 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
This study aimed to assess the ability of two Lebanese duckweed species, (Lemna minor and L. gibba), to grow under in vitro conditions on three nutritive solutions, Murashige Skoog (MS), Schenk-Hildebrand (SH) and Algal Assay Procedure (AAP). Plant growth of both species, expressed as doubling time, differed significantly (p < .05) between the tested media with best results obtained on SH for L. minor after 7 days of culture and on AAP for L. gibba after 2.7 days for both species. Growth index was significantly higher (P < .05) for L. minor on SH reaching 41.6 after 21 days of culture whereas L. gibba exhibited its highest growth index of 15.51 on AAP. These results indicate the efficiency of SH and AAP media in promoting the vegetative proliferation of L. minor and L. gibba to further provide a good source of duckweed material for phytoremediation applications.
1. Introduction
Lemna (duckweed) is a small-sized freshwater floating macrophyte from the family Lemnaceae. The individual plant consists of a leaf-like structure, a frond, connected to a fine rootlet [Citation1]. The species inhabits stagnant to gently flowing surface waters and reproduces mainly vegetatively giving rise to genetically uniform plant populations [Citation1]. Lemna sp. are commonly cultured for wastewater treatment in the Mediterranean climate [Citation2–5].
Two Lemna species are reported to be naturally found in Lebanese water bodies and precisely in Litani River, Bekaa [Citation6–8]. In spite of the number of reported studies on pollution concerning the Lebanese fresh waterbodies [Citation9–11], few studies are concerned with the phytoremediation using macrophytes of the polluted waterbodies [Citation12]. These efforts were mostly limited to the investigation of bio-filtration in the wetland in the Upper Litani River basin to evaluate the constructed wetland performance and determine the treatment efficiency and improvement of the water quality [Citation13].
Duckweeds grow rapidly, and this is vital for their ability to colonize open water surfaces in nature [Citation1,Citation14] whereby accumulation of the biomass is often coupled with nutrient and heavy metals removal from wastewater [Citation3,Citation4,Citation15]. When conditions, i.e. water temperature, pH, light and nutrient concentrations, are optimal, duckweed often demonstrates near exponential growth rates and double the biomass in between 2 and 3 days depending on the environmental conditions [Citation16]. Vegetative growth in Lemna sp exhibits cycles of senescence and rejuvenation under constant nutrient availability and consistent climatic conditions [Citation14].
Axenic cultures of duckweeds established under in vitro conditions are often used for biological research and stock-culture maintenance. Lemna plants can be grown in aseptic nutritive solutions that supply essential macro- and micro-nutrients. Several basic solutions were reported for the proliferation of Lemna species, e.g. Murashige and Skoog (MS) [Citation17], Schenk and Hildebrandt (SH) [Citation18] and Algal Assay Procedure (AAP) [Citation19]. These nutrient solutions have been widely used in plant tissue culture [Citation16] and in ecotoxicological studies according to the standardized methods and protocols [Citation19,Citation20]. Duckweeds require many nutrients and minerals to support its optimal growth. Duckweeds appear to be able to concentrate many macro and micro minerals from water [Citation21]. Duckweeds have developed many mechanisms to concentrate trace minerals from slowly decaying plant materials for growth which is often more affected by the concentrations of nitrogen, phosphorous, potassium and sodium levels [Citation21]. There is a large number of nutritive media in use for duckweed cultivation which implies that duckweed can adapt to a broad range of conditions, while the physiological responses are not always the same [Citation22].
In vitro culture, if properly optimized, can satisfy all requirements for Lemna proliferation. It provides a reliable approach for a sustainable and high rate multiplication of Lemna, which in turn may enhance remediation ability by providing a tool to understand the uptake of heavy metals for phytoremediation applications [Citation23].
This study aimed to investigate the growth potential under in vitro conditions of two Lebanese duckweed species L. minor L. and L. gibba L., collected from two different sites at the Litani River Ghouzeil River and Haouche Harimeh tributaries, respectively, in Bekaa region. The proliferation ability is assessed under axenic and controlled conditions, in response to different nutritive solutions MS, SH and AAP. Such proliferation system is the first step towards enabling trials for future phytoremediation research.
2. Materials and methods
2.1. Pre-culture of plant materials and culture conditions
Plants of L. minor and L. gibba collected respectively from Ghouzeil River and Haouche Harimeh tributaries (Bekaa) in October 2017. Plant individuals were introduced in the laboratory where they were subject to thorough washing with tap water for 10 times. Fronds were then surface-sterilized for one minute by immersion in 0.5% sodium hypochlorite solution, before being rinsed five times with sterile distilled water. Sterilized plants were periodically transferred to 500-ml Erlenmeyer flasks containing 250 ml of sterile culture nutritive solutions. Three nutritive solutions were tested: MS [Citation17], SH [Citation18] and Algal Assay Procedure (AAP) [Citation19] differing by their mineral composition (Table ). Daughter plants derived from single mother frond were kept and maintained as stock cultures while being exposed to continuous light conditions (24 h) at 100 μmol/m2/s light intensity from fluorescent light tubes (18W/54) at 25°C [Citation20]. Only individual plants with three fronds were used for the experiments of proliferation.
Table 1. Composition of nutrient solutions utilized for the cultivation of L. minor and L. gibba.
2.2. In vitro growth proliferation
Fronds of both species were chosen randomly from a mixture of at least four flasks of pre-cultured plants. Cultures were grown aseptically under the same conditions described above, in three replicates of 10 plants of three fronds each per medium. Subcultures are renewed every seven days to prohibit nutrient limitation. Lemna proliferation was monitored on the different growth media based on the visible fronds number and fresh weight scored during the experiment and at t = 0 and 2, 5, 7, 9, 12, 14, 19, and 21 days of culture and using the following variables:
Doubling time (DT) determined in terms of frond numbers at different days of culture according to the formula of Zeigler et al. [Citation14]:
where RGR is relative growth rate per day [Citation24]:
where N0 = initial number of fronds, Nt = number of fronds scored at t days of culture, and t = days of culture.
As well the doubling time after 7 days of culture was recorded according to the OECD guidelines [Citation19].
Growth index (GI) calculated according to Khellaf et al. [Citation25]:
where FW0 = initial fresh weight (biomass production) of fronds and FWt = fresh weight of fronds (biomass production) scored on different days of culture.
2.3. Data analysis
All quoted data give the mean values obtained from six replicate measurements, together with the Standard Error (SE). Data were analysed using ANOVA and Duncan test. The regression equations for the best-fitted prediction model for growth index variable were built in function with the time of cultivation. Statistical analysis was performed with the statistical software processor IBM SPSS 22. Differences between treatments were considered as statistically significant at P < .05.
3. Results
Different effects of the nutrient media SH, MS and AAP on the vegetative growth of L. minor and L. gibba during in vitro cultivation are demonstrated in this study. Many constituents are commonly present in SH, MS and AAP media at different concentrations, including KNO3, MgSO4, KH2PO4, H3BO3, NaMoO4 and Na2EDTA (Table ). With the exception of ZnSO4.7H2O, the macro and micro nutrients concentrations in SH are higher than those in MS solutions. SH also contains the CoCl2.6H2O while this micro-nutrient is absent in MS [Citation22]. The nutrient solution AAP recommended by the OECD guidelines [Citation19] has higher concentrations of macro and micro nutrients. Moreover, NaHCO3 is added to AAP to stabilize the medium and is actually a good bicarbonate source needed for growth.
3.1. Fronds number and doubling time
In terms of fronds number, L. minor and L. gibba recorded different proliferation responses along the cultures on the different nutritive media (Figure ). Lemna minor displayed a higher proliferation rate on SH medium with 176 ± 4.9 and 389 ± 13.5 newly developed fronds by the 7th and 14th day of start respectively (Figure ). Whereas, the proliferation rate recorded 129 ± 3.18 and 227 ± 3.21 on AAP and 100 ± 1.6 and 72 ± 3.84 fronds on MS medium during the same corresponding time intervals. For L. gibba, higher proliferation rate was found on AAP media with 174 ± 4.06 and 342 ± 1.76 newly developed fronds after 7th and 14th day respectively vs. 115 ± 2.85 and 168 ± 9.13 new fronds obtained on SH medium and 119 ± 1.4 and 131 ± 0.67 fronds on MS medium during the same time interval. Remarkably, an exponential frond proliferation is recorded up to the 30th day of culture for L. minor on SH medium (1096 ± 17.9 fronds) and for L. gibba on AAP medium (650 ± 12.3 fronds).
Figure 1. Mean fronds number and SE of L. minor and L. gibba after 21 days of cultivation on SH, MS and AAP media.
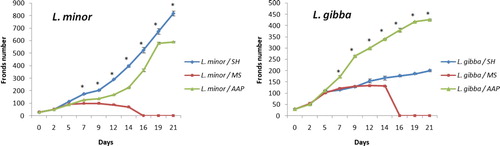
The DT increased for both species along cultures on the three media, resulting from the deceleration of the frond proliferation, with significant effects recorded for both species and nutritive media (Table ). As to L. minor, DT passed from 2.5 at day 5 to 4.4 and above on both SH and AAP while DT becomes nul after day 14 on MS medium. Similarly, for L. gibba, DT increased along cultures from 2.4 at day 5 to 5.4 reached at day 21 on AAP medium vs. 7.6 on SH medium, while DT was nul after day 14 on MS medium.
Table 2. Mean values and standard errors of DT of proliferation of L. minor and L. gibba along 21 days of culture.
At seven days of culture, doubling time of frond number of both L. minor and L. gibba differed between the different test media (Table ; Figure ). Lowest growth was found on MS medium for L. minor and L. gibba where average DT values ranged from 3.3–4.0 days. Best doubling time was found on SH medium for L. minor and on AAP for L. gibba with 2.7 days.
Figure 2. The doubling time (DT in days) of L. minor and L. gibba plants in different nutrient media (SH, MS and AAP) based on frond number after 7 days of culture. Line indicates a DT of 2.5 days according to OECD guidelines [Citation19] for the growth inhibition test for Lemna spp.
![Figure 2. The doubling time (DT in days) of L. minor and L. gibba plants in different nutrient media (SH, MS and AAP) based on frond number after 7 days of culture. Line indicates a DT of 2.5 days according to OECD guidelines [Citation19] for the growth inhibition test for Lemna spp.](/cms/asset/e541dc1b-73af-4d9c-a2a9-fa7aacbc5b3d/tusc_a_1597450_f0002_oc.jpg)
3.2. Fresh weight and growth index
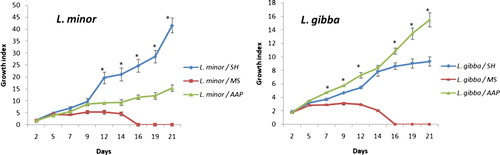
The ANOVA analysis shows that the fresh weight (biomass production) of L. minor and L. gibba, determined by GI, is relatively similar on SH, MS and AAP media after five days of culture, before becoming significantly higher (P < .05) on SH medium later on (Figure ). The growth index of both species decreases in MS medium where the proliferation ceased after 14 days of culture.
Figure 3. Growth index of L. minor and L. gibba on SH, MS and AAP media on day 2, 5, 7, 9, 12, 14, 16, 19 and 21.
Lemna minor displayed a higher biomass production on SH medium of 196.43 ± 4.2 mg and 597.63 ± 35 mg of fresh weight by the 7th and 14th day of start respectively. While the fresh weight was 166.53 ± 6.7 mg and 282.73 ± 36.3 mg on AAP and 114.13 ± 8.2 mg and 124.63 ± 21.6 mg on MS medium by the 7th and 14th day of culture. For L. gibba, higher biomass production was found on AAP media with 459.7 ± 22.6 mg and 813.6 ± 15.4 mg fresh weight after 7th and 14th day respectively vs. 305.6 ± 5.5 mg and 643.3 ± 47.5 mg obtained on SH medium and 242.9 ± 10.3 mg and 171.7 ± 12.9 mg on MS medium during the same time interval (Figure ).
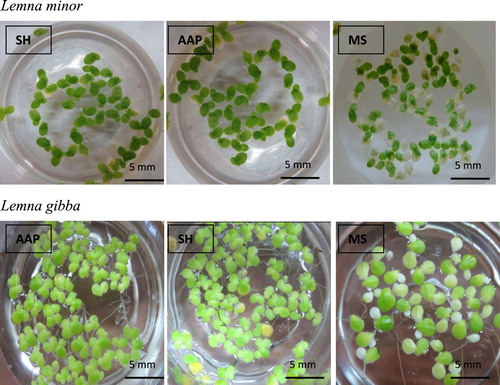
Figure 4. Proliferation of L. minor and L. Gibba on different nutrient media (SH, MS and AAP) after 7 days of culture (bar = 5 mm).
After 21 days of cultivation, the growth index of L. minor on SH medium was 41.6 ± 3.08 vs. 15.4 ± 1.33 recorded on AAP medium (about 2.7 times higher than on AAP medium). For L. gibba, GI on AAP medium at 21 days was 15.51 ± 1.04 vs. 9.3 ± 0.6 recorded on SH medium (about 1.66 times higher than on SH medium).
The linear regression model built to describe growth index as a function of cultivation time and on different nutrient media resulted in the below equations (1-6); R2 ranged from 0.40 - 0.95 for both species.
For L. minor:
(1)
(1)
(2)
(2)
(3)
(3)
For L. gibba:
(4)
(4)
(5)
(5)
(6)
(6)
The growth index of L. minor in the three different media showed that the coefficients for time (days) were 1.96, 0.23, 0.65 for SH, MS and AAP media respectively; whereas, the coefficients for time (days) for L. gibba were 0.43, 0.026 and 0.71 for SH, MS and AAP media respectively. This confirms that L. minor displayed maximum GI on SH, with an expected increase of 1.96 average for every additional day in time. As to L. gibba, it displayed its highest GI on AAP medium, for every additional day in time GI is expected to increase by an average of 0.71.
4. Discussion
The effects of SH, MS and AAP nutrient solutions on the in vitro vegetative growth of L. minor and L. gibba are investigated. Proliferation of axenically grown L. minor and L. gibba is determined based on the frond doubling time and growth index.
Our results indicate that the biomass production of L. minor and L. gibba in SH and AAP solutions, respectively, are significantly higher than those obtained in MS. These results are in line with the suggestions of OECD [Citation19] which recommends the growth of L. gibba on AAP, while MS is rather suitable for maintaining stock cultures of Lemna species [Citation16, Citation22]. Actually, the macro and micro nutrients concentrations are generally higher in SH than MS solutions [Citation22]. It is also worthy noted that the high concentration of phosphate in SH and AAP normally accelerates Lemna proliferation and its frond doubling time while this process remains slower in MS medium. Horemans et al. [Citation26] found that L. minor growth rates were similar in SIS-medium and K-medium and generally decreased with lowering phosphate concentrations.
Previous works showed that L. minor and other duckweeds grow on many diluted inorganic salt solution with essential macro-and micro nutrients such as SH, MS and Hoagland [Citation16], with however distinct growth and physiological responses according to the composition of the nutritive solution [Citation14,Citation27]. Yu et al. [Citation28] reported an efficient frond proliferation for L. minor in SH solution.
Other examples are reported in the literature where floating plant species exhibited different average growth rates across the tested media. Lemon et al. [Citation29] examined the growth of L. minor, Spirodela polyrhiza, and Wolffia borealis, at high nutrients levels (33% v/v Hutner’s medium) and found that W. borealis has the highest growth rate, while S. polyrhiza has the lowest in terms of frond proliferation. Lemon et al. [Citation29] explained that differences in growth rates can be the result of faster development and release of daughter fronds and a longer life span. Ge et al. [Citation30] recorded a growth cycle of 27 days for L. minor when measuring the biomass production and starch accumulation grown in SH medium under sterile conditions compared to wastewater. The biomass increased almost linearly between day 6 and 21 with an average growth rate of 14.1 g dry weight per day and a doubling time of 2.1 days [Citation30]. Patel et al. [Citation31] inspected the effects of MS, B5 and White media on the rates of frond proliferation of L. gibba based on the frond doubling time after 28 days of cultivation. Plant responses of L. gibba to White medium exhibited a higher biomass production and rapid frond proliferation in in vitro culture.
Lemna minor exhibited a better growth index on SH medium as resulted from the regression model (regression coefficient 1.96, R2 = 0.89) while L. gibba grew better on AAP medium (regression coefficient 0.71, R2 = 0.95). This could be a good predictive model for the growth of duckweed for further studies for phytoremediation purposes for nutrients and heavy metal uptake. The growth index of L. minor and L. gibba reached after 21 days 41.6 and15.51, respectively, on SH on AAP media. Similar results were also recorded by Ziegler et al. [Citation14] for five floating plant genera grown in SH medium. Conversely, Zhang et al. [Citation32] indicated that the nutrient concentrations had no significant influence on the growth rate of L. minor if the nitrogen concentration was between 1 and 5 mg/L in the culture medium. They pointed that the kinetics of duckweed growth and nutrient uptake may provide insights into the uptake mechanisms and facilitate the predictive modelling of nutrient uptake in nature [Citation32]. As stated by Goulet et al. [Citation33] the composition of the plant nutrient medium, e.g. the higher amounts of phosphate necessary to sustain L. minor growth, could greatly influence heavy metal uptake. The nutrient medium described in the guidelines for a standard L. minor and L. gibba growth inhibition test [Citation19] is indeed rich in phosphate and other ions.
Following 14 days of cultivation in MS medium, the biomass of our both species did not increase further, indicating that the growth potential for duckweed in MS culture was not more than 14 days. MS medium is recommended to provide a slower proliferation needed for stock culture cultivation, while SH medium is suggested by Appenroth [Citation22] to be an optimized culture medium for L. minor, and AAP is recommended by OECD [Citation19] to grow L. gibba. The duckweed plants grown in the SH and AAP media had a longer growth cycle due to proper nutrient ingredients. When grown in MS medium, the duckweed biomass was usually lower compared to that in the SH and AAP media, due to the low nutrient levels.
Frond number DT increased for both species along cultures on the three media, resulting from the decrease of the frond proliferation, with significant effects recorded for both species and nutritive media. Horemans et al. [Citation26] stated that growing plants for longer periods in any of the different experimental media resulted in lower doubling time for the tested media. In other similar studies, Kittiwongwattana and Vuttipongchaikij [Citation27] found that under experimental conditions, L. minor cultivated in Hoagland medium displays a faster proliferation rate than in MS medium with 2.3 days doubling time and a growth index of 24.03, around 2.2 times higher than in MS medium.
The rapid growth of duckweed together with the simplicity of in vitro cultivation, have contributed to the importance of the use of duckweed as an experimental plant model [Citation2, Citation4, Citation14, Citation24, Citation34, Citation35]. Lemna species are commonly used for bioassays for heavy metals and nutrients uptake over a standardized 7-day growth inhibition test according to the standardized methods and protocols [Citation19,Citation20] with specified DT of Lemna species of 2.5 days as a validity criterion.
Based on our observation, at seven days culture, DT of both L. minor and L. gibba differed between the different test media. Lowest growth was found on MS medium which has lower phosphate concentration, for both L. minor and L. gibba (3.3 and 4.0 days, respectively). These values were all significantly higher than those obtained at 2.5 days, which is the validity criteria indicated in the OECD guidelines [Citation19] for the growth inhibition test for Lemna spp. Best growth was found on SH medium for L. minor and on AAP for L. gibba with 2.7 days. Similarly, lower growth was previously recorded for L. minor with the decrease of phosphate concentrations contained in the nutritive solutions as evidenced by a doubling time significantly higher than 2.5 days [Citation26].
Depending on the conditions of the growth medium, species of duckweed differ in growth rates and biomass production. These species-specific differences are important because nutrient uptake differs depending on the source of nutrient loading and other various factors [Citation14, Citation36].
5. Conclusion
The findings of this study showed that SH and AAP media may be efficiently used for growing Lebanese duckweeds when high biomass production is needed. In contrast, the slower growth rate can be obtained by using MS medium which may therefore be used for sustaining stock cultures. Additionally, the effects of media on plant growth may also be a determining factor for the selection of medium that can be used in further investigations. Moreover, the variation in growth rates of different Lemna sp. provides a particularly good understanding of how different the response and productive potentials of the various duckweeds can be. Therefore, further research tackling the responses of Lemna sp. to nutrients will have practical recommendations for both management and phytoremediation applications.
Acknowledgements
The authors would like to acknowledge the valuable guidance and kind support of Dr. F. As-sadi and Dr. L. Itani for the processing and statistical analysis of the data.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
H. Ghanem http://orcid.org/0000-0003-3904-6711
S. Baydoun http://orcid.org/0000-0003-1820-4995
H. Abou Hamdan http://orcid.org/0000-0002-3615-2538
S. Korfali http://orcid.org/0000-0002-2038-510X
L. Chalak http://orcid.org/0000-0003-1301-982X
References
- Landolt E. Biosystematic investigation on the family of duckweeds: the family of Lemnaceae a monograph study. 1986. Veroffentlichungen des Geobotanischen Institutes ETH, StiftungRubel, Zurich, Switzerland.
- Hegazy AK, Kabiel HF, Fawzy M. Duckweed as heavy metal accumulator and pollution indicator in industrial wastewater ponds. Desal Wat Treat. 2009;12:400–406. doi: 10.5004/dwt.2009.956
- Allam A, Tawfik A, Negm A, et al. Treatment of drainage water containing pharmaceuticals using duckweed (Lemna gibba). En Proc. 2015;74:973–980. doi: 10.1016/j.egypro.2015.07.734
- Allam A, Tawfik A, El-Saadi A, et al. Potentials of using duckweed (Lemna gibba) for treatment of drainage water for reuse in irrigation purposes. Desal Wat Treat. 2016;57(1):459–467.
- Shammout M, Zakaria H. Water lentils (duckweed) in Jordan irrigation ponds as a natural water bioremediation agent and protein source for broilers. Eco Eng. 2015;83:71–77. doi: 10.1016/j.ecoleng.2015.05.041
- Ismail H, Abou-Hamdan H, Kobaissi A, et al. Investigation on macrophyte development in Litani River (Lebanon) subjected to human disturbances. Eco Medi. 2009;35:31–39.
- Tohme G, Tohme H. Illustrated Flora of Lebanon, National Council for Scientific Research Publication. 2nd edition. Beirut: CNRS; 2014.
- Abou-Hamdan H, Ismaïl H, Amacha N, et al. Preliminary assessment of macrophytic community in Qaraoun reservoir. Lebanon. Int J Sc Res (IJSR. 2014;3:1132–1141.
- Saadeh M, Semerjian L, Amacha N. Physicochemical evaluation of the upper Litani River watershed, Lebanon. Sci World J. 2012;2012:462–467. doi: 10.1100/2012/462467
- Haydar C, Nehme N, Awad S, et al. Physiochemical and microbial assessment of water quality in the Upper Litani River Basin. Lebanon. J Env and Earth Sc. 2014;4:87–98.
- Korfali K, Jurdi M, Amacha N. Sources and levels of metals in the Upper Litani Basin soils: Lebanon. J Env Sci Eng. 2014;3:55–71.
- Abou-Hamdan H, Ismail H, Mouawad R, et al. Macrophytic communities of Ghouzaiel, a Mediterranean river of Bekaa plain (Lebanon) with anthropic disturbances. Int J Inno Res Sc Eng. 2014;2:430–442.
- Amacha N, Karam F, Jerdi M, et al. Assessment of the efficiency of a pilot constructed wetland on the remediation of water quality; case study of Litani River. Lebanon. Env Pollut Clim Chan. 2017;1:119–125.
- Ziegler P, Adelmann K, Zimmer S, et al. Relative in vitro growth rates of duckweeds (Lemnaceae) – the most rapidly growing higher plants. Plant Biol. 2015;17:33–41. doi: 10.1111/plb.12184
- Landesman L, Fedler C, Duan R, et al. Eutrophication: causes, consequences and control. In: Ansari S, editor. Plant nutrient phytoremediation using duckweed. Berlin: Springer Science + Business Media B.V.; 2011. p. 341–354.
- Stomp A. The duckweeds: A valuable plant for biomanufacturing. Biotech Ann Rev. 2005;11:69–99. doi: 10.1016/S1387-2656(05)11002-3
- Murashige T, Skoog F. A revised medium for rapid growth and bio assay with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x
- Schenk RU, Hildebrandt AC. Medium and techniques for induction and growth of Monocotyledonous and Dicotyledonous. Plant Cell Cult. 1972;50:199–204.
- OECD 221. Guidelines for the testing of chemicals. Revised proposal for a new guideline 221. Lemna sp. Growth Inhibition Test. 2006.
- ISO 20079:2006. Water quality – determination of the toxic effect of water constituents and waste water to duckweed (Lemna minor) – Duckweed growth inhibition test. 2007. BS EN ISO ICS 13.060.70.
- FAO. Duckweed: A tiny aquatic plant with enormous potential for agriculture and environment. Rome: FAO production book; 1999.
- Appenroth KJ. Media for in vitro cultivation of duckweed. Inter St Com Du Res App (ISCDRA). 2015;3:180–203.
- Czako M, Feng X, He Y, et al. In vitro propagation of wetland monocots for phytoremediation. In: Mackova M., Dowling D., Macek T., editors. Phytoremediation Rhizoremediation. Dordrecht: Springer; 2006. p. 217–225.
- Naumann B, Eberius M, Appenroth KJ. Growth rate based dose–response relationships and EC-values of ten heavy metals using the duckweed growth inhibition test (ISO 20079) with Lemna minor L. clone St. J Plant Phys. 2007;164:1656–1664. doi: 10.1016/j.jplph.2006.10.011
- Khellaf N, Zerdaoui M, Faure O. Growth response of the duckweed Lemna minor to heavy metal pollution. Iran J Environ Health Sci Eng. 2010;6:161–166.
- Horemans N, Hees MV, Senen E, et al. Influence of nutrient medium composition on uranium toxicity and choice of the most sensitive growth related endpoint in Lemna minor. J Env Radio. 2016;151:427–437. doi: 10.1016/j.jenvrad.2015.06.024
- Kittiwongwattana C, Vuttipongchaikij S. Effects of nutrient media on vegetative growth of Lemna minor and Landoltia punctata during in vitro and ex vitro cultivation. Maejo Inter J Sci Tech. 2013;7(01):60–69.
- Yu C, Sun C, Yu L, et al. Comparative analysis of duckweed cultivation with sewage water and SH media for production of fuel ethanol. Plos One. 2014;9(12):e115023. doi: 10.1371/journal.pone.0115023
- Lemon GD, Posluszny U, Husband BC. Potential and realized rates of vegetative reproduction in Spirodela polyrhiza, Lemna minor, and Wolffia borealis. Aqu Bot. 2001;70:79–87. doi: 10.1016/S0304-3770(00)00131-5
- Ge X, Zhang N, Phillips GC, et al. Growing Lemna minor in agricultural wastewater and converting the duckweed biomass to ethanol. Biores Tech. 2012;124:485–488. doi: 10.1016/j.biortech.2012.08.050
- Patel H, Ramchandra S, Patel PK. Effect of different nutrient medium on growth of Lemna gibba during in vitro culture. Inter J Pharma and Bio Sci. 2015;6(4):193–198.
- Zhang K, Chen YP, Zhang TT, et al. The logistic growth of duckweed (Lemna minor) and kinetics of ammonium uptake. Env Tech. 2014;35(5):562–567. doi: 10.1080/09593330.2013.837937
- Goulet RR, Thompson YPA, Serben KC, et al. Impact of environmentally based chemical hardness on uranium speciation and toxicity in six aquatic species. Env Tox Chem. 2015;34(3):562–574. doi: 10.1002/etc.2834
- Radic S, Stipanicev D, Cvjetko P, et al. Duckweed Lemna minor as a tool for testing toxicity and genotoxicity of surface waters. Ecotox Env Safety. 2011;74:182–187. doi: 10.1016/j.ecoenv.2010.06.011
- Mkandawire M, Teixeira daSilva JA, Dudel EG. The Lemna bioassay: Contemporary issues as the most standardized plant bioassay for aquatic ecotoxicology. Crit Rev Env Sc Tech. 2014;44(2):154–197. doi: 10.1080/10643389.2012.710451
- McCann MJ. Response diversity of free-floating plants to nutrient stoichiometry and temperature: growth and resting body formation. PeerJ. 2016;4:e1781. doi: 10.7717/peerj.1781
