ABSTRACT
We initiated a program to synthesize thiazolidinone derivatives as antitubercular agent by preparing hybrid molecules having the similar features of reported potent antitubercular agents. We desire to state the advancement and execution of a methodology allowing for the synthesis of some new (Z)-5-(substituted benzylidene)-2-((substituted phenyl) amino)thiazol-4(5H)-one analogues with antitubercular activity. A highly efficient protocol was developed for the synthesis with excellent yields as well as evaluated in vitro for their antimycobacterial activity against Mycobacterium tuberculosis MTB H37Ra and M. bovis BCG strains. Among these synthesized compounds 6a, 6c, 6e, 6f and 6i showed marginal antitubercular activity in the series along with no significant cytotoxicity against the MCF-7 and A549 human cancer cell lines.
GRAPHICAL ABSTRACT
1. Introduction
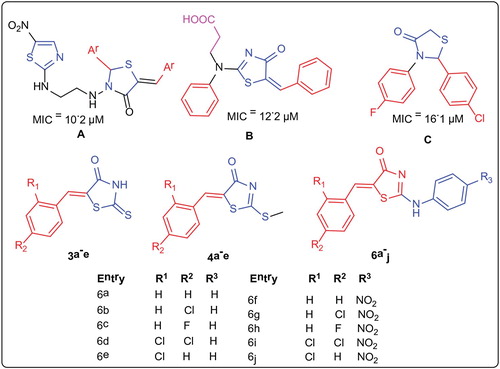
The thiazole nucleus is a crucial constituent for medicinal agents having anticancer, antimicrobial and anti-inflammatory activities [Citation1,Citation2]. The thiazole ring system is seen in a wide range of drugs used as cardiotonic, fungicidal, for the treatment of HIV infection, mental retardation in children, age-related neurodegenerative brain damage (Alzheimer's disease), Parkinson's disease. Thiazole is considered as the key nucleus in the class of heterocyclic compounds. Biologically potent as well as dynamic molecules such as Sulfathiazol (antimicrobial drug), Ritonavir (antiretroviral drug), Abafungin (antifungal drug), Bleomycine and Tiazofurin (antineoplastic drug) contain thiazoles. Thiazole pharmacophore along the combination with other rings possibly will provide biologically active compounds [Citation3,Citation4]. Thiazoles also show anti-tuberculosis activity. Tuberculosis (TB) is caused by the pathogen Mycobacterium tuberculosis (MTB), which is most infectious cause of humanity universally. Even though effective anti-TB drugs, such as isoniazid and rifampicin, are accessible, problems emerge as MTB develops resistance not only against the first line but also with the second line drugs. Hence there is an imperative need to develop novel anti-TB agents, which are synthetically possible, have less adverse effects and shorter duration of treatment [Citation5]. The thiazolidinone and its derivatives are central part of biologically active compounds with various applications and uses [Citation6–8] such as antibacterial [Citation9–11], antifungal [Citation12–14], anticancer [Citation15], antitubercular [Citation16,Citation17] and antimalarial [Citation18]. For years many medicinal chemists are in search of biologically active alkaloids containing substituted amine ring systems for their whole or partial synthesis. As by considering the medicinal and biological importance of thiazolidinone moiety, we initiated a program to synthesize thiazolidinone derivatives as an antitubercular agent by preparing hybrid molecules having the similar features of reported potent antitubercular agents [Citation19] (Figure ).
In continuation of our work [Citation20–29] here, we desire to state the advancement and execution of a methodology allowing for the synthesis of some new (Z)-5-(substituted benzylidene)-2-((substituted phenyl) amino)thiazol-4(5H)-one analogues. We have reported reaction under conventional method, which proceeds in short reaction time and give good to excellent yield. The structures of compounds 6a–j were checked by IR, 1H NMR, 13C NMR, Mass spectral analysis and elemental analysis.
2. Experimental
2.1. General
Rhodanine, substituted benzaldehyde, substituted amines, anhydrous sodium acetate, triethylamine, dichloromethane, iodomethane and various solvents were commercially available. The major chemicals were purchased from Sigma Aldrich and Avra labs. Reaction courses were monitored by TLC on silica gel precoated F254 Merck plates. Developed plates were examined with UV lamps (254 nm). All compounds were purified by column chromatography. IR spectra were recorded on an FT-IR (Bruker). Melting points were recorded on SRS optimelt, melting point apparatus and are uncorrected. The 1H NMR spectra were recorded on a 400 MHz varian NMR spectrometer. The 13C NMR spectra were recorded on a 400 MHz varian NMR spectrometer. The chemical shifts are reported as δppm units. The following abbreviations are used: singlet (s), doublet (d), triplet (t), quartet (q), multiplet (m) and broad (br). HRMS spectra were taken with micromass-QUATTRO-II of WATER mass spectrometer. Elemental analysis was performed on a Perkin-Elmer EAL-240 elemental analyser.
2.2. Synthesis
2.2.1. General procedure for the synthesis of (Z)-5-substituted benzylidene-2- thioxothiazolidin-4-one (3a–e)
In a 50-mL round bottom flask, the compound substituted 2-thioxothiazolidin-4-one 1 (1 mmol), benzaldehyde 2a–e (1 mmol), glacial acetic acid (1 mL), anhydrous sodium acetate (1 mmol) were added to the reaction mixture. The mixture was stirred under reflux condition up to 2 h. The progress of the reaction was monitored by TLC (10% methanol: chloroform). After completion of the reaction, the reaction mixture was poured into the ice-cold water. The precipitate was filtered off and washed with water (3×15 mL), dried and purified by recrystallization in ethanol as solvent to give 90–94% yield.
(Z)-5-benzylidene-2-thioxothiazolidin-4-one (3a).
Colour: Orange solid. Yield: 90%. M.P. 204–206°C. 1H NMR: δ ppm = 7.40–7.42 (m, 5H, Ar–CH), 7.70 (s, 1H, =CH), 13.90 (bs, 1H, NH). 13C NMR: δppm = 125.5, 128.5, 130.7, 130.9, 131.4, 133.5, 169.4, 194.7. IR νmax/cm–1: 1670 (C=O), 1600 (C=C), 1585 (C=N), 1230 (C=S), 1192(C–N). ES-MS m/z: 221.0578.
(Z)-5-(4-chlorobenzylidene)-2-thioxothiazolidin-4-one (3b).
Colour: Yellow solid. Yield: 92%. M.P. 131–133°C. 1H NMR (400 MHz, CDCl3): δ ppm = 7.25–7.27 (d, 2H, Ar–H), 7.70–7.72 (d, 2H, Ar–H), 7.90 (s, 1H, =CH), 13.72 (s, 1H, NH). 13C NMR (100 MHz, CDCl3): δ ppm = 116.3, 128.4, 129.0, 133.3, 133.5, 143.4, 168.4, 193.6. IR νmax/cm–1: 3010 (NH), 2827 (CH–Ar), 1693 (C=O), 1580 (C=C), 1462 (C=N), 1183 (C=S), 1034 (C–N). ES-MS m/z: 255.1025.
(Z)-5-(4-fluorobenzylidene)-2-thioxothiazolidin-4-one (3c).
Colour: Yellow solid. Yield: 92%. M.P. 226–228°C. 1H NMR: δ ppm = 7.01–7.03 (d, 2H, Ar–H), 7.55–7.57 (d, 2H, Ar–H), 8.10 (s, 1H, =CH), 10.01 (bs, 1H, NH). 13C NMR: δ ppm = 115.3, 116.3, 130.4, 130.8, 143.4, 162.3, 168.3, 193.6. IR νmax/cm–1: 3010 (NH), 2837 (CH–Ar), 1702 (C=O), 1554 (C=C), 1483 (C=N), 1224 (C=S), 1083 (C–N). ES-MS m/z: 239.2932.
(Z)-5-(2,4-dichlorobenzylidene)-2-thioxothiazolidin-4-one (3d).
Colour: Yellow solid. Yield: 92%. M.P. 230–232°C. 1H NMR (400 MHz, DMSO-d6): δ ppm = 7.33–7.35 (d, 1H, Ar–CH), 7.60–7.62 (d, 1H, Ar–CH), 7.70 (s, 1H, Ar–CH), 7.90 (s, 1H, =CH), 12.81 (bs, 1H, NH). 13C NMR (100 MHz, DMSO-d6): δ ppm = 116.0, 125.2, 126.2, 128.1, 130.3, 131.1, 136.4, 143.2, 168.3, 193.3. IR νmax/cm–1: 3040 (NH), 2922 (CH–Ar), 1715 (C=O), 1576 (C=C), 1432 (C=N), 1183 (C=S), 1042 (C–N). ES-MS m/z (%): 288.9255.
(Z)-5-(2-chlorobenzylidene)-2-thioxothiazolidin-4-one (3e).
Colour: Yellow solid. Yield: 94%. M.P. 180–182°C. 1H NMR (400 MHz, CDCl3): δ ppm = 7.25–7.27 (m, 4H, Ar–H), 7.78 (s, 1H, =CH), 12.72 (s, 1H, NH). 13C NMR (100 MHz, CDCl3): δ ppm = 116.3, 126.4, 127.8, 129.0, 129.4, 133.3, 134.5, 143.4, 168.4, 193.6. IR νmax/cm–1: 3021 (NH), 2827 (CH–Ar), 1683 (C=O), 1560 (C=C), 1464 (C=N), 1188 (C=S), 1036 (C–N). ES-MS m/z: 254.9625.
2.2.2. General procedure for the synthesis of (Z)-5-substituted benzylidene-2-(methylthio)thiazol-4(5H)-one (4a–e)
In a 50-mL round bottom flask, the compound (Z)-5-substituted benzylidene-2-thioxothiazolidin-4-one (3a–e) (1 mmol), triethylamine (1.2 mmol), iodomethane (1.5 mmol), ethanol (1 mL) stirred at room temperature up to 10–15 min. The progress of the reaction was monitored by TLC (15% methanol: chloroform). After completion of the reaction, the reaction mixture was concentrated in-vacuo. The residue was washed with water (3×10 mL) to afford the crude product. The crude product was recrystallized using ethanol as solvent to give yield in the range 90–95%.
(Z)-5-benzylidene-2-(methylthio)thiazol-4(5H)-one (4a).
Colour: Orange solid. Yield: 94%. M.P. 145–147°C. 1H NMR: δ ppm = 2.85 (s, 3H, S-CH3), 7.60–7.62 (m, 5H, Ar–CH), 7.90 (s, 1H, =CH). 13C NMR: δ ppm = 14.4, 126.5, 128.6, 131.4, 132.9, 133.5, 135.7, 152.3, 162.7, 169.2. IR νmax/cm–1: 3026 (CH–Ar), 1694 (C=O), 1590 (C=C), 1462 (C=N), 1151 (C–S), 979 (C–N). ES-MS m/z: 235.0023.
(Z)-5-(4-chlorobenzylidene)-2-(methylthio)thiazol-4(5H)-one (4b).
Colour: Yellow solid. Yield: 95%. M.P. 160–162°C. 1H NMR (400 MHz, CDCl3): δ ppm = 2.75 (s, 3H, SCH3), 7.60–7.62 (d, 2H, Ar–H), 7.70–7.72 (d, 2H, Ar–H), 8.60 (s, 1H, =CH). 13C NMR (100 MHz, CDCl3): δ ppm = 14.0, 128.6, 129.3, 132.4, 133.1, 133.5, 152.4, 162.2, 167.1. IR νmax/cm–1: 3015 (CH–Ar), 1709 (C=O), 1580 (C=C), 1462 (C=N), 1149 (C–S), 974 (C–N). ES-MS m/z: 269.0526.
(Z)-5-(4-fluorobenzylidene)-2-(methylthio)thiazol-4(5H)-one (4c).
Colour: Yellow solid. Yield: 92%. M.P. 143–145°C. 1H NMR: δ ppm = 2.80 (s, 3H, SCH3), 7.50–7.52(d, 2H, Ar–H), 7.70–7.72 (d, 2H, Ar–H), 7.90 (s, 1H, =CH). 13C NMR: δ ppm = 14.2, 115.4, 130.4, 132.3, 132.4, 133.5, 152.2, 162.1, 167.2. IR νmax/cm–1: 1700 (C=O), 1594 (C=C), 1483 (C=N), 1153 (C–S), 824 (C–N). ES-MS m/z: 253.0505.
(Z)-5-(2,4-dichlorobenzylidene)-2-(methylthio)thiazol-4(5H)-one (4d).
Colour: Yellow solid. Yield: 90%. M.P. 163–165°C. 1H NMR (400 MHz, DMSO-d6): δ ppm = 2.33 (s, 3H, S–CH3), 7.32–7.34 (d, 1H, Ar–CH), 7.40–7.42 (d, 1H, Ar–CH), 7.60 (s, 1H, Ar–CH), 7.95 (s, 1H, =CH). 13C NMR (100 MHz, DMSO-d6): δ ppm = 14.2, 126.3, 127.6, 129.3, 129.9, 132.3, 133.1, 134.3, 152.2, 162.3, 167.5. IR νmax/cm–1: 2922 (CH–Ar), 1716 (C=O), 1572 (C=C), 1433 (C=N), 1180 (C=S), 1040 (C–N). ES-MS m/z: 302.9320.
(Z)-5-(2-chlorobenzylidene)-2-(methylthio)thiazol-4(5H)-one (4e).
Colour: Yellow solid. Yield: 94%. M.P. 170–172°C. 1H NMR (400 MHz, CDCl3): δ ppm = 2.34 (s, 3H, S–CH3), 7.32–7.34 (m, 4H, Ar–H), 7.90 (s, 1H, =CH). 13C NMR (100 MHz, CDCl3): δ ppm = 14.3, 126.4, 127.8, 129.3, 129.9, 132.3, 133.5, 134.4, 152.4, 162.8, 167.6.
IR νmax/cm–1: 2827 (CH–Ar), 1683 (C=O), 1512 (C=C), 1424 (C=N), 1134 (C=S), 1036 (C–N). ES-MS m/z: 268.9725.
2.2.3. General procedure for the synthesis of (Z)-5-(substituted benzylidene)-2-((substituted phenyl)amino)thiazol-4(5H)-one (6a–j)
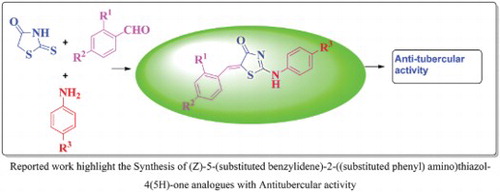
In a 50-mL round bottom flask, the compound (4a–e) (1 mmol), substituted amines (5a–b) (1 mmol), potassium carbonate (1 mmol) and methanol (1 mL) were added to the reaction mixer and stirred for 5–7 h at room temperature. The progress of the reaction was monitored by TLC (20% methanol: chloroform). After completion of the reaction, the reaction mixture was concentrated in-vacuo. The compounds (6a–j) were recrystallized from ethanol and get pure compounds as yellowish solids.
(Z)-5-benzylidene-2-(phenylamino)thiazol-4(5H)-one (6a).
Colour: Orange solid. Yield: 92%. M.P. 214–216°C. 1H NMR: δ ppm = 6.40–6.42 (d, 2H, Ar–CH), 6.80–6.82 (t, 3H, Ar–CH), 7.40–6.42 (m, 5H, Ar–CH), 7.90 (s, 1H, =CH), 10.90 (s, 1H, NH). 13C NMR: δ ppm = 120.5, 122.4, 127.9, 128.6, 128.8, 129.6, 132.9, 135.7, 139.4, 152.3, 158.3, 162.7. IR νmax/cm–1: 3026 (NH), 1715 (C=O), 1590 (C=C), 1462 (C=N),1151 (C–S), 979 (C–N). ES-MS m/z: 280.0716. Anal. calcd. for C16H12N2OS: C, 68.55; H, 4.31; N, 9.99; S, 11.44. Found: C, 68.53; H, 4.33; N, 9.97; S, 11.46.
(Z)-5-(4-chlorobenzylidene)-2-(phenylamino)thiazol-4(5H)-one (6b).
Colour: Orange solid. Yield: 94%. M.P. 142–144°C. 1H NMR: δ ppm = 6.40–6.42 (m, 5H, Ar–CH), 7.44–7.46 (d, 2H, Ar–CH), 7.68–7.70 (d, 2H, Ar–CH), 7.95 (s, 1H, =CH). 11.20 (s, 1H, NH). 13C NMR: δ ppm = 120.5, 122.5, 127.9, 128.7, 129.0, 129.5, 133.3, 133.5, 139.4, 152.3, 158.3, 167.7. IR νmax/cm–1: 3015 (NH), 1710 (C=O), 1520 (C=C), 1411 (C=N), 1152 (C–S), 974 (C–N). ES-MS m/z: 314.0720. Anal. calcd. for C16H11ClN2OS: C, 61.05; H, 3.52; N, 8.90; S, 10.19. Found: C, 61.07; H, 3.54; N, 8.92; S, 10.21.
(Z)-5-(4-fluorobenzylidene)-2-(phenylamino)thiazol-4(5H)-one (6c).
Colour: Orange solid. Yield: 94%. M.P. 212–214°C. 1H NMR: δ ppm = 6.40–6.42 (m, 5H, Ar–CH), 7.44–7.46 (d, 2H, Ar–CH), 7.68–7.70 (d, 2H, Ar–CH), 7.95 (s, 1H, =CH), 12.20 (s, 1H, NH). 13C NMR: δ ppm = 120.5, 122.5, 127.9, 128.7, 129.0, 129.5, 133.3, 133.5, 139.4, 152.3, 158.3, 167.7. IR νmax/cm–1: 3010 (NH), 1710 (C=O), 1490 (C=C), 1422 (C=N), 1121 (C–S), 979 (C–N). ES-MS m/z: 299.3503. Anal. calcd. for C16H11FN2OS: C, 64.41; H, 3.72; N, 9.39; S, 10.75. Found: C, 64.43; H, 3.74; N, 9.41; S, 10.77.
(Z)-5-(2,4-dichlorobenzylidene)-2-(phenylamino)thiazol-4(5H)-one (6d).
Colour: Orange solid. Yield: 92%. M.P. 245–247°C. 1H NMR: δ ppm = 6.60–6.62 (m, 5H, Ar–CH), 7.60 (bs, 1H, NH). 7.80–7.82 (m, 3H, Ar–CH), 7.95 (s, 1H, =CH). 13C NMR: δ ppm = 120.9, 122.4, 125.9, 126.8, 128.9, 129.5, 130.3, 131.5, 132.3, 136.4, 139.4, 152.0, 158.3, 167.7. IR νmax/cm–1: 3026 (NH), 1715 (C=O), 1590 (C=C), 1462 (C=N),1151 (C–S), 979 (C–N). ES-MS m/z: 349.3509. Anal. calcd. for C16H10Cl2N2OS: C, 55.03; H, 2.89; N, 8.02; S, 9.18. Found: C, 55.05; H, 2.91; N, 8.04; S, 9.21.
(Z)-5-(2-chlorobenzylidene)-2-(phenylamino)thiazol-4(5H)-one (6e).
Colour: Yellow solid. Yield: 96%. M.P. 220–222°C. 1H NMR: δ ppm = 7.01–7.03 (d, 2H, Ar–H), 7.20–7.22 (t, 2H, Ar–H), 7.40–7.42 (m, 4H, Ar–H), 7.60–7.62 (t, 1H, Ar–H), 7.90 (s, 1H, =CH), 12.20 (bs, 1H, NH). 13C NMR: δ ppm = 120.4, 122.4, 126.7, 127.8, 129.3, 129.5, 129.9, 132.6, 133.0, 134.0, 139.4, 152.3, 158.1, 167.2. IR νmax/cm–1: 1700 (C=O), 1594 (C=C), 1483 (C=N), 1153 (C–S), 824 (C–N). ES-MS m/z: 315.4505. Anal. calcd. for C16H11ClN2OS: C, 61.05; H, 3.52; N, 8.90; S, 10.19. Found: C, 61.07; H, 3.54; N, 8.92; S, 10.21.
(Z)-5-benzylidene-2-((4-nitrophenyl)amino)thiazol-4(5H)-one (6f).
Colour: Orange solid. Yield: 96%. M.P. 165–167°C. 1H NMR: δ ppm = 6.64–6.66 (d, 2H, Ar–CH), 7.20–7.22 (m, 5H, Ar–CH), 7.90 (s, 1H, =CH), 8.01–8.03 (d, 2H, Ar–CH), 10.75 (s, 1H, NH). 13C NMR: δ ppm = 114.5, 124.4, 127.9, 128.6, 128.8, 129.6, 132.9, 137.7, 145.4, 152.3, 158.3, 162.7. IR νmax/cm–1: 3018 (NH), 1720 (C=O), 1690 (C=C), 1468 (C=N), 1151 (C–S), 975 (C–N). ES-MS m/z: 325.0520. Anal. calcd. for C16H11N3O3S: C, 59.07; H, 3.41; N, 12.92; S, 09.86. Found: C, 59.09; H, 3.43; N, 12.90; S, 09.88.
(Z)-5-(4-chlorobenzylidene)-2-((4-nitrophenyl)amino)thiazol-4(5H)-one (6g).
Colour: Orange solid. Yield: 94%. M.P. 180–182°C. 1H NMR: δ ppm = 6.62–6.64 (d, 2H, Ar–CH), 7.40–7.42 (d, 2H, Ar–CH), 7.65–7.67 (d, 2H, Ar–CH), 7.85 (s, 1H, =CH), 8.10–8.12 (d, 2H, Ar–CH), 10.95 (bs, 1H, NH). 13C NMR: δ ppm = 114.5, 124.4, 127.9, 128.6, 128.8, 129.0, 132.9, 133.7, 137.4, 152.1, 158.2, 162.2. IR νmax/cm–1: 3046 (NH), 1716 (C=O), 1580 (C=C), 1462 (C=N), 1155 (C–S), 979 (C–N). ES-MS m/z: 359.0122. Anal. calcd. for C16H10ClN3O3S: C, 53.41; H, 2.80; N, 11.68; S, 08.91. Found: C, 53.39; H, 2.83; N, 11.70; S, 08.88.
(Z)-5-(4-fluorobenzylidene)-2-((4-nitrophenyl)amino)thiazol-4(5H)-one (6h).
Colour: Orange solid. Yield: 92%. M.P. 223–225°C. 1H NMR: δ ppm = 6.61–6.63 (d, 2H, Ar–CH), 7.41–7.43 (d, 2H, Ar–CH), 7.70–7.72 (d, 2H, Ar–CH), 7.86 (s, 1H, =CH), 8. 05–8.07 (d, 2H, Ar–CH), 10.92 (bs, 1H, NH). 13C NMR: δ ppm = 114.5, 115.4, 124.9, 130.4, 130.8, 132.9, 137.7, 145.4, 152.3, 158.3, 162.7, 167.2. IR νmax/cm–1: 3012 (NH), 1712 (C=O), 1560 (C=C), 1442 (C=N), 1123 (C–S), 924 (C–N). ES-MS m/z: 343.0425. Anal. calcd. for C16H10FN3O3S: C, 55.97; H, 2.94; N, 12.24; S, 09.34. Found: C, 55.99; H, 2.93; N, 12.26; S, 09.38.
(Z)-5-(2,4-dichlorobenzylidene)-2-((4-nitrophenyl)amino)thiazol-4(5H)-one (6i).
Colour: Orange solid. Yield: 92%. M.P. 193–195°C. 1H NMR: δ ppm = 6.44–6.46 (d, 2H, Ar–CH), 7.30–7.32 (d, 1H, Ar–CH), 7.48 (s, 1H, Ar–CH), 7.60–7.62 (d, 1H, Ar–CH), 8.01 (s, 1H, =CH), 8.05–8.07 (d, 2H, Ar–CH), 10.90 (bs, 1H, NH). 13C NMR: δ ppm = 114.3, 124.5, 125.4, 126.9, 128.6, 130.8, 131.6, 132.9, 136.7, 137.9, 145.4, 152.3, 158.3, 167.7. IR νmax/cm–1: 3023 (NH), 1718 (C=O), 1592 (C=C), 1460 (C=N), 1150 (C–S), 971 (C–N). ES-MS m/z: 392.9730. Anal. calcd. for C16H9Cl2N3O3S: C, 48.75; H, 2.30; N, 10.66; S, 08.13. Found: C, 48.77; H, 2.33; N, 10.67; S, 08.15.
(Z)-5-(2-chlorobenzylidene)-2-((4-nitrophenyl)amino)thiazol-4(5H)-one (6j).
Colour: Orange solid. Yield: 94%. M.P. 110–112°C. 1H NMR: δ ppm = 6.64–6.66 (d, 2H, Ar–CH), 7.32–7.34 (m, 4H, Ar–CH), 7.92 (s, 1H, =CH), 8.00–8.02 (d, 2H, Ar–CH), 10.88 (bs, 1H, NH). 13C NMR: δ ppm = 114.5, 124.7, 126.9, 127.6, 129.3, 129.6, 132.3, 133.2, 134.7, 137.4, 145.5, 152.3, 158.3, 167.7. IR νmax/cm–1: 3010 (NH), 1710 (C=O), 1591 (C=C), 1452 (C=N), 1144 (C–S), 975 (C–N). ES-MS m/z: 359.0125. Anal. calcd. for C16H10ClN3O3S: C, 53.41; H, 2.80; N, 11.68; S, 08.91. Found: C, 53.43; H, 2.82; N, 11.67; S, 08.95.
2.3. Biological activity
All the chemicals such as sodium salt XTT, DMSO, sulfanilic acid, sodium nitrate, HCl, NEED and rifampicin were purchased from Sigma-Aldrich, USA. Dubos medium was purchased from DIFCO, USA. Compounds were dissolved in DMSO (10 µg/mL) and it was used as stock solution for further antimycobacterial testing. Microbial strains such as MTB H37Ra (ATCC 25177) and M. bovis BCG (ATCC 35734) were obtained from AstraZeneca, India. The stock culture was maintained at −80°C and subcultured once in a liquid medium before inoculation into an experimental culture. Cultures were grown in Dubos media (enrichment media). Mycobacterium pheli medium (minimal essential medium) was used for antimycobacterial assay. It contains 0.5 g KH2PO4, 0.25 g trisodium citrate, 60 mg MgSO4, 0.5 g aspargine and 2 mL glycerol in distilled water (100 mL) followed by pH adjustment to 6.6. All the newly synthesized compounds were screened in vitro against two Mycobacterium species such as MTB H37Ra and M. bovis BCG. Both species of Mycobacterium were grown in Mycobacterium pheli medium. Screening of MTB H37Ra was done by using XTT reduction menadione assay (XRMA) and M. bovis BCG screening was done by using NR (nitrate reductase) assay, both of them were developed [Citation30]. Briefly 2.5 mL of these inhibitor solutions were added in a total volume of 250 mL of Mycobacterium pheli medium consisting of bacilli. The incubation was terminated on the 8th day for active MTB culture. The XRMA and NR was then carried out to estimate viable cells present in different wells of the assay plate. The optical density was read on a micro plate reader (Spectramax plus384 plate reader, Molecular Devices Inc.) at 470 nm filter for XTT and at 540 nm filter for NR against a blank prepared from cell-free wells. Absorbance given by cells treated with the vehicle alone was taken as 100% cell growth. Initially primary screening was done at 30, 10 and 3 µg/mL. Compounds showing 90% inhibition of bacilli at or lower than 30 µg/mL were selected for further dose response curve. All experiments were performed in triplicates and the quantitative value was expressed as the average ± standard deviation. MIC50 values of selected compound were calculated from their dose response curves by using Origin 6 software. % Inhibition was calculated by using following formula: % Inhibition = [(absorbance of compound − absorbance of test)/(absorbance of control − absorbance of Blank)] × 100, where control is the medium with bacilli along with vehicle and blank is cell free medium.
3. Results and discussion
We have synthesized title compounds of (Z)-5-(substituted benzylidene)-2-((substituted phenyl) amino)thiazol-4(5H)-one analogues (6a–j) (, Table ). Also reported the synthesis of (Z)-5-(substituted)-2-thioxothiazolidin-4-one (3a–e) and (Z)-5-(substituted)-2-(methylthio)thiazol-4(5H)-one (4a–e) (, Table ). We had also screened the bases, solvents, reaction time and yield of model reaction (Z)-5-benzylidene-2-(phenylamino)thiazol-4(5H)-one (6a) (, Table ).
Scheme 1. Synthesis of (Z)-5-(substituted benzylidene)-2-((substituted phenyl)amino) thiazol-4(5H)-one (6a–j)a. aReaction condition: (a) Sodium acetate (1 mmol), 1 mL acetic acid, reflux 2–4 h. yields 90–44%, (b) iodomethane (1.5 mmol), triethylamine (1.2 mmol), 1 mL ethanol, stirring room temperature, 10–15 min. yields 90–95%. (c) Methanol, potassium carbonate, stirring at room temperature, 5–7 h.
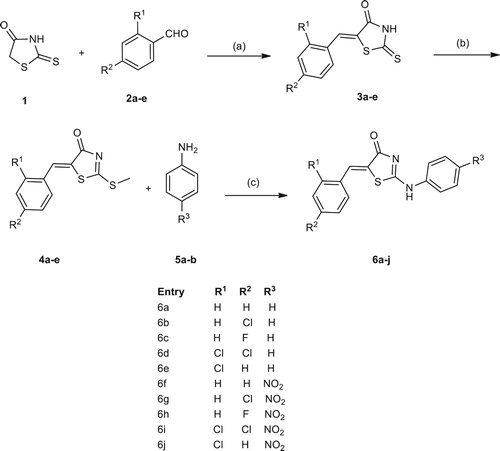
Table 1. Synthesis of (Z)-5-(substituted)-2-thioxothiazolidin-4-one (3a–e)a and (Z)-5-(substituted)-2-(methylthio)thiazol-4(5H)-one (4a–e)a
Table 2. Screening of solvent for the synthesis of compounds (6a).
In view of chemistry and easily available solvent, it was decided to prefer methanol as solvent in our initial study for optimization of the solvent. During this study, the model reaction was performed using methanol as a reaction medium at room temperature.
The desired product 6a was obtained in 90%, taking only 5 h in methanol at room temperature (Table , entry 6). Other solvents like ethanol give good yield 65% in 6 h (Table , entry 1). Among these, the solvents like THF, DMF, DCM, toluene gave a very low yield at 20%, 30%, 40%, 25% respectively. Methanol as a solvent was selected for further reaction. We have also screened different types of bases, reaction time and yield of the product. The model reaction (Z)-5-benzylidene-2-(phenylamino)thiazol-4(5H)-one (6a) (, Table ). All of these yields were generally low before further optimizations. To increase the efficiency of the reaction, the effects of different bases were investigated (Table , entries 7–10). The yield obtained with potassium carbonate as a base and methanol as solvent was high (90%). Sodium carbonate, triethylamine and dimethylamine gave lower yields with methanol solvents (40%, 25% and 35% respectively). All the reactions were carried out in equimolar amounts of each compound in 1 mL of solvent. From the above conclusion, we decided to carry out the reaction in methanol as a solvent and potassium carbonate as a base. This condition was worked as a standard protocol for the further reactions (Table ).
Table 3. Synthesis of (Z)-5-(substituted benzylidene)-2- ((substituted phenyl)amino)thiazol-4(5H)-one (6a–j)a.
The antitubercular activity of synthesized analogues 6a–j were determined by measuring the growth of inhibition against an avirulent strain of MTB H37Ra (ATCC 25177) and M. bovis BCG (ATCC 35743) in liquid M. pheli medium. In a preliminary screening, the antimycobacterial activity of the synthesized analogues (Table ) was assessed at concentrations of 30, 10 and 3 μg/mL using an established XTT Reduction Menadione Assay (XRMA) antitubercular screening protocol [Citation30, Citation31] using first-line antitubercular drugs rifampicin and isoniazid as a reference standard (Table ).
Table 4. In vitro antitubercular activity against MTB H37Ra and M. Bovis BCG of compound 6a–j.
Among the synthesized analogues, in particular, the compounds 6a, 6c, 6e, 6f and 6i exhibited MIC50 values 20, 5.5, 7.5, 8.9 and 15 μg/mL, respectively against MTB H37Ra, which shows that, these are marginal antitubercular agents. Also the compounds 6a, 6c, 6e active against M. bovis BCG strain with MIC50 values 25, 7.7, 21.5 μg/mL respectively.
Among the synthesized analogues, in particular, the compounds 6e and 6f exhibited MIC90 values 26, and 25 μg/mL, respectively against MTB H37Ra, which shows that, these are marginal antitubercular agents. Also the compounds 6a and 6i active against M. bovis BCG strain with MIC90 values 15 and 12 μg/mL respectively which shows that these are marginal antitubercular agents. It may be noted that compared to standard RIF and INH above compounds shows better activity against M. Bovis BCG and MTB H37Ra.
Among the synthesized analogues, in particular, the compounds 6c, 6d, 6f, 6i and 6j exhibited MIC90 values 20 μg/mL against MTB H37Rv, which shows that, these are marginal antitubercular agents. Also the compounds 6c, 6d, 6e, 6f, 6i and 6j exhibited MIC50 values 10, 18, 7.0, 10, 15, 17 μg/mL respectively against MTB H37Rv, which shows that these are marginal antitubercular agents.
3.1. Cytotoxicity assay
These findings inspired us to evaluate synthesized analogues cytotoxicity. The cytotoxicity results are presented in Table , where many compounds exhibit less cytotoxicity activity compared to Adriamycin as positive control. The compounds 6a, 6c, 6d and 6i were the less toxic, with MIC50 values ranging from 34.2 to 45.6 µM. On the A-549 cell line the compounds which showed lower toxicity were 6b, 6f, 6 g (MIC50 = 23.6, 46.3, 46.6 µM, respectively).
Table 5. In vitro cytotoxicity of compounds towards the MCF-7 and A-549 cells, after 24 h.
The synthesized analogues 6a–j were assayed for their cytotoxic effects in two different cell lines, MCF-7 and A549 using MTT assay [Citation32, Citation33]. The cell lines were maintained under standard cell culture conditions under 5% CO2 at 37°C in 95% air humidified environment. Each concentration was tested in duplicates in a single experiment. MIC50 values were calculated using OriginPro Software.
4. Conclusion
An effective method was developed which provided an easy access to a new series (Z)-5-(substituted benzylidene)-2-((substituted phenyl) amino)thiazol-4(5H)-one analogues. The mild reaction conditions, good to excellent yields, ease of workup and easily available substrates make the reactions attractive for the preparation of compounds 6a–j. In vitro antitubercular studies revealed that the compounds 6a, 6c, 6e, 6f and 6i showed marginal antitubercular activity in the series along with no cytotoxicity against the MCF-7 and A-549 human cancer cell lines.
Acknowledgements
We acknowledge financial support from the Council of Scientific and Industrial Research (CSIR), New Delhi, India (File No. 02(0288)/17/EMR-II). RS thank Dr. R. J. Barnabas, Principal, Ahmednagar College, Ahmednagar 414001, MS, India.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Rohini N. Shelke http://orcid.org/0000-0002-2053-4785
Dattatraya N. Pansare http://orcid.org/0000-0002-0419-3538
Aniket P. Sarkate http://orcid.org/0000-0002-1998-1772
Kshipra S. Karnik http://orcid.org/0000-0002-8118-2963
Shankar R. Thopate http://orcid.org/0000-0001-5092-0196
References
- Holla BS, Rao BS, Sarojini BK, et al. Synthesis and studies on some new fluorine containing triazolothiadiazines as possible antibacterial, antifungal and anticancer agents. Eur J Med Chem. 2006;41:657–663. doi: 10.1016/j.ejmech.2006.02.001
- Sarkate AP, Lokwani DK, Karnik KS, et al. Novel 2-(nitrooxy)ethyl 2-(4-(substituted phenyl)-2-((substituted phenyl)amino)thiazol-5-yl)acetate as anti-inflammatory, analgesic and nitric oxide releasing agents: synthesis and molecular docking studies. Ant Infl Ant All Agen Med Chem. 2017;16:153–167.
- Pawar CD, Sarkate AP, Karnik KS, et al. Synthesis and antimicrobial evaluation of novel ethyl 2-(2-(4-substituted) acetamido)-4-subtituted-thiazole-5-carboxylate derivatives. Bioorg Med Chem Lett. 2016;26:3525–3528. doi: 10.1016/j.bmcl.2016.06.030
- Nikalje APG, Tiwari SV, Sarkate AP, et al. Imidazole-thiazole coupled derivatives as novel lanosterol 14-α demethylase inhibitors: Ionic liquid mediated synthesis, biological evaluation and molecular docking study. Med Chem Res. 2018;27:592–606. doi: 10.1007/s00044-017-2085-5
- Zumla A, Chakaya J, Centis R, et al. Tuberculosis treatment and management—an update on treatment regimens, trials, new drugs, and adjunct therapies. Lan Resp Med. 2015;3:220–234. doi: 10.1016/S2213-2600(15)00063-6
- Hongyu Z, Wu S, Zhai S, et al. Design, synthesis, cytoselective toxicity, Structure–activity relationships, and pharmacophore of thiazolidinone derivatives targeting drug-resistant lung cancer cells. J Med Chem. 2008;51:1242–1251. doi: 10.1021/jm7012024
- Diurno MV, Mazzoni O, Calignano PE, et al. Synthesis and antihistaminic activity of some thiazolidin-4-ones. J Med Chem. 1992;35:2910–2912. doi: 10.1021/jm00093a025
- Solomon VR, Haq W, Srivastava K, et al. Synthesis and antimalarial activity of side chain modified 4-aminoquinoline derivatives. J Med Chem. 2007;50:394–398. doi: 10.1021/jm061002i
- Rida SM, Labouta IM, Salama HM, et al. Syntheses and in vitro antimicrobial evaluation of some benzimidazol-2-ylmethylthioureas, benzimidazol-2-ylacetylthiosemicarbazides and products of their condensation with monochloroacetic acid. Pharmazie. 1986;41:475–478.
- Bhat AR, Singh D. Synthesis and biological activities of 4-thiazolidinones and dihydro-3-(2h)-thiophenones. Ind J Pharm Sci. 1988;50:169–171.
- Bonde CG, Gaikwad NJ. Synthesis and preliminary evaluation of some pyrazine containing thiazolines and thiazolidinones as antimicrobial agents. Bioorg Med Chem. 2004;12:2151–2161. doi: 10.1016/j.bmc.2004.02.024
- Giri S, Shukla AK, Nizamuddin. Synthesis of some 3-(5'-aryloxymethyl-1’,3’,4'-thiadiazol-2'-yl)-2-aryl-4-thiazoldinones and their antifungal activity. Ind J Pharm Sci. 1990;52:108–110.
- Capan G, Ulusoy N, Ergenc N, et al. New 6-phenylimidazo[2,1-b]thiazole derivatives: synthesis and antifungal activity. Monat Fur Chemie. 1999;130:1399–1407.
- Karali N, Illhan E, Gursoy A, et al. New cyclohexylidenehydrazide and 4-aza-1-thiaspiro[4.5]decan-3-one derivatives of 3-phenyl-4(3H)-quinazolinones. Farmaco. 1998;53:346–349. doi: 10.1016/S0014-827X(98)00032-9
- Menicagli R, Samaritani S, Signore G, et al. In vitro cytotoxic activities of 2-alkyl-4,6-diheteroalkyl-1,3,5-triazines: new molecules in anticancer research. J Med Chem. 2004;47:4649–4652. doi: 10.1021/jm0495374
- Pathak RB, Chovatia PT, Parekh HH. Synthesis, antitubercular and antimicrobial evaluation of 3-(4-chlorophenyl)-4-substituted pyrazole derivatives. Bioorg Med Chem Lett.. 2012;22:5129–5133. doi: 10.1016/j.bmcl.2012.05.063
- Babaoglu K, Page MA, Jones VC, et al. Novel inhibitors of an emerging target in Mycobacterium tuberculosis; substituted thiazolidinones as inhibitors of dTDP-rhamnose synthesis. Bioorg Med Chem Lett. 2003;13:3227–3230. doi: 10.1016/S0960-894X(03)00673-5
- Melato S, Prosperi D, Coghi P, et al. A combinatorial approach to 2,4,6-trisubstituted triazines with potent antimalarial activity: combining conventional synthesis and microwave-assistance. Chem Med Chem. 2008;3:873–876. doi: 10.1002/cmdc.200700344
- Samadhiya P, Sharma R, Srivastava SK, et al. Synthesis and biological evaluation of 4-thiazolidinone derivatives as antitubercular and antimicrobial agents. Arab J Chem. 2014;7:657–665. doi: 10.1016/j.arabjc.2010.11.015
- Pansare DN, Mulla NA, Pawar CD, et al. One pot three components microwave assisted and conventional synthesis of new 3-(4-chloro-2-hydroxyphenyl)-2-(substituted) thiazolidin-4-one as antimicrobial agents. Bioorg Med Chem Lett. 2014;24:3569–3573. doi: 10.1016/j.bmcl.2014.05.051
- Tiwari SV, Saijas JA, Vazquez-Tato MP, et al. Ultrasound mediated one-pot, three component synthesis, docking and ADME prediction of novel 5-amino-2-(4-chlorophenyl)-7-substituted phenyl-8, 8a-dihydro-7H-[1,3,4] thiadiazolo [3,2-α]pyrimidine- 6-carbonitrile derivatives as anticancer agents. Molecules. 2016;21(8). 894 (1–13). doi: 10.3390/molecules21080894
- Pansare DN, Shinde DB. A facile synthesis of (Z)-5-(substituted)-2-(methylthio)thiazol-4(5H)-one using microwave irradiation and conventional method. Tet Lett. 2014;55:1107–1110. doi: 10.1016/j.tetlet.2013.12.113
- Shelke RN, Pansare DN, Khade MC, et al. Synthesis and anticancer evaluation of new benzenesulfonamide derivatives. Eur Chem Bull. 2019;8(1):1–6. doi: 10.17628/ecb.2019.8.1-6
- Pansare DN, Shelke RN, Khade MC, et al. New thiazolone derivatives: design, synthesis, anticancer and antimicrobial activity. Eur Chem Bull. 2019;8(1):7–14. doi: 10.17628/ecb.2019.8.7-14
- Shelke RN, Pansare DN, Khade MC, et al. Synthesis of 2-((5-benzylidene-4-oxo-4,5-dihydrothiazol-2-yl)-substituted amino acids as anticancer and antimicrobial agents. Eur Chem Bull. 2019;8(2):63–70. doi: 10.17628/ecb.2019.8.63-70
- Pansare DN, Shelke RN, Pawar CD. A facile synthesis of (Z)-2-((5-(4-chlorobenzylidene)-4-oxo-4,5-dihydrothiazol-2-yl)amino)substituted acid using microwave irradiation and conventional method. Lett Org Chem. 2017;14(7):517–524. doi: 10.2174/1570178614666170524142722
- Pansare DN, Shelke RN, Shinde DB. A facial synthesis and anticancer activity of (Z)-2-((5-(4-nitrobenzylidene) -4-oxo-4,5-dihydrothiazol-2-yl)amino) substituted acid. J Het Chem. 2017;54(6):3077–3086. doi: 10.1002/jhet.2919
- Pansare DN, Shinde DB. A facile synthesis of novel series (Z)-2-((4-oxo-5-(thiophen-2-yl methylene)-4,5-dihydro thiazol-2-yl)amino) substituted acid. J Saudi Chem Soc. 2017;21:434–440. doi: 10.1016/j.jscs.2015.10.005
- Sarkate AP, Bahekar SS, Wadhai VM, et al. Microwave-assisted synthesis of nonsymmetrical aryl ethers using nitro-arenes. Synlett. 2013;24:1513–1516. doi: 10.1055/s-0033-1338869
- Singh U, Akhtar S, Mishra A, et al. A novel screening method based on menadione mediated rapid reduction of tetrazolium salt for testing of anti-mycobacterial agents. J Micro Meth. 2011;84:202–207. doi: 10.1016/j.mimet.2010.11.013
- Khan A, Sarkar S, Sarkar D. Bactericidal activity of 2-nitroimidazole against the active replicating stage of Mycobacterium bovis BCG and Mycobacterium tuberculosis with intracellular efficacy in THP-1 macrophages. Int J Antimicro Agen. 2008;32:40–45. doi: 10.1016/j.ijantimicag.2008.02.022
- Doyen CM, Moshkin YM, Chalkley GE, et al. Subunits of the histone chaperone CAF1 also mediate assembly of protamine-based chromatin. Cell Rep. 2013;4:59–65. doi: 10.1016/j.celrep.2013.06.002
- Ciapetti G, Cenni E, Pratelli L, et al. In vitro evaluation of cell/biomaterial interaction by MTT assay. Biomaterials. 1993;14:359–364. doi: 10.1016/0142-9612(93)90055-7