution of DPPH was prepared by dissolving 0.004 g of DPPH crystalline solid in 100 mL of analytical grade methanol and stored at 4°C. A 0.2 mL of DPPH solution was added to 2 mL of the extract. After 30 min of incubation in the dark, the absorbance at 517 nm using a UV-Vis Spectrophotometer (Hitachi U-1800, Japan) was recorded. Methanol was used as the blank. The analysis was performed thrice. The percentage of DPPH radical inhibition was determined using EquationEq. (3)(3)
(3) . The DPPH inhibitory activity of V. amygdalina leaf extract was expressed as IC50 (concentration in µg/mL of the extracts required to inhibit 50% of the DPPH radical formation) by preparing four different concentrations 100, 200, 300, and 500 µg/mL of the extracts. The IC50 values were calculated from the linear regression between the percentage of inhibition and concentrations.
(3)
(3) where Acontrol is the mixture of methanol and DPPH solution; and Asample is the mixture of sample extract and DPPH solution.
2.7.2 ABTS assay
ABTS assay was carried out using the procedure used in the previous study [Citation19]. In concise, ABTS radical cation was prepared by mixing 7 mM ABTS stock solution with 2.45 mM potassium persulfate in equal quantities, the mixture was left in dark at room temperature for 12 h until the reaction was completed and the absorbance was stabled. The fresh working solution of ABTS radical cation was prepared by diluting 2 mL ABTS+• solution with 120 mL methanol to obtain an absorbance of 1.1 ± 0.02 at 734 nm using UV-Vis Spectrophotometer (Hitachi U-1800, Japan). A 0.15 mL of V. amygdalina leaves extract (at concentrations 100, 200, 400 and 500 µg/mL) was mixed with 2.85 mL of ABTS+• solution and allowed to stand in the dark for 2 h at room temperature. Thereafter, the absorbance was determined at 734 nm using a UV-Vis Spectrophotometer (Hitachi U-1800, Japan). Methanol was used as the blank. The results were expressed in milligram gallic acid per gram dry weight (mg GAE/g d.w.). EquationEq. (4)(4)
(4) was used for determining the percentage of inhibition. The ABTS+• inhibitory activity of V. amygdalina leaf extract was expressed as IC50 (concentration in µg/mL of the extracts required to inhibit 50% of the ABTS+• radical formation) by preparing four different concentrations 100, 200, 300, and 500 µg/mL of the extracts. The IC50 values were calculated from the linear regression between the percentage of inhibition andconcentrations.
(4)
(4) where Acontrol is the mixture of methanol and ABTS+• solution; and Asample is the mixture of sample extract and ABTS+• solution.
2.8. Gas chromatography-mass spectroscopy (GC-MS) analysis
The chemical components and their percentage of abundance in the extracts were evaluated using a gas chromatography-mass spectrometry analysis. Alara et al. [Citation19] procedure with some modification was followed. Briefly, the V. amygdalina leaf extracts were analysed using an Agilent GC-MS equipment (Agilent 5975C inert, USA) coupled with a mass spectrometer MS 597C and equipped with a DB-1MS capillary column (30 m tubular column length × 0.25 mm i.d. × 0.25 µm film thickness) and an autosampler. The operating conditions for the analysis were as follows: initial temperature of 35°C which was raised to 95°C at the rate of 3°C/min for 10 min, then raised to 270°C at the rate 10°C/min and finally to 300°C at the rate 3°C/min which was maintained for 3 min; column flow rate of 0.8 mL/min; carrier helium at a flow rate of 1.0 mL/min; split ratio 10:1; the ionization voltage of 70 eV; run time 67.5 min; and sample injection volume was 1 µL solution of extract (5 mg/mL). The components were identified by comparing the obtained spectra with those on the NIST 05a library database, and the percentages of abundance were determined with the total ion chromatogram. 1 mL sample was prepared by diluting the extract with analytical absolute ethanol at a ratio of 1:20 (w/v).
2.9. Fourier transform infrared spectroscopy analysis
The V. amygdalina leaf extracts were subjected to FTIR analysis for the determination of present functional groups. Fourier transform infrared spectrometry (Nicolet iS5 iD7 ATR; Thermo Scientific, Germany) equipped with OMNIC software was used in the analysis. The plant samples were analysed to obtain IR spectra in the scanning wave number ranging from 500–4000 cm−1 with a resolution of 4 cm−1 [Citation21]. The spectra of the observed compounds, group frequencies and characteristics bonds from the extracts were compared with the table of expected absorption bands for the molecule's various groups and bonds.
2.10. Scanning electron microscopy and elemental analysis
The scanning electron microscopy (SEM) was used to observe the changes in the morphology of V. amygdalina leaves before and after Soxhlet and MAE techniques. The samples were mounted on an SEM stub and the morphologies were observed in SEM (HITACHI TM3030Plus, Japan). The analyses were examined under the high vacuum condition at an accelerated voltage of 15 keV, 100x magnification and an analytical working distance of 2 mm. Likewise, the elemental analysis of the extracts was analysed using Energy-Dispersive X-ray Spectroscopy (EDX). The detection of the elements in the V. amygdalina leaf extract was carried out using silicon (lithium) detector cooled with liquid nitrogen. EDX Shimadzu software package was used to determine the intensity of each element counts per second from the sample X-ray spectrum deconvolution.
2.11. Statistical analysis
All the experiments were carried out thrice and the average results were selected. The statistical analyses were performed using one-way ANOVA and the results were expressed as mean ± SD.
3. Results and discussion
3.1. Phytochemical analysis
The pharmacological effects of V. amygdalina leaf may perhaps be due to the presence of several secondary metabolites such as flavonoids, terpenoids, alkaloids, tannins, saponins, glycosides, steroids, and phenols. The preliminary phytochemical analyses of the ethanolic extracts from V. amygdalina leaf using Soxhlet and MAE techniques are shown in Table .
Table 1. Preliminary phytochemicals screening of the V. amygdalina leaf extracts.
The presences of flavonoids, terpenoids, alkaloids, tannins, saponins, glycosides, steroids, and phenols were observed in both extracts (Table ). These secondary metabolites had been studied to have many biological properties [Citation19,Citation8]. Moreover, anthraquinones were absent in the V. amygdalina leaf extracts. The presence of flavonoids had been reported to be associated with the naturally occurring phenolic compounds that possess antioxidant properties in the human diet [Citation20,Citation21]. In different studies, the presence of terpenoids and steroids in V. amygdalina possess different biological activities which include anti-inflammatory, anticancer, anti-diabetes, antibacterial, analgesic, hepatoprotective, and antioxidant [Citation8,Citation22]. The presence of alkaloids could be well correlated with antimicrobial activities. Thus, this species is expected to have many medicinal uses.
3.2. TPC, TFC and antioxidant activities of the extracts
Phenolics and flavonoids are the plant secondary metabolites that possess several pharmacological activities which include antioxidant, anti-inflammatory, antimicrobial, anticancer, and among biological activities [Citation23]. The TPC, TFC and antioxidant activities ofV. amygdalina leaf using Soxhlet and MAE techniques are presented in Table . The highest TPC (114.03 ± 1.25 mg GAE/g d.w.) and TFC (96.29 ± 1.70 mg QE/g d.w.) were obtained for extracts from MAE. This might be due to the shorter extraction time with a higher yield in the MAE technique [Citation24]. Furthermore, there exists a clear correlation between TPC, TFC and antioxidant activity of the extracts whereby a higher phenolic and flavonoid contents resulted in stronger antioxidant activity. The IC50 values of DPPH and ABTS inhibitions were higher in Soxhlet than MAE, implying a lesser antioxidant activity of the extracts from Soxhlet extraction techniques. This implies that the method of extracting phenolic compounds from the plant matrix plays an important role in determining the antioxidant activity of the extracts.
Table 2. The TFC, TPC and antioxidant activity of V. amygdalina leaf using Soxhlet and MAE techniques.
3.3. Components identification from the extracts of V. amygdalina leaf
The identified chemical components from GC-MS chromatogram analysis of the ethanolic extract ofV. amygdalina leaf using Soxhlet and MAE techniques are shown in Table . A total number of 23 and 9 chemical components belonging to hydrocarbons, alcohols, esters, fatty acids, and ketones were identified from V. amygdalina leaf extracts through MAE and Soxhlet extraction, respectively. For MAE, the most prevailing compounds were phytol, a terpenoids compound (56.30%), 4-methyl-vinyl butyrate (7%), and (z,z,z)-methyl ester-9,12,15-octadecatrienoic acid (6.94%) which belongs to unsaturated fatty acids. Similarly, the most abundant chemical compounds in extract using the Soxhlet technique were phytol (50.57%) and (z,z,z)-methyl ester-9,12,15-octadecatrienoic acid (17.60%). Phytol was found to be a strong antioxidant, possessing anti-microbial, anti-inflammatory, and anti-proliferative activity. Phytol, a precursor of synthetic vitamin E and vitamin K, was found to be cytotoxic against breast cancer cell lines MCF7 [Citation25]. This chemical component had previously been reported as components of V. amygdalina leaf but interesting, the identified phytol from this study was in abundance compared to the previous studies [Citation17,Citation19,Citation24]. This may be attributed to the geographical location where the plant samples were harvested and the extraction methods. Moreover, the presence of higher percentages of terpenoids and unsaturated fatty acids showed the anti-inflammatory and antioxidant potential of V. amygdalina leaf [Citation17,Citation19].
Table 3. Chemical composition of V. amygdalina leaf using MAE and Soxhlet techniques.
In addition to antimicrobial activity, the squalene was also reported to have anticancer, antioxidant, chemopreventive, pesticide, anti-tumor, and sunscreen properties. The coumarin compound (8-methyloctahydrocoumarin) had antioxidant and anti-inflammatory properties [Citation17,Citation19]. The presence of aromatics and fatty acids in the ethanolic extracts revealed the pharmacological properties of V. amygdalina leaf. Thus, this study has revealed the presence of major bioactive compounds that can serve as a basis for determining the possible health benefits of this plant leading to further biological and pharmacological studies.
3.4. Structural analysis of V. amygdalina leaf extracts
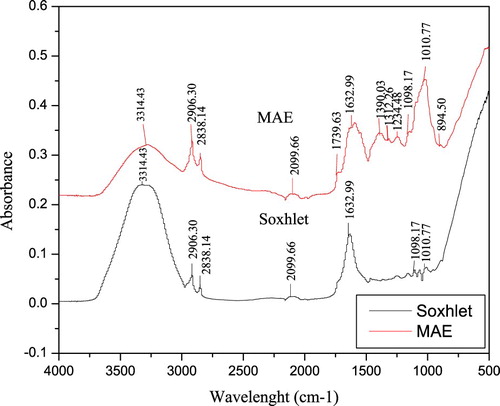
Figure shows the characteristic fingerprinting of ethanolic extracts from V. amygdalina leaf using Soxhlet and MAE techniques. In general, each band in the spectra corresponds to a functional group responsible for IR absorption [Citation26]. There are 7 and 12 peaks identified from the extracts using Soxhlet and MAE, respectively. The bands between the wavelengths of 1800–750 cm−1 showed the biochemical composition, especially the compounds associated with lipid, carbohydrate, protein secondary structures, lipid, and phenolic compounds in the plant extract. The band at 894.50 cm−1 in MAE might be caused by ring C–H deformation which could reflect structural information on phenolic compounds [Citation27]. Moreover, the stretching peak at 1010.17 cm−1 is associated with the presence of an O–C-C stretch of esters. A group of bands appearing in the wavelength region of 1300–1000 cm−1 is due to C–O stretching vibration of esters, free fatty acids of alcohols. The presence of a minor peak at 1739 cm−1 revealed the presence of C = O stretching vibration in carbonyl groups that characterized abundance of the terpenoids and the stretched C–H absorption band at 2838.14 and 2906.30 cm−1 show the presence of methylene compounds. The distinguished peak at 1312.26 cm−1 is due to the plane C–O stretching vibration combined with the ring stretch of phenyl [Citation28]. The presence of higher wavelength (lower frequency) at peak of 3314.43 cm−1 is due to N-H stretching of proteins and O-H stretching of carbohydrate and water. In addition, the peak at 2906.30 cm−1 is due to the CH2 anti-symmetric stretch of methyl groups mainly from lipids [Citation28]. Thus, it can be deduced that MAE has higher potential comparing to Soxhlet in the extraction of bioactive compounds from V. amygdalina leaf.
3.5. Morphological and elemental analyses of V. amygdalina leaf before and after extraction
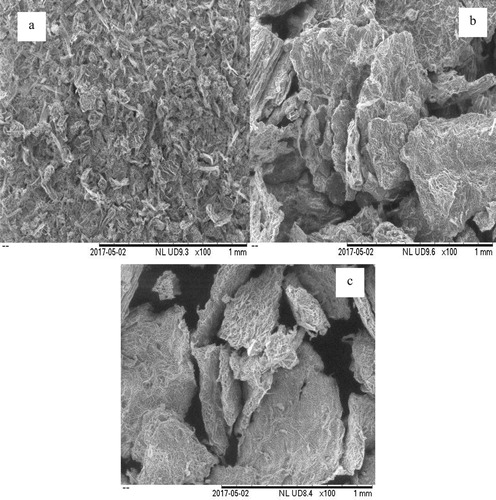
The morphological analyses of the powdered sample of V. amygdalina leaf before extraction and the residues after MAE and Soxhlet extraction are shown in Figure . Before extraction, the cell wall of the plant was firmly packed (Figure (a)). In contrast, the fractural changes in the physical structure of the plant sample were obvious after the extraction whereby the physical morphology of the V. amygdalina leaf after MAE was destroyed, showing that the microwave irradiation played an important role in breaking the vegetal cell wall (Figure (b)). Therefore, it can be deduced that the molecular movement and rotation of liquids associated with a permanent dipole resulted in rapid heating of the extracting solvent [Citation16]. Moreover, the physical structure of the sample after Soxhlet extraction was severely damaged (Figure (c)). This might be due to the effect caused by localized heating from the long period of extraction [Citation10].
Figure 2. SEM analysis of V. amygdalina leaf before extraction (a); after MAE (b); after Soxhlet extraction (c).
The results of the elemental analysis of V. amygdalina leaf of the extracts using MAE and Soxhlet are shown in Figure and Table . Carbon, oxygen, aluminium, silicon, phosphorus, chlorine, potassium, calcium, sulphur, and iron are present in the extracts (Figure ). The presence of these elements in the plant extracts made them responsible for curing several diseases [Citation29]. Amongst all these element, carbon and oxygen were present in a higher amount while silicon and potassium in moderate concentrations. On the contrary, aluminium, phosphorus, calcium, chlorine, iron, and sulphur were present in trace quantities. It was observed that toxic elements such as Hg, Cd and Sb were not detected in the extracts. Iron was not detected in the extract from Soxhlet. Moreover, the concentration of carbon in the Soxhlet extraction was higher than that of MAE, this might be because of localized heating generated from Soxhlet extraction that may lead to the degradation of the plant sample and produced more carbon residue. Other elements detected in MAE extract were higher than extract from Soxhlet. Therefore, it can be deduced that the MAE technique possesses a higher capacity to extract more nutrients compared to Soxhlet extraction technique.
Figure 3. EDS spectra of ethanolic extract from V. amygdalina leaf using MAE (a) and Soxhlet extraction (b).
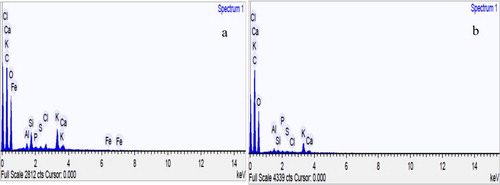
Table 4. Nutrient composition (%) in V. amygdalina leaf after MAE and Soxhlet extraction.
The calcium and potassium found in V. amygdalina leaf extracts showed it potent in muscle contraction and synthesis of some protein. In addition, calcium helps in teeth and bone development, blood clotting, regulate heart rhythm, and lower blood pressure [Citation30]. Furthermore, chlorine acts as an electrical charge when dissolving body fluids and the regulation of body pH. Thus, V. amygdalina leaf can be used as pharmaceutical and therapeutic agents.
4. Conclusions
This study clearly revealed that phytochemical and elemental composition of V. amygdalina leaf varied with the method of extraction whereby extract through MAE contained more extractable metabolites compared to Soxhlet extraction technique. Moreover, both methods of extraction showed a considerable antioxidant activity with a varying amount due to differences in their phytochemical compositions from the GC-MS analysis. Thus, this study suggests the use of MAE in extracting higher yields of bioactive compounds from V. amygdalina leaf which may possibly be utilized as a therapeutical source for managing several diseases.
Acknowledgement
We acknowledge the support of Universiti Malaysia Pahang, Malaysia through the grant number RDU180329.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Oluwaseun Ruth Alara http://orcid.org/0000-0002-5114-2100
References
- Akinmoladun AC, Ibukun EO, Afor E, et al. Chemical constituents and antioxidant activity of Alstonia boonei. African J Biotechnol. 2007;6(10):1197–1201.
- Qi Z. WHO traditional medicine strategy 2014–2023: Background and progress in the last decade. Glob Heal Hist Semin Tradit Med Ayurveda. 2015: 1–28.
- Khoddami A, Wilkes MA, Roberts TH. Techniques for analysis of plant phenolic compounds. Molecules. 2013;18(2):2328–2375. doi: 10.3390/molecules18022328
- Alara OR, Abdurahman NH, Abdul Mudalip SK, et al. Phytochemical and pharmacological properties of Vernonia amygdalina: a review. J Chem Eng Ind Biotechnol. 2017;2:80–96.
- Ijeh II, Ejike CECC. Current perspectives on the medicinal potentials of Vernonia amygdalina Del. J. Med Plants Res. 2011;5(7):1051–1061.
- Eyong EU, Agiang MA, Atangwho IJ, et al. Phytochemicals and micronutrients composition of root and stem bark extracts of Vernonia amygdalina Del. J. Med Med Sci. 2011;2(6):900–903.
- Alara OR, Abdurahman NH, Ukaegbu CI, et al. Dataset on LC-Q-TOF/MS tentative identification of phytochemicals in the extract of Vernonia amygdalina leaf through positive ionization. Data Brief. 2018;21:1686–1689. doi: 10.1016/j.dib.2018.10.159
- Ngatu NR, Okajima MK, Yokogawa M, et al. Anti-allergic effects of Vernonia amygdalina leaf extracts in hapten-induced atopic dermatitis-like disease in mice. Allergol Int. 2012;61(4):597–607. doi: 10.2332/allergolint.11-OA-0393
- Yeap SK, Ho WY, Beh BK, et al. Vernonia amygdalina, an ethnomedical used green vegetable with multiple bio-activities. J Med Plants Res. 2010;4(25):2787–2812.
- Alara OR, Abdurahman NH, Ukaegbu CI. Soxhlet extraction of phenolic compounds from Vernonia cinerea leaves and its antioxidant activity. J Appl Res Med Aromat Plants. 2018;11:12–17.
- Alara OR, Abdurahman NH, Ukaegbu CI, et al. Vernonia cinerea leaves as the source of phenolic compounds, antioxidants, and anti-diabetic activity using microwave-assisted extraction technique. Ind Crop Prod. 2018;122:533–544. doi: 10.1016/j.indcrop.2018.06.034
- Raut P, Bhosle D, Janghel A, et al. Emerging microwave assisted extraction (MAE) techniques as an innovative green technologies for the effective extraction of the active phytopharmaceuticals. Res J Pharm Technol. 2015;8(6):655–666.
- Alara OR, Abdurahman NH, Olalere OA. Optimization of microwave-assisted extraction of flavonoids and antioxidants from Vernonia amygdalina leaf using response surface methodology. Food Bioprod. Process. 2018;107:36–48. doi: 10.1016/j.fbp.2017.10.007
- Bargah R. Preliminary test of phytochemical screening of crude ethanolic and aqueous extract of Moringa pterygosperma Gaertn. J Pharmacogn Phytochem. 2015;4(1):7–9.
- Saravanan S, Parimelazhagan T. In vitro antioxidant, antimicrobial and anti-diabetic properties of polyphenols of Passiflora ligularis Juss. fruit pulp. Food Sci Hum Wellness. 2014;3(2):56–64. doi: 10.1016/j.fshw.2014.05.001
- Dahmoune F, Spigno G, Moussi K, et al. Pistacia lentiscus leaves as a source of phenolic compounds: microwave-assisted extraction optimized and compared with ultrasound-assisted and conventional solvent extraction. Ind Crops Prod. 2014;61:31–40. doi: 10.1016/j.indcrop.2014.06.035
- Dahmoune F, Nayak B, Moussi K, et al. Optimization of microwave-assisted extraction of polyphenols from Myrtus communis L. leaves. Food Chem. 2015;166:585–595. doi: 10.1016/j.foodchem.2014.06.066
- Oriakhi K, Oikeh EI, Ezeugwu N, et al. Comparative antioxidant activities of extracts of Vernonia amygdalina and Ocimum gratissimum leaves. J Agric Sci. 2013;6(1):13–20.
- Alara OR, Abdurahman NH, Abdul Mudalip SK, et al. Effect of drying methods on the free radicals scavenging activity of Vernonia amygdalina growing in Malaysia. J King Saud Univ - Sci. 2017. doi:10.1016/j.jksus.2017.05.018.
- Atangwho IJ, Egbung GE, Ahmad M, et al. Antioxidant versus anti-diabetic properties of leaves from Vernonia amygdalina Del. growing in Malaysia. Food Chem. 2013;141(4):3428–3434. doi: 10.1016/j.foodchem.2013.06.047
- Ashokkumar R, Ramaswamy M. Phytochemical screening by FTIR spectroscopic analysis of leaf extracts of selected Indian medicinal plants. Int J Curr Microbiol Appl Sci. 2014;3(1):395–406.
- Erasto P, Grierson DS, Afolayan AJ. Evaluation of antioxidant activity and the fatty acid profile of the leaves of Vernonia amygdalina growing in South Africa. Food Chem. 2007;104:636–642. doi: 10.1016/j.foodchem.2006.12.013
- Shah SR, Ukaegbu CI, Hamid HA, et al. Evaluation of antioxidant and antibacterial activities of the stems of Flammulina velutipes and Hypsizygus tessellatus (white and brown var. extracted with different solvents). J Food Meas Charact. 2018;12:1947–1961. doi: 10.1007/s11694-018-9810-8
- Tatke Y, Jaiswal P. An overview of microwave assisted extraction and its applications in herbal research. Res J Med Plant. 2011;5(1):21–31. doi: 10.3923/rjmp.2011.21.31
- Casuga FP, Castillo AL, Corpuz MJAT. GC-MS analysis of bioactive compounds present in different extracts of an endemic plant Broussonetia luzonica (Blanco) (Moraceae) leaves. Asian Pac J Trop Biomed. 2016;6(11):957–961. doi: 10.1016/j.apjtb.2016.08.015
- Rohman A, Che Man YB. The optimization of FTIR spectroscopy combined with partial least square for analysis of animal fats in quartenary mixtures. Spectroscopy. 2011;25(3–4):169–176. doi: 10.1155/2011/825121
- Li Y, Han L, Ma R, et al. Effect of energy density and citric acid concentration on anthocyanins yield and solution temperature of grape peel in microwave-assisted extraction process. J Food Eng. 2012;109(2):274–280. doi: 10.1016/j.jfoodeng.2011.09.021
- Lu X, Wang J, Al-Qadiri HM, et al. Determination of total phenolic content and antioxidant capacity of onion (Allium cepa) and shallot (Allium oschaninii) using infrared spectroscopy. Food Chem. 2011;129(2):637–644. doi: 10.1016/j.foodchem.2011.04.105
- Mushtaq T, Bahadur A, Shah Z, et al. Elemental and nutritional analysis and ethnomedicinal study of selected wild plants species of District Swabi, Khyber Pakhtunkhwa. Pakistan J Pharm Res. 2012;5(9):4910–4913.
- Ragavendran P, Sophia ARCD, Starlin T, et al. Elemental analysis of Aerva lanata (L. by EDX method). Int Res J Pharm. 2012;3(7):218–220.