Abstract
Background: Prostate cancer has become the most common cancer among African – American men and the second leading cause of cancer death in men worldwide. Many anti-malignant agents have been isolated from different plant species with minimal or no side effects thus it holds future promise as a resort as cancer biotherapeutics when compared with other treatment methods including synthetic drugs. Aim: This current investigation was aimed at evaluating the anti-proliferative efficacy of the ethanolic extract of Annonamuricata leaf on annexin 7 gene of malignant prostatic hyperplasia induced male wistar rats. Materials and Methods: Sub-chronic daily oral gavage exposure of the test substances to experimental animals lasted for a period of 28 days. Monosodium glutamate and L-arginine (90:22.5 mg/kg/b.wt) with purity 98% were administered concomitantly to the male wistar rats in various treatment groups. A total of 25 male wistar rats of about 6 weeks old weighing between 250–282 grams were used for this investigation. Quantitative and qualitative phytochemicals screening of ethanolic extract of Annona muricata leaf were also carried out. Hematoxylin and eosin staining were used in the histological assay of the prostate tissues. The prostate specific antigen (PSA) values were determined using standard protocol and polymerase chain reaction (PCR) was used to amplify annexin-7 gene. 1.5% agarose gel was used to separate the amplicons into bands of varying patterns. Result: Qualitative analysis demonstrated the presence of alkaloids, Saponins, flavonoids, tannins, cardiac glycoside, reducing sugar, phenol and triterpenes. Quantitative screening of the extract unveiled that alkaloid was present in the highest amount while Cardiac glycosides had the least concentration. The prostate histological assessment revealed a dose-dependent disruption in normal prostate tissue architecture. There was statistically significant difference P ≤ 0.05 in the body weight and prostate specific antigen (PSA) values of the male wistar rats in the experimental groups during the period of this investigation. The varying amplicon band patterns obtained from the treatment groups indicates possible MSG and L-ARG induced mutation in annexin 7 gene. The DNA amplicon bands observed in the positive control without treatments had some degree of similarity with bands obtained from the amplicons in the various treatment groups that were administered with both carcinogens and ethanolic leaf extract while thin bands were observed for the negative control group that was administered with carcinogens alone. Conclusion: This investigation has demonstrated that ethanolic extract of A. muricata leaf could be used as a potent ethno-chemopreventive agent against L-arginine and monosodium glutamate induced malignant prostatic hyperplasia in male wistar rats.
1. Introduction
Prostate cancer is a state of malignancy within the prostate tissue which result from tumour-promoting effects of endogenous hormones and growth factors which are associated with the stimulation of cellular growth and inhibition of apoptosis, thus aberrant cells within the prostate tissue replicate more rapidly than the normal cells forming malignant tumours. Malignant hyperplasia is the abnormal enlargement of an organ or tissue resulting from uncoordinated growth of cells which is usually a preneoplastic response to stimulus and the initial stage in cancer development [Citation1,Citation2]. According to [Citation3] recent research on cancer, prostate cancer and other kinds of cancers are preventable, thus chemoprevention of cancer can be described as the reversion or suppression of the early precancerous stages that leads to tumour formation by the use of natural, synthetic or biological agents and these agents target any of the major cancer development stages such as initiation, promotion and progression through molecular mechanisms [Citation4,Citation5].
The common treatment routine for prostate cancer includes surgery, chemotherapy and radiotherapy. Radiotherapy decrease the survival rate of cancer patients because it targets all dividing cells and exert significant negative side effects. This is coupled with the high cost of therapy which makes it unaffordable for low and middle class income earners hence the need for ethno-medicinal research on cancer treatment remedies worldwide. Many anti-malignant agents have been isolated from different plant species which hold future promise as a resort for cancer treatment with low side effect when compared with other treatment methods including synthetic drugs [Citation6].
Monosodium glutamate (MSG) a sodium salt of glutamic acid which is used widely globally to improve food taste and flavour. MSG plays a pivotal role in the signal transduction in the nervous system of all complex living organisms. It can alter the antioxidant status of the brain and mitochondria lipid peroxidation [Citation7,Citation8]. Glutamate concentration [Citation9] in systemic circulation is relatively stable and abnormal circulatory levels in men indicate pathological condition such as prostate cancer. Arginine (L-arginine) is a precursor for the biosynthesis of nitric oxide (NO), proteins, urea, creatine, agmatine and vasopressin [Citation10]. It has vital effects on the endocrine function mainly the adrenal and pituitary secretions in humans and animals. It has being reported to promote cancer growth in humans and animals by stimulating polyamine formation, which acts as growth factor for cancer [Citation11,Citation12]. Concomitant administrations of L-arginine and monosodium glutamate have being earlier reported to exert prostate dysfunction in male wistar rats [Citation13].
Annona muricata Linn. also known as sour soup or graviola is a tropical tree with heart shaped edible fruits and belong to the family Annonaceae. Leaf extract of this plant have been reported to possess antioxidants, anti-inflammatory, anti-analgesic, anti-tumour and anti-cancer properties. Annonaceous acetogenins, one of the bioactive components of this plant in numerous in-vitro and in-vivo studies have shown to exert high apoptotic activities by triggering the intrinsic and extrinsic apoptotic pathways, which eventually leads to execution phase through caspase activation [Citation14,Citation15]. This bioactive compound has demonstrated selective toxicity between normal and malignant cells thus have become a potent compound for cancer ethno-chemotherapy [Citation16,Citation17].
Annexin 7 is a candidate tumour suppressor gene (TSG) implicated in prostate cancer and function in a number of cellular processes involving calcium signalling [Citation18]. It’s the most evolutionary conserved member of the annexin gene family and located on human chromosome 10 in the q21 region [Citation19]. ANX7 a biomarker gene in prostate cancer progression is down regulated during prostate malignancy condition. Gleason score reflects the pathological aggressiveness of prostate tumour under the microscope and its rated on a scale from 2 to 10 thus lower score of 2–4 indicate less aggressive cancer while 7–10 suggest a more aggressive tumour [Citation20]. Prostate specific antigen (PSA) is a glycoprotein in the blood encoded by a gamma-seminoprotein or kalllikrein-3 (KLK3) produced only by prostate cells and values obtained after assay always reflects the state of the prostate. High PSA levels more likely indicate the presence of prostate cancer thus 4 ng/ml is usually considered normal and values above are regarded high indicating likelihood of malignancy within the prostate tissue although in some cases values lower than 4 ng/ml have being reported during prostate malignancy [Citation21].
The increasing prevalent rate of prostate malignancy among Nigerian men and unaffordability of the available treatment plans to low income individuals thus this study was aimed at assessing the antiproliferative potential of ethanolic leaf extract of A. muricata on annexin 7 gene of malignant prostatic hyperplasia induced in male wistar rats.
2. Materials and methods
2.1. Test substances and DNA extraction materials
The test substances, Arginine (L-ARG) and Monosodium Glutamate (MSG) purity greater than 98% (>98%) were purchased from Moscow chemical store, Gbagada, Lagos state, Nigeria. The DNA extraction kit and primers were manufactured by Jena Bioscience chemical company, Germany.
2.2. Plant material identification and extraction
The plant was identified and authenticated by a taxonomist Mr. Oyebanji, O.A with voucher specimen number LUH 6330 at the Herbarium unit of the Botany Department University of Lagos, Nigeria where the specimen were deposited. Annona muricata leaves were washed using running tap water and air-dried at room temperature (± 28°C). The dried leaves were blended by using an electric blender which had been sterilized with ethanol (70%). About 1.5 kg of Annona muricata powder was soaked in 70% ethanol in the ratio of 1:6 for 72 h with occasional shaking, as recommended [Citation22]. The mixture was filtered using a sieve cloth and whatman No.1.filter to obtain the filtrate. The greenish filtrate was concentrated using water bath at a temperature of 40°C to obtain 55.2 g of concentrated extract. The concentrate was used to prepare the different concentrations used for experimental animals’ treatment and phytochemical screening.
2.3. Experimental animal
A total of 25 isogenic strain of male albino rats (Rattus norvegicus), weighing between 250–282 g and about 6 weeks old similar to those earlier used [Citation23] earlier. They were obtained from the department of the Zoology university of Lagos. All animals were housed in an animal facility in Zoological and Botanical garden, University of Lagos and maintained on standard food (Pfizer products, pellets) and water ad libitum. After two weeks of acclimatization, they were allotted randomly to one of the five oral exposure groups based on body weight in a completely randomized design for a period of four weeks (28 days). The cages were cleaned daily, followed by the replacement of water and food. Approval was obtained from the University of Lagos Ethics Committee Guidelines for experiment with whole animals which is in accordance with the code of Ethics of The World Medical Association for experimental animals [Citation24].
3. Methodology
3.1. Experimental design
About 25 male albino rats of isogenic strain (R. norvegicus) weighing between 250–282 g and about 6 weeks old similar to those earlier used by Larbies et al. [Citation23]. They weredivided into five treatment groups each consisting of five rats as presented in the table below Table .
Table 1. Experimental animals grouping and treatment plan dosage.
3.2. Animal sample collection and analysis
At the end of the experiment, 16 hr after the last feeding, the rats were sacrificed to obtain blood samples at 9:15 am, by retro orbital sinus venipuncture using sterile capillary tubes (containing no anticoagulant) as earlier described [Citation25]. Excision to obtain the prostate was carried out immediately and samples were kept in sterile bottles some containing formaldehyde or normal saline prior to histological and molecular analysis respectively. Blood samples were centrifuged for 10 min at 3,000 rpm at room temperature and the serum was kept in a deep freezer for biochemical assays. The prostate sections were stained and mounted using hematoxylin and eosin and viewed at a microscopic magnification of HE-200X [Citation26].
3.3. Phytochemical screening of the plant extract
Physiochemical screening (Qualitative and Quantitative analysis) of the ethanolic leaf extract of A. muricata was carried out using standard assay protocol as described [Citation27].
3.4. Test for prostate specific antigen (PSA)
The standard prostate specific antigen (PSA) assay protocol used by university of Lagos Teaching Hospital (LUTH) was adapted. At the end of the assay PSA values for each sample was determine using a microtiter plate reader, read the optical density at 450 nm within 15 min. The values obtained were expressed in ng mL−1.
3.5. DNA extraction and polymerase chain reaction (PCR)
The DNA extraction was carried using Jena Bioscience genomic DNA preparation kit from whole blood, animal and plant, the protocol was based on spin column using the manufacturer’s manual guide. The prostate samples were grinded with buffer solution to form homogenous mixtures which were pipetted into different eppendorf tubes. The DNA kit manufacturer’s protocol was followed to obtained high purity genomic DNA from the prostate tissue samples which were stored at −20°C prior to polymerase chain reaction exercise.
3.6. Primer
The sequences used for this work were obtained [Citation28]. The primer sets were:
Forward primer (A7P3F) – 5’ CACCTGGGCTGTGACGCTGCT 3’ and
Reverse primer (A7P3R) - 5’ CCCTCCTACTGGCCCACAATAGCC 3’.
3.7. PCR amplification of annexin VII (ANXA7) gene and agarose gel electrophoresis (AGE)
The Amplification was performed using Peltier Thermal Cycle PTC-100 from MJ Research in a total reaction volume of 50 µl containing 10 µl master mix, 34.6 µl double distilled-water, 0.2 µl each of forward and reverse primer and 5 µl of DNA. Amplification was done with an initial denaturation temperature of 94°C for 3 min followed by 30 cycles: denaturation at 94°C for 1 min, annealing at 61°C for 1 min, extension at 72°C for 4 min and 1 cycle of final extension at 72°C for 15 min. Amplicon were separated in 1.5% agarose using SYBR safe dye, at 100 V for 1hr 30 min.
3.8. Statistical analysis method
The data obtained was analysed using SPSS version 16.0 windows software and the results were expressed as mean ± standard error. Significant differences were established by using one-way Analysis of Variance (ANOVA) and post-hoc test of multiple comparism was used to determine between group differences. A difference is considered significant at P < 0.05.
4. Results
Phytochemical parameters: Table : the result of the quantitative assay demonstrated that phenol was present in the highest amount while cardiac glycosides had the least concentration. Table : qualitative analysis of phytochemicals in the ethanolic extract of A. muricata leaf revealed the presence of alkaloids, saponin, flavonoids, tannins, cardiac glycoside, reducing sugar, phenol and triterpenes while steroid and terpenoids were absent.
Table 2. Shows the result phytochemicals qualitative assay of ethanolic extract of A. muricata leaf.
Table 3. Quantitative assay of selected phytochemicals presents in ethanolic extract of A. muricata leaf.
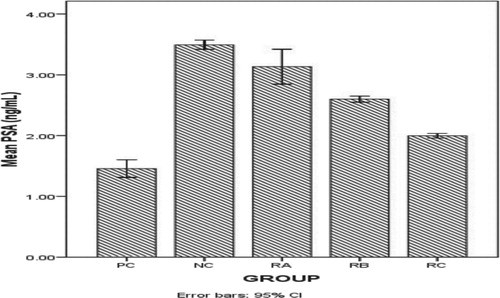
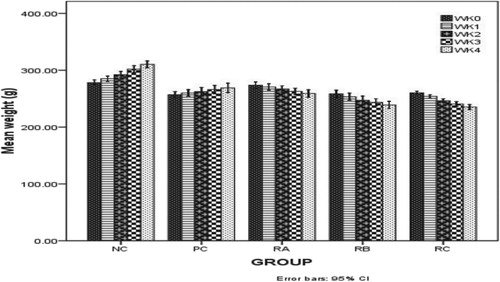
Prostate specific antigen (PSA) and body weight Figure : shows the effects of the treatments on prostate specific antigen (PSA) values in the various treatment groups. Table : shows the effect of different treatments on the weight of the male wistar rats throughout the period of this investigation. Figure : shows the weight trend of experimental animals during treatment.
Table 4. Shows the effect of treatments on the body weight of male wistar rats in treatment groups.
Histopathology examination of the prostate tissue: (Group PC): histological section through a normal prostate tissue showing cuboidal epithelial cells, many fibro-muscular stroma, large gland unit and few epithelial nuclei. (Group NC): histological section of prostate tissue of male wistar rats revealing the presence of many epithelial nuclei, few stroma, small lumen and irregular gland units. (Group RA): shows the histological section of prostate tissue in male wistar rats unveiling the presence of small gland units, few stroma, few epithelial nuclei and small size lumen. : histological section of prostate tissue obtained from the male wistar rats in group RB revealing the presence of gland units, fibro muscular stroma, few epithelial nuclei and lumen of medium size. : histological section of prostate tissue of male wistar rats from group RC showing the presence of large lumen, few epithelial nuclei, medium size gland units and many fibro muscular stroma.
Plate 1-3. (HE-200X): photomicrographs obtained from histopathological examination of prostate tissue sections from experimental groups PC, NC and RA. Group PC (positive control): Distilled water + Food only.NC: (negative control): ARG+ MSG 90:22.5 mg kg−1 b.wt. RA: ARG+ MSG+ extract: 90:22.5:100 mg kg−1 b.wt.. Key: [ð] indicate lumen, [★] shows the gland unit while [○] shows the stroma and epithelial nuclei.
![Plate 1-3. (HE-200X): photomicrographs obtained from histopathological examination of prostate tissue sections from experimental groups PC, NC and RA. Group PC (positive control): Distilled water + Food only.NC: (negative control): ARG+ MSG 90:22.5 mg kg−1 b.wt. RA: ARG+ MSG+ extract: 90:22.5:100 mg kg−1 b.wt.. Key: [ð] indicate lumen, [★] shows the gland unit while [○] shows the stroma and epithelial nuclei.](/cms/asset/9092c9b3-f956-4073-aa42-ab217dec688b/tusc_a_1595358_f0003_oc.jpg)
Plate 4-5. (HE-200X): Photomicrographs obtained from the histopathological examination of sections of prostate tissue from experimental groups RB and RC. Group RB: ARG+ MSG+ extract: 90:22.5:200 mg kg−1 b.wt and group RC: ARG+ MSG+ extract: 90:22.5 + 300 mg kg−1 b.wt. Key: [ð] indicate lumen [★] shows the gland unit while [○] shows the stroma and epithelial nuclei.
![Plate 4-5. (HE-200X): Photomicrographs obtained from the histopathological examination of sections of prostate tissue from experimental groups RB and RC. Group RB: ARG+ MSG+ extract: 90:22.5:200 mg kg−1 b.wt and group RC: ARG+ MSG+ extract: 90:22.5 + 300 mg kg−1 b.wt. Key: [ð] indicate lumen [★] shows the gland unit while [○] shows the stroma and epithelial nuclei.](/cms/asset/4572a66f-a4ff-42a9-8c3e-7a3ee7534cab/tusc_a_1595358_f0004_oc.jpg)
DNA electrophoresis: Figure : . DNAgel electrophoretic band patterns of PCR amplified Annexin VII gene obtained from the prostate tissue of the male wistar rats.
Plate 6. DNA gel electrophoretic band pattern of PCR amplified annexin VII gene using 100 bp molecular marker [M]. Lane 1–2 (PC1-PC2), 3–4 (RB1-RB2), 5–6 (RA1-RA2), 7–9 (RC1-RC3), -ve (NC1-NC2). PC: (positive control): Distilled water + Food only. NC: ARG+ MSG 90:22.5 mg kg−1 b.wt. RA: ARG+ MSG+ extract: 90:22.5:100 mg kg−1 b.wt. RB: ARG+ MSG+ extract: 90:22.5:200 mg kg−1 b.wt. RC: ARG+ MSG+ extract: 90:22.5 + 300 mg kg−1 b.wt.
![Plate 6. DNA gel electrophoretic band pattern of PCR amplified annexin VII gene using 100 bp molecular marker [M]. Lane 1–2 (PC1-PC2), 3–4 (RB1-RB2), 5–6 (RA1-RA2), 7–9 (RC1-RC3), -ve (NC1-NC2). PC: (positive control): Distilled water + Food only. NC: ARG+ MSG 90:22.5 mg kg−1 b.wt. RA: ARG+ MSG+ extract: 90:22.5:100 mg kg−1 b.wt. RB: ARG+ MSG+ extract: 90:22.5:200 mg kg−1 b.wt. RC: ARG+ MSG+ extract: 90:22.5 + 300 mg kg−1 b.wt.](/cms/asset/9b288991-93da-44fb-a765-1283929067a8/tusc_a_1595358_f0005_ob.jpg)
5. Discussion
The high prevalence and mortality rate of prostate cancer among Nigerian men and men of African ancestry coupled with the lengthy period required for the development of the metastatic disease lead to research intervention on the chemotherapeutic prevention of prostate cancer [Citation29,Citation30]. Earlier reports have shown that anti-cancer activity of some compounds in sub-pharmaceutical doses could disrupt or slow the development of metastatic disease [Citation31,Citation32]. This present study evaluated the effect of ethanolic extract of A. muricata leaf on the ANXA7 gene of malignant prostatic hyperplasia induced male wistar rats. There was significant difference P ≤ 0.05 in the concentration of prostate specific antigen (PSA) values observed in the different treatment groups thus this demonstrates the potential of concomitant administration of L–ARG and MSG to elicit prostate dysfunction such as prostate cancer in male wistar rats. It’s in accordance with an earlier report that concomitant oral administration of L-ARG and MSG adversely affected the functionality of the prostate gland which was determined by elevated values of serum total and prostatic acid phosphates (TAP and PAP). Elevated TAP and PAP levels have being one of the characteristics of male serum with metastatic prostate cancer earlier used before the invention of prostate specific antigen (PSA) [Citation13,Citation14].
The significant P ≤ 0.05dose dependent decrease in the PSA value observed in group NC, RA, RB and RC could be attributed to the bioactive compounds present in the leaf extract of A. muricata (Tables and ) [Citation33–35] which account for the anti-proliferative efficacy exhibited by the extract in amelioration of the neoplastic effects exerted by the carcinogens as observed in the prostate specific antigen values. Phytochemicals derived from plants have been reported in various in-vivo and in-vitro studies to interfere with stabilization of microtubule structure thus inhibiting mitosis and cancer cell propagation. Polyphenols act by scavenging free radical and limit cancer cell proliferation by reducing DNA methylation through the inhibition of DNA methyltransferase together with reactivation of silenced tumour suppressor genes [Citation36–39].
The significant increase in body weigh P ≤ 0.05 recorded in Table for group NC may be as a result of the potential link between MSG intake and obesity as MSG has being reported to affect energy balance by disrupting the hypothalamic signalling cascade of leptin and actin [Citation40,Citation41]. The significant reduction P ≤ 0.05 in the weight of the male wistar rats in group RA, RB and RC may be due to possible toxicity of A. muricata leaf extract, this was earlier reported to be due to reduced food intake resulting from the secondary feeling of fullness and loss of appetite after the administration of the extract [Citation10] thus its effect masked the MSG effect in determining the weight of the experimental animal throughout the treatment period.
The features depict by the photomicrograph of the prostate tissue obtained from group PC is in conformity with the report [Citation42] indicating the feature of a normal prostate tissue histological section without any neoplastic change. The histological abnormalities observed in form of precancerous hyperplasia in the photomicrograph of the prostate tissue specimen obtained from groups, NC, RA, RB and RC suggest possible neoplastic change in the prostate tissue which usually marks the initiation and progression of the process of carcinogenesis. This have being earlier reported [Citation43] that similar precancerous lesion in the histological feature of the prostate tissue section obtained from male wistar rats treated with high dose of prolactin for 4 weeks. The changes in features of precancerous lesion observed in the treatment groups RA, RB and RC suggest emending effect of the A. muricata leaf extracts. This is in tandem with the recent in-vitro studies [Citation44] which indicate the crude extract of Annona muricata alone can be used as an alternative chemotherapy against pancreatic, prostate and breast cancer [Citation45]. The mechanism of action of the leaf extract of the leaf extract on cancer cells implies the disruption of mitochondrial membrane to arrest cells in Go/G1 phase and the induction of apoptosis by down regulating Bcl-2 and Bax proteins. Bcl-2 proteins are anti-apoptotic proteins while the Bax proteins mediates the leakage of pro-apoptotic factors including cytochrome C,Ca+2and mitochondrial Smac/DIABLO into the cytosol through dimerization and translocation to the outer mitochdrial membrane [Citation46,Citation47].
The amplicon from group NC showed a very faint band while those in the groups RA, RB and RC had varying band patterns and thickness similar to those in the group PC. These variations observed in group NC, RA, RB and RC can be attributed to possible mutagenic effect of MSG and L-ARG in inducing mutation in the cells exposed to these carcinogens which usually occur in genes regulating DNA damage responses, protoncogens and tumour suppressor gene such as annexin 7, a tumour suppressor gene (TSG) which is down regulated in prostate malignancy. Mutation in this gene is a hallmark in prostate cancer progression because it plays a vital role in maintaining cavin homeostasis, regulating cytoskeleton and cell motility [Citation48–50].
MSG induced DNA damage in testis of male albino rats determined using comet assay has been earlier reported by previous studies to be attributed to oxidative damage and changes in lipid peroxidation which play the vital role in toxicity and carcinogenesis of many carcinogens [Citation51–54]. The similarity observed between the positive control group (PC) and other groups RA, RB and RA can be attributed to the ability of the ethanolic leaf extract to reduce the degree of possible DNA damage induced by these carcinogens. The two bands produced by the amplicons from these different groups suggest the heterozygous nature of the annexin 7 gene alleles.
6. Conclusion
The results obtained from this investigation revealed that concomitant administration of L-arginine and monosodium glutamate in male wistar rats may lead to possible mutation in annexin 7, a tumour suppressor gene (TSG) that has been implicated in prostate malignancy. The ethanolic extract of A. muricata leaf exerted anti-proliferative efficacy on L-arginine and monosodium glutamate induce malignant prostatic hyperplasia in male wistar rats thus it can serve as biotherapeutics for such disease condition. High consumption of monosodium glutamate in men should be cautioned, especially among older men with high risk of prostate malignancy to prevent possible enhancement of the process of initiation and progression of the disease. However further research is recommended to determine the detailed mechanism of prostate carcinogenesis trailed by L-arginine and monosodium glutamate.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Andriole GL, Crawford ED, Grubb RL. Mortality results from a randomized prostate- cancer screening trial. N Engl J Med. 2009;360:1310–1319.
- Prostate Cancer Organization (PCO). Understanding the PSA test: a guide for men concerned about prostate cancer. [cited 2019 Jan 16]. Available from: <www.prostatecanceruk.org > 2019.
- America Cancer Society. Chemotherapy for prostate cancer. [cited 2019 Jan 16]. Available from: <www.cancer.org > 2019.
- Anne A, Grippo KC, Ben R, et al. Analysis of flavonoids phytoestrogens in botanical and ephedra-containing dietary supplements. Ann Pharmacother. 2007;41(9):1375–1382.
- National Cancer Institute (NCI). What you need to know about prostate cancer. [cited 2019 Jan 16]. Available from: <http://www.cancer.gov/cancertopics/wyntk/prostate.pdf > 2019.
- National Cancer Institute. Surveillance, epidemiology and end results program: prostate cancer statistics [cited 2019 Jan 17]. Available from: <www.seer.cancer.gov > 2016.
- Singh K, Ahluwalia P. Study on effect of monosodium glutamate administration on some antioxidant enzymes in arterial tissue of adult mice. J Nutr Sci Vitaminol. 2003;49:145–148.
- Rang HP, Ritter JM., Dale MM. Pharmacology. 5th ed. Edinburgh: Churchill Livingstone; 2003.
- Husarova V, Ostatnikova D. Monosodium glutamate toxic effects and their implications for human intake. A review. J. Med Res. 2013;4:8–10.
- David JT, Derek JV, Yuipanqui AC, et al. Effect of Arginase II on L-arginine depletion and cell growth in murine cell lines of renal cell carcinoma. J. Ha Oncol. 2008;1(14):1756–1770.
- Jeremy N, Appleton D. Arginine: clinical potential of semi amino acid. Altern Med Rev. 2002;7(6):512–522.
- Wu G, Moris SM. Arginine metabolism: nitric oxide and beyond. Biochem J. 1998;336:1–17.
- Egbuonu AC, Ejikeme PM, Ezeanyika LUS, et al. Combined oral arginine and monosodium glutamate exposure Induces Adverse response on the prostate function and testis Histology of male wistar rats. Br J Pharm Res. 2013;3(2):247–258.
- Egbuonu AC, Ejikeme PM, Obasi LN. Monosodium glutamate: Potentials at inducing prostate pathologies in male wistar rats. Afr J Biotechnol. 2010;9(36):5950–5954.
- Chang FR, Liaw CC, Lin CY. New adjacent bis-tetrahydrofuran annonaceous acetogenins from Annona muricata. Plant Medication. 2003;69:241–246.
- Sohiel ZM, Mehran F, Sonia N, et al. Annona muricata: A Review of Its Traditional Uses, isolated acetogenins and biological activities. Int J Mol Sci. 2015;16:15625–15658.
- Kossouoh C, Mouduchirou M, Adjakidje V. Essential oil chemical composition of Annona muricata L. leaves from Benin. J Essent Oil Res. 2007;19:307–309.
- Olufemi TO, Olayiwola BS. Increased Incidence of prostate cancer in Nigeria. J Natl Med Assoc. 1999;91(3):159–164.
- Woo HD, Kim J. Dietary flavonoids intake and risk of stomach and colorectal cancer. World J Gastroenterol. 2013;19(7):1011–1019.
- Cardo VM, Arden KC, Cavenee WK, et al. Is annexin 7 a tumor suppressor gene in prostate cancer? Pharmacogenomics J. 2001;1:92–94.
- CancerCouncil Report. Understanding Prostate Cancer. [cited 2019 Jan 18]<www.cancercouncil.com.au > 2013.
- Suleiman H, Roslida AH, Fezah O, et al. Chemopreventive potential of Annona muricata L leaves on Chemically-induced Skin Papillomagenesis in Mice. Asian Pac J Cancer P. 2012;13:2533–2539.
- Larbies C, Arthur FK, Woode E, et al. Evaluation of Acute and Sub-chronic toxicity of Annona muricata (Linn) Aqueous extract on animals. Eur J Exp Biol. 2011;1(14):115–124.
- World Medical Association Declaration of Helsinki. Ethical principles of medical research involving human subjects. 59th WMA General Assembly, Seoul, 2008.
- Egbuonu ACC, Obidoa O, Ezeokonkwo CA, et al. Hepatic effect of low dose of oral administration of monosodium glutamate in male albino rats. Afr J Biotechnol. 2009;8:3031–3035.
- Lena C, Olga V, Mena G, et al. Prostate Histology: Learning tissue component Histogram for cancer Detection and Classification. IEEE Trasaction on Med Imag. 2013;32(10):1804–1818.
- Ayoola GA, Coker HA, Adesegun SA, et al. Phytochemical screening and antioxidant activities of some selected medicinal plants used for malaria therapy in south western Nigeria. Trop J Pharm Res. 2008;7(3):1019–1024.
- Yap Lim H. Over expression of wild type ANXA7 tumor suppressor gene targets cancer- related microRNAs in human prostate cancer cells (M.Sc dissertation, University of Malaya, Malaysia). [cited Jan 30] Available from: <www.umcancer.webs.com > 2013.
- Stephen OI, Olufumilade AO, Muftau JB, et al. Prevalence and characteristics of prostate cancer among participants of a community-based screening in Nigeria using serum prostate specific antigen and digital rectal examination. Pan African Med J. 2013;15:129–136.
- Ebugle GA, Ekanem IA, Omoronyia OE, et al. Prostate cancer incidence in Calabar –Nigeria. Br J Med Med Res. 2016;14(5):1–10.
- Pitcham P, Ogawa K, Suzuki S, et al. Momordica charantia leaf extract suppresses rat prostate cancer progression in vitro and in vivo. Cancer Sci. 2010;101(10):2234–2240.
- Roslida AH, Tay CE, Zuraini A. Anti-inflammatory and antinouceptive activities of the ethanolic extract of Annona muricata leaf. Int J Natural Remedies. 2010;10:97–104.
- Jiang J, Hu C. Evodiamine: a novel anticancer alkaloid from Evodia rutaecarpa. Molecules. 2009;14:1852–1859.
- Minari JB, Okeke U. Chemopreventive effect of Annona muricata on DMBA-induced cell proliferation in the breast tissues of female albino mice. Egypt J Med Hum Genet. 2014;15:327–334.
- Ye F, Xui L, Yi J, et al. Anticancer activity of Scutellaria baicalensis and its potential mechanism. J Altern Complem Med. 2002;8:567–572.
- Jisun OH, Lynn H, Yong-Seob J, et al. Therapeutic effectiveness of anticancer phytochemicals on cancer stem cells. JToxins. 2016;8:199–210.
- Graham JG, Quinn ML, Fabricant DS, et al. Plant use against cancer –extension of the work of Jonathan Hartwell. J Ethnopharmacol. 2000;73:347–377.
- Amin A, Gali-Muhtasib H, Ocker M, et al. Overview of major classes of plantderived anticancer drugs. Int J Biomed Sci 2009;5:1–11.
- Diederich M, Cerella C. Non-canonical programmed cell death mechanisms triggered by natural compounds. Semin.Cancer Biol. 2016;20(2):89–97.
- He K, Du S, Xun P, et al. Consumption of monosodium glutamate in Relation to issues of Overweight in Chinese Adult: China Health and Nutrition Survey (CHNS). Am J Clin Nutr. 2011;93(6):1328–1336.
- Hermanussen M, Tresguerres JAF. Does high glutamate intake cause obesity? J Pediatr Endoc M. 2003;16(7):965–968.
- Husarova V, Ostatnikova D. Monosodium glutamate Toxic effects and Their Implications for human intake. A review. J Med Res. 2013;4:8–10.
- Herrera-Covarrubias GA, Coria-Avila P, Chavarria-Xicotencent P, et al. Long-Term administration of prolactin or Testosterone induced similar precancerous prostate Lesions In rats. Exp J Oncol. 2015;37(1):13–18.
- Torres MP, Rachagari V, Purohit I. Graviola: a novel promising natural-derived drug that inhibit tumorigenic and metastasis of prostate, pancreatic and breast cancer cell lines in-vivo and in-vitro through altering cell metabolism. Cancer Lett. 2012;323(1):29–40.
- Jee YK, Thien TP, Dao KS, et al. Annona muricata leaf extract triggered intrinsic Apoptotic pathway to Attenuatecancer features of triple Negative Breast Cancer MDA –MD -23 Cells.2018. [cited 2019 Jan 30]<http://doi.org/10.1155/2018/7972916 >.
- Pieme CA, Kumar MS, Dongamo BM, et al. Anti-proliferative and induction of apoptosis by Annonaceae extract on human cancer cells. BMC Complement Altern Med. 2014;14(1):516–521.
- Asare GA, Afriyies RA, Ngala H, et al. Antiproliferative activities of aqueous leaf extract of Annona muricata L. on the prostate, BPH-1 cells and some target genes. JIntergr.Can.Ther. 2015;14(1):65–74.
- Koochekpour S, Majumdar S, Azabdaftari G, et al. Serum glutamate levels correlate with Gleason score and glutamate blockade decrease proliferation, migration, invasion and apoptosis in prostate cancer cells. Clin Cancer Res. 2012;18:5888–5901.
- Xin W, Rhodes DR, Ingold C. Deregulation of the annexin protein family is associated with prostate cancer progression. Am J Pathol. 2003;162:255–261.
- Liu W, Shen JJ, Tanzillo SA. Annexin II expression is reduced in prostate cancer cells and its re-expression inhibits prostate cancer cell migration. Oncogene. 2003;22:1475–1485.
- Ismail NH. Assessment of DNA damage in testis from young wistar male rats treated with monosodium glutamate. Life Sci J. 2012;9(1):1930–1939.
- Schinella GR, Tournier HA, Prieto JM, et al. Antioxidant activity of anti-inflammatory plant extract. Life Sci. 2002;70:1023–1033.
- Palovic V, Pavlovic D, Kocic D, et al. Effect of monosodium glutamate on oxidative stress and apoptosis in rat thymus. J. Mol Cel Bi. 2007;303:161166.
- Farmorbi EO, Onyema OO. Monosodium glutamate induces oxidative damage and genotoxicity in rats: Modulatory role of vitamin C, vitamin E and quercetin. Hum. Exp. Toxicol. 2006;25:251–259.