?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The present paper reports a simple theoretical study of the pressure dependence of compressibility of bulk and the nano crystalline SnO2 of three different sizes (3, 8 & 14 nm) by using Equation of state model. The present work demonstrates the effect of size and pressure on compression and shows that the compressibility of nano-crystals depends upon size. The comparison of present results with available experimental data proves the validity of present model at bulk as well as nano scale. It is also found in this study that compressibility increases with decreasing particle size which is consistent with other available experimental and theoretical data.
1. Introduction
Over the past decade, nanomaterials have been the subject of enormous interest. These materials, notable for their extremely small size, have the potential for wide-ranging industrial, biomedical, and electronic applications. Nanomaterials can be metals, ceramics, polymeric materials, or composite materials. Nanocrystalline materials with particle size of 1–100 nm are of current interest because they show noble physical and chemical properties that may differ from those of the corresponding bulk materials [Citation1]. The chemical preparation method plays a significant role in controlling the size distribution, structure and crystallinity of these particles. In nanocrystals, approximately half of the atoms are on the surface and it is important to understand the surface structure to develop any device using these materials. The surface structure and shape of a nanoparticle is determined by minimization of the surface energy. This is accomplished by maximizing the amount of low-energy and low-index facets of the crystals. In case of nanomaterials size plays an important role in explaining the various physical properties such as melting temperature, hardness, sintering ability and electronic structure [Citation2,Citation3]. One way of inducing changes in the size, shape and structure of nanoparticles is to apply high pressure and that’s why high-pressure studies in case of nanomaterials are important.
Because of their shape and size-dependent physical and chemical properties [Citation4,Citation5] metal oxide semiconductor nanomaterials become one of the most important classes of materials, in different fields of science and technology. Among different metal oxide nanomaterials, SnO2 which is an n type semiconductor with a direct band gap of 3.6 eV at 300 K [Citation6] has attracted more attention of the researchers, because of its ample applications in lithium batteries [Citation7,Citation8], super capacitors [Citation9,Citation10], sensors for toxic gases [Citation11–13], photo-catalysis [Citation14,Citation15], field emitters [Citation16], transistors [Citation17] and catalysis [Citation18]. Reports suggest that the applications of SnO2 nanostructures mainly depend on their morphologies and structural features.
Several theoretical studies [Citation19–22] on size-dependent elastic and thermodynamic properties of nanomaterials are motivated from Qi model [Citation23] which is based on the relation between cohesive energy per mole of the nanocrystalline solids and that of bulk material. In the present work our aim is to analyze the mechanical properties especially the volume compression (V/Vo) of nanocrystalline n-SnO2 of three different sizes (3, 8, and 14 nm) as well as bulk SnO2 by using Tait’s equation of state. Although, the Tait’s equation has been widely used in most of the bulk materials [Citation2,Citation24] however, this equation has not been used so far for the combined study of compression behaviour in different sized nanomaterial and its bulk counterpart.
2. Method of analysis
By assuming the fact that in quasi-harmonic approximation the product of the volume thermal expansion coefficient (α) and the isothermal bulk modulus (K) is constant under the effect of pressure [Citation25] we have:
(1)
(1)
Differentiation of Equation (1) with respect to volume (V) at constant temperature, gives:
(2)
(2)
Anderson-Gruneisen parameter (δT) may be defined as [Citation26]:
(3)
(3) where P is the pressure.
From Equations (2) and (3), we have:
(4)
(4)
Anderson-Gruneisen parameter δT and η = V/V0 (where V0 is the initial volume) are related by the relation [Citation27]:
(5)
(5) where A is a constant for a given solid.
In view of Equation (5), Equation (4) can be written as
(6)
(6)
Integrating above equation, we get
(7)
(7) where K0 is the bulk modulus at zero pressure.
The isothermal bulk modulus may also be defined as [Citation27]:
(8)
(8)
In view of Equation (8), Equation (7) may be written as
(9)
(9)
The integration of Equation (9) gives:
(10)
(10)
Here, we have considered that at zero pressure volume is V0 and with the application of pressure P volume becomes V = V0 i.e. reference pressure is zero.
The constant A in Equation (10) may be determined by substituting the initial conditions, viz at V = V0, (where
is the Anderson–Gruneisen parameter at zero pressure) in Equation (5) as
(11)
(11)
Substituting the value of K from Equation (8) in Equation (4) we get:
(12)
(12) where
is the first order pressure derivative of bulk modulus.
In view of Equation (12) one may consider [Citation28] and hence:
(13)
(13) where
is the first order pressure derivative of the bulk modulus at zero pressure.
Substituting the value of A in Equation (10) and taking the natural log, we get the following final form of Tait’s equation of state [Citation29]:
(14)
(14) where V is the volume of the solid at pressure P, keeping the temperature constant. V0 is the initial volume at P = 0 and at room temperature. K0 and
are the isothermal bulk modulus and its first order pressure derivative.
The beauty of this equation of state is that it requires less number of input parameters and provides a simple and straight forward approach to predict the relative compression in solids at high pressure. To test the validity of this equation of state in nanomaterials, (as in bulk materials), we have, therefore, employed Tait’s equation of state to predict the compression behaviour of nanomaterials.
3. Result and discussion
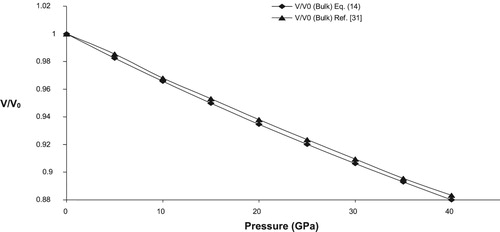
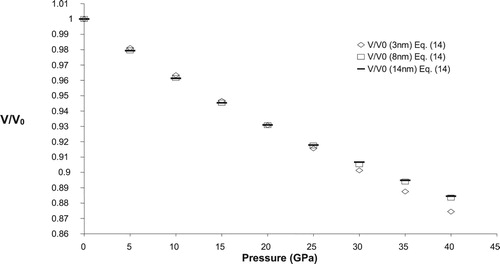
To calculate volume compression (V/V0) at different pressure, the present formulation requires two input parameters viz. bulk modulus (K0) and its pressure derivative at zero pressure (). The experimental values of these input parameters are given in Table along with the corresponding references. For bulk SnO2 high pressure compression behaviour is calculated from Equation (14) and is shown in Figure along with the experimental data points. A good agreement between calculated and experimental data shows the validity of present model to predict the pressure volume relationship in case of bulk materials.
Table 1. Experimental values of the equation of state parameters, K0 (GPa) and 
 for SnO2 [Citation30].
for SnO2 [Citation30].
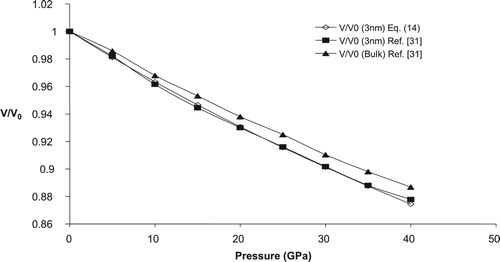
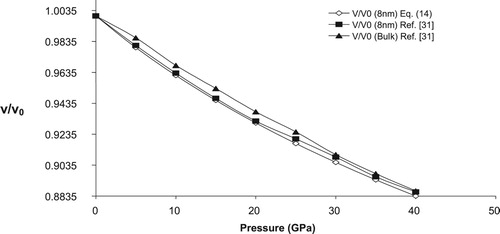
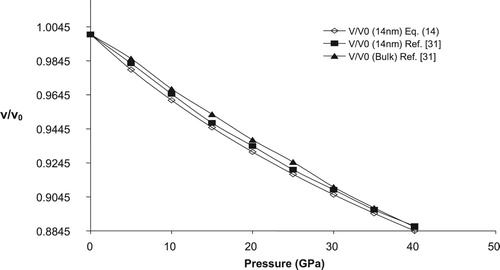
To check the applicability of the present model in nano regime we have calculated the variation of relative volume change with pressure for three n-SnO2 (3, 8 & 14 nm). Figures – shows the pressure dependence of relative change of volume for three different sized nano crystalline SnO2 (3, 8 & 14 nm), respectively along with the available experimental data. A reasonable harmony between present model and experimental values further confirm the legitimacy of present model in nano regime also. For comparison purpose we have also plotted the volume compression of bulk SnO2 in Figures –. From these figures it may be observed that as size of the nanomaterial increases the volume compression approaches the value of its bulk counterpart, at higher pressures. The calculated relative volume change with pressure for 3, 8 & 14 nm n-SnO2 is given in Figure and from here it may be revealed that the compressibility of the nanomaterials increases with decreasing particle size and thus lower size nanomaterial is more compressible. This observation may be attributed to the fact that decrease in grain size increases the surface to volume ratio and hence the cohesive energy and the bulk modulus of a nanocrystalline solid will decrease with decrease in grain size as both the cohesive energy and the bulk modulus are the parameters to describe the rigidity or bond strength of materials. This is also validated by the experimental work of He et al. [Citation30] as they have reported the value of K0 to be 252, 248, 246 and 233 GPa for bulk, 14, 8 & 3 nm n-SnO2 respectively, keeping to be fixed at 4.
It is pertinent to mention here that He et al. [Citation30] have observed a phase transition from rutile to cubic phase at respective pressures 23 ± 2 GPa, 29 ± 2 GPa and 30 ± 2 GPa for bulk, 14 nm nanocrystalline and 8 nm nanocrystalline SnO2 while for 3 nm n-SnO2 there was no phase transition upto 39 GPa. The present model is unable to predict the phase transition as well to study the volume compression in cubic phase it requires input parameters viz. K0 and of that phase which are unavailable in the literature (to the best of our knowledge) so we have restricted ourselves to the study of rutile phase of SnO2 only.
4. Conclusion
In brief it may be concluded that although the present model based on the quasi-harmonic approximation, i.e. the product of bulk modulus and the coefficient of volume thermal expansion remains constant, is unable to predict the phase transition pressure (if phase transition occur in higher pressure range) but can explain the volume compression properties of bulk materials and its nano counterpart with same potential.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Kuldeep Kholiya http://orcid.org/0000-0001-5919-1030
Kailash Pandey http://orcid.org/0000-0003-2546-9334
References
- Alivisatos AP, Barbara PF, Castleman AW, et al. From molecules to materials: current trends and future directions. Adv Mater. 1998;10: 1297–1336. doi: 10.1002/(SICI)1521-4095(199811)10:16<1297::AID-ADMA1297>3.0.CO;2-7
- Schiotz J, DiTolla FD, Jacobsen KW. Softening of nanocrystalline metals at very small grain sizes. Nature. 1998;391: 561–563. doi: 10.1038/35328
- Qadri SB, Skelton EF, Dinsmore HJZ, et al. The effect of particle size on the structural transitions in zinc sulfide. J Appl Phys. 2001;89: 115–119. doi: 10.1063/1.1328066
- Chen JS, Lou XW. SnO2-based nanomaterials: synthesis and application in lithium-ion batteries. Small. 2013;9: 1877–1893. doi: 10.1002/smll.201202601
- Liu Y, Jiao Y, Zhang Z, et al. Hierarchical SnO2 nanostructures made of intermingled ultrathin nanosheets for environmental remediation, smart gas sensor, and supercapacitor applications. ACS Appl Mater Interfaces. 2014;6: 2174–2184. doi: 10.1021/am405301v
- Batzill M, Diebold U. The surface and materials science of tin oxide. Prog Surf Sci 2005;79: 47–154. doi: 10.1016/j.progsurf.2005.09.002
- Lou XW, Li CM, Archer LA. Designed synthesis of coaxial SnO2 @carbon hollow nanospheres for highly reversible lithium storage. Adv Mater. 2009;21: 2536–2539. doi: 10.1002/adma.200803439
- Meduri P, Pendyala C, Kumar V, et al. Hybrid tin oxide nanowires as stable and high capacity anodes for Li-ion batteries. Nano Lett. 2009;9: 612–616. doi: 10.1021/nl802864a
- Pusawale SN, Deshmukh PR, Lokhande CD. Chemical synthesis of nanocrystalline SnO2 thin films for supercapacitor application. Appl Surf Sci. 2011;257: 9498–9502. doi: 10.1016/j.apsusc.2011.06.043
- Faraji S, Ani FN. Microwave-assisted synthesis of metal oxide/hydroxide composite electrodes for high power supercapacitors – a review. J Power Sources. 2014;263: 338–360. doi: 10.1016/j.jpowsour.2014.03.144
- Zhang J, Guo J, Xu H, et al. Reactive-template fabrication of porous SnO2 nanotubes and their remarkable gas-sensing performance. ACS Appl Mater Interfaces. 2013;5: 7893–7898. doi: 10.1021/am4019884
- Sun YH, Dong PP, Lang X, et al. Comparative study of electrochemical performance of SnO2 anodes with different nanostructures for lithium-ion batteries. J Nanosci Nanotechno. 2015;15: 5880–5888. doi: 10.1166/jnn.2015.10282
- Jiao Z, Chen D, Jiang Y, et al. Synthesis of nanoparticles, nanorods, and mesoporous SnO2 as anode materials for lithium-ion batteries. J Mater Res 2014;29: 609–616. doi: 10.1557/jmr.2014.32
- Zhang Y, Kolmakov A, Lilach Y, et al. Electronic control of chemistry and catalysis at the surface of an individual tin oxide nanowire. J Phy Chem B. 2005;109: 1923–1929. doi: 10.1021/jp045509l
- Kar A, Sain S, Kundu S, et al. Influence of size and shape on the photocatalytic properties of SnO2 nanocrystals. Chem Phys Chem. 2015;16: 1017–1025. doi: 10.1002/cphc.201402864
- Li Z, Wang H, Qin Z. A rapid and efficient method to prepare aligned SnO2 nanorod arrays for field-emission application. Vacuum. 2009;83: 1340–1343. doi: 10.1016/j.vacuum.2009.04.036
- Dattoli EN, Wan Q, Guo W, et al. Fully transparent thin-film transistor devices based on SnO2 nanowires. Nano Lett. 2007;7: 2463–2469. doi: 10.1021/nl0712217
- Wang X, Fan H, Ren P. Self-assemble flower-like SnO2/Ag heterostructures: correlation among composition, structure and photocatalytic activity. Colloids Surf A. 2013;419: 140–146. doi: 10.1016/j.colsurfa.2012.11.050
- Kumar R, Kumar M. Size dependence of thermoelastic properties of nanomaterials. Inter J Nanosci. 2010;9: 537–542. doi: 10.1142/S0219581X10007113
- Kumar R, Kumar M. Effect of size on cohesive energy, melting temperature and debye temperature of nanomaterials. Indian J Pure & Appl Phys. 2012;50:329–334.
- Bhatt JC, Kholiya K. Effect of size on the elastic and thermodynamic properties of nanomaterials. Indian J Pure & Appl Phys. 2014;52:604–608.
- Chandra J, Kholiya K. Diameter-dependent thermodynamic and elastic properties of metallic nanoparticles. Modern Phys Lett B. 2015;29: 1550025. doi: 10.1142/S0217984915500256
- Qi WH. Size effect on melting temperature of nanosolids. Physica B. 2005;368: 46–50. doi: 10.1016/j.physb.2005.06.035
- Bai HY, Luo LL, Jin D, et al. Particle size and interfacial effect on the specific heat of nanocrystalline Fe. J Appl Phys. 1996;79: 361–364. doi: 10.1063/1.360838
- Shanker J, Kumar M. Thermodynamic approximations in high-pressure and high-temperature physics of solids. Phys Stat Sol B. 1993;179: 351–356. doi: 10.1002/pssb.2221790209
- Kushwah SS, Kumar P, Shanker J. Analysis of pressure - volume - temperature relationship for some alkali halide crystals. Physica B. 1996;229: 85–90. doi: 10.1016/S0921-4526(96)00507-8
- Anderson OL, Isaak DG, Oda H. High-temperature elastic constant data on minerals relevant to geophysics. Rev Geophys. 1992;30: 57. doi: 10.1029/91RG02810
- Kumar M. High pressure equation of state for solids. Physica B. 1995;212: 391–394. doi: 10.1016/0921-4526(95)00361-C
- Hayward ATJ. Compressibility equations for liquids: a comparative study. Brit J App Phys. 1967;18: 965–977. doi: 10.1088/0508-3443/18/7/312
- He Y, Liu JF, Chen W, et al. High-pressure behavior of SnO2 nanocrystals. Phys Rev B. 2005;72: 212102. doi: 10.1103/PhysRevB.72.212102