Abstract
A new simple, accurate, precise RP-HPLC [reverse phase high performance liquid chromatography] method was developed for the simultaneous estimation of the Emtricitabine, Bictegravir and Tenofovir alafenamide in pharmaceutical dosage form. Chromatogram was run through Denali C18 column (150 mm × 4.6 mm, 5 µm); mobile phase containing buffer and acetonitrile in the ratio of 50:50 was pumped through column at a flow rate of 1 ml/min [Buffer: 0.1% at pH 2.2, temperature 30°C]. Optimized wavelength was 272 nm. Retention times of Emtricitabine, Bictegravir and Tenofovir alafenamide were found to be 2.303, 3.219 and 3.754 min respectively. The %RSD of the Emtricitabine, Bictegravir and Tenofovir alafenamide were found to be 0.7, 0.8 and 0.4, respectively. The %recovery was obtained as 99.89%, 100.65% and 100.38% for Emtricitabine, Bictegravir and Tenofovir alafenamide, respectively. This method was accurate, precise and sensitive; hence, could be employed for routine quality control of Emtricitabine, Bictegravir and Tenofovir alafenamide in pharmaceutical industries and drug testing laboratories.
1. Introduction
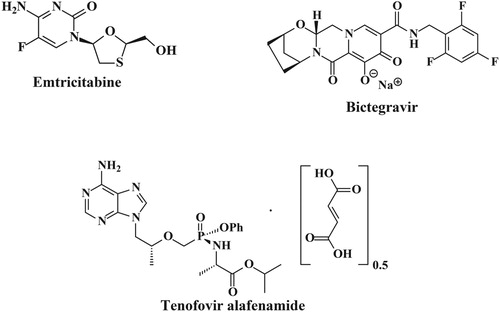
Emtricitabine [Figure ] is a nucleoside reverse transcriptase inhibitor (NRTI) for the treatment of HIV infection in adults. Emtricitabine is an analogue of cytidine. The drug works by inhibiting reverse transcriptase, the enzyme that copies HIV RNA into new viral DNA. Chemically it is known as 4-Amino-5-fluoro-1-((2R,5S)-2-hydroxymethyl-[1,3]-oxathiolan-5-yl)-1H-pyrimidin-2-one [Citation1]. Bictegravir [Figure ] is a recently approved investigational drug that has been used in trials studying the treatment of HIV-1 and HIV-2 infection. Chemically it is known as (1S,11R,13R)-5-Hydroxy-3,6-dioxo-N-(2,4,6-trifluorobenzyl)-12-oxa-2,9-diazatetracyclo [11.2.1.02,11.04,9] hexadeca-4,7-diene-7-carboxamide. It has been approved for HIV-1 monotherapy combined with 2 other antiretrovirals in a single tablet [Citation2]. Tenofovir alafenamide fumarate (TAF) [Figure ] is a nucleotide reverse transcriptase inhibitor (NRTI) and a novel ester prodrug of the antiretroviral Tenofovir. Tenofovir mimics normal DNA building blocks, but is lacking a 3'-OH molecule required for phosphodiester bond linkage. By competing with regular nucleotides for incorporation into proviral DNA and prevention of the formation of the 5’ to 3’ phosphodiester linkage required for DNA elongation, Tenofovir causes early chain termination and prevents proviral DNA transcription. Chemically it is known as propan-2-yl-(2S)-2-{[(S)({[(2R)-1-(6-amino-9H-purin-9-yl)propan-2-yl]oxy} methyl) (phenoxy)phosphoryl] amino} propanoate [Citation3]. Although Tenofovir (available as Tenofovir disoproxil fumarate) has a good safety profile and efficacy, and is currently a cornerstone of HIV antiviral treatment, its use has been associated with nephrotoxicity and reduced bone mineral density. In comparison, TAF has been shown to have improved antiviral efficacy, enhanced delivery of TFV into peripheral blood mononuclear cells and lymphatic tissues, a higher barrier to resistance, and an improved safety profile. Improved renal safety is likely attributable to lower circulating plasma concentrations of tenofovir and therefore less exposure and damage to bone and the kidneys, where tenofovir is metabolized. Because HIV antiretroviral therapy is usually life-long, reduced toxicity and improved efficacy results in better patient outcomes and improved adherence in the long term [Citation4].
The literature survey reveals that there is no analytical method available for the estimation of Emtricitabine, Bictegravir and Tenofovir alafenamide in pharmaceutical dosage forms. The reported methods available for the estimation of Emtricitabine and Tenofovir alafenamide are RP-HPLC [Citation5–9 reverse phase high performance liquid chromatography method ], Tenofovir Disoproxil Fumarate and Emtricitabine RP-HPLC [Citation9–11 reverse phase high performance liquid chromatography Method ], Application of UV Spectrophotometric Methods for Simultaneous Estimation of Emtricitabine and Tenofovir Alafenamide Fumarate in Bulk [Citation12], spectrofluorimetric analysis [Citation13], reverse phase high performance liquid chromatography [Citation14,Citation15. Since there are no official reported methods on high performance liquid chromatographic methods for the simultaneous estimation of Emtricitabine, Bictegravir and Tenofovir alafenamide in the public domain, we have planned to develop a simple, precise, economic and accurate stability indicating RP-HPLC reverse phase high performance liquid chromatography] method development and validation for the estimation of emtricitabine, bictegravir and tenofovir alafenamide in pharmaceutical dosage form.
2. Materials and methods
2.1. Instrumentation and chromatographic conditions
The analysis was performed on a high performance liquid chromatography system consists of waters 2695 with 2996 module Photo Diode Array detector equipped with a quaternary solvent delivery pump, automatic sample injector and column thermostat. The data acquisition and analysis was performed by using Empower2 software. The chromatographic separation was performed on Denali C18 column (150 mm × 4.6 mm, 5 µm). The flow rate was kept at 1 ml/min. The column temperature was maintained at 30°C. The mobile phase containing 0.1% Orthophosphoric acid buffer and Acetonitrile in the ratio of 50:50 results acceptable less retention time and good resolution between Emtricitabine, Bictegravir and Tenofovir alafenamide. The method was optimized at 272 nm. Data acquisition and processing was performed by using empower 2 system software. The run time was taken as 6 min. All the determinations are carried out at an ambient temperature.
2.2. Materials
Active pharmaceutical ingredients Emtricitabine, Bictegravir and Tenofovir alafenamide were obtained as a gift sample from Spectrum Pharma research solutions, Hyderabad. The pharmaceutical dosage form (Biktarvy® – Symphony Pharma Limited, Guwahati, Assam) was purchased from a local pharmacy. The solvents used in this work were of HPLC [reverse phase high performance liquid chromatography] grade and obtained from Merck Specialties Private Limited, Mumbai. Symphony Pharma Limited.
2.3. Preparation of standard stock solutions
An accurately weighed amount of Emtricitabine (50 mg), Bictegravir (12.5 mg) and Tenofovir alafenamide (6.25 mg) working standards were transferred into 25 ml clean dry volumetric flasks, dissolved in 15 ml of diluent, sonicated for 30 min and made up to the final volume with the same diluent. 1 ml of each stock solution was pipetted out and transferred into 10 ml volumetric flaks and made up with same diluent.
2.4. Preparation of sample stock solutions
5 tablets were accurately weighed and the average weight of each tablet was calculated. The weight equivalent of one tablet was transferred into 100 ml clean dry volumetric flask, dissolved in 60 ml of diluent, sonicated for 25 min and made up to the final volume with the same diluent followed by filtering. The filtered 1 ml sample of stock solution was transferred into 10 ml fresh dried volumetric flask and made up with same diluents.
2.5. Preparation of buffer
Preparation of 0.1% ortho phosphoric acid Buffer: 1 ml of ortho phosphoric acid solution was transferred into a 1000 ml volumetric flask followed by addition of milli-Q water into the same to make the final volume up to 1000 ml by using milli-Q water.
3. Method validation
The method was validated according to ICH guidelines in terms of: Linearity, Accuracy, Precision, Limit of detection, Limit of quantification, Robustness and Stability indicating capability.
3.1. System suitability parameters
The system suitability parameters were determined by preparing standard solutions of Emtricitabine, Bictegravir and Tenofovir alafenamide and the solutions were injected six times and the parameters like peak tailing, resolution and USP plate count were determined.
3.2. Linearity
The linearity of the method is determined by preparing three individual series of solutions in the range of Emtricitabine (50–300 µg/ml), Bictegravir (12.5–75 µg/ml) and Tenofovir alafenamide (6.25–37.5 µg/ml). The obtained peak areas are plotted against concentration.
3.2.1. Preparation of linearity solutions
Preparation of Standard stock solutions: Accurately weighed and transferred 50 mg, 12.5 mg & 6.25 mg of Emtricitabine, Bictegravir and Tenofovir Alafenamide working Standards into a 25 ml, 25 ml & 25 ml clean dry volumetric flasks, add 15 ml of diluent, sonicated for 30 min. Flasks were made up with water and acetonitrile (50:50) and labelled as Standard stock solution 1, 2 and 3.
From three stock solutions pipette out 0.25 ml, 0.5 ml, 0.75 ml, 1.0 ml, 1.25 ml, 1.50 ml into 10 ml volumetric flask to get 25%, 50%, 75%, 100%, 125%, 150% of standard solutions.
3.3. Precision
3.3.1. Method precision (repeatability)
The method precision/ repeatability can be determined by injecting six working standard solutions and six sample injections. The areas of all the injections were taken and standard deviation, %Relative standard deviation, %assay were calculated.
3.3.2. Intermediate precision
The intermediate precision can be determined by injecting six working standard solutions and six sample injections on different days by different operators or by different instruments. The areas of all the injections were taken and standard deviation, %Relative standard deviation, %assay were calculated. The results obtained were within the acceptance criteria.
3.4. Accuracy
Accuracy is tested by the standard addition method at three different levels 50, 100 and 150%. The percentage recoveries of Emtricitabine, Bictegravir and Tenofovir Alafenamide present in the pharmaceutical dosage form were calculated.
3.4.1. Preparation of 50% spiked solution
0.5 ml of sample stock solution was taken into a 100 ml volumetric flask and made up with diluents followed by filtration with HPLC [reverse phase high performance liquid chromatography] and labelled as accuracy 50% Sample stock solution. 1 ml from each standard stock solution was pipetted out and taken into a 10 ml volumetric flask, to that 1 ml of the above filtered 50% sample stock solution was spiked and made up with diluents.
3.4.2. Preparation of 100% spiked solution
1 ml of sample stock solution was taken into a 100 ml volumetric flask and made up with diluents followed by filtration with HPLC [reverse phase high performance liquid chromatography] and labelled as Accuracy 100% Sample stock solution. 1 ml from each standard stock solution was pipetted out and taken into a 10 ml volumetric flask, to that 1 ml of the above filtered 100% sample stock solution was spiked and made up with diluents.
3.4.3. Preparation of 150% spiked solution
1.5 ml of sample stock solution was taken into a 100 ml volumetric flask and made up with diluents followed by filtration with HPLC [reverse phase high performance liquid chromatography] and labelled as Accuracy 150% Sample stock solution. 1 ml from each standard stock solution was pipetted out and taken into a 10 ml volumetric flask, to that 1 ml of the above filtered 150% sample stock solution was spiked and made up with diluents.
3.5. Limit of detection and limit of quantification
Limit of detection (LOD) and limit of quantification (LOQ) of Emtricitabine, Bictegravir and Tenofovir alafenamide were determined by calibration curve method. Solutions of Emtricitabine, Bictegravir and Tenofovir alafenamide were prepared in linearity range and injected in triplicate. Average peak area of three analyses was plotted against concentration.
3.6. Method robustness
The Robustness of the developed method was determined by making small deliberate changes in flow rate (± 1 ml/min), column temperature (± 5%), organic mobile phase ratio (± 10%), along with the optimized method.
3.7. Forced degradation studies
The stability studies were conducted by exposing the drug product to different stress conditions like acid, alkali, peroxide, thermal, photolytic and hydrolytic conditions.
3.7.1. Oxidation
To 1 ml of stock solution of Emtricitabine, Bictegravir and Tenofovir alafenamide, 1 ml of 20% hydrogen peroxide (H2O2) was added separately. The solutions were kept for 30 min at 60°C. For HPLC [reverse phase high performance liquid chromatography] study, the resultant solution was diluted to obtain 200 µg/ml, 50 µg/ml and 25 µg/ml of all components and 10 µl were injected into the system and the chromatograms were recorded to assess the stability of sample.
3.7.2. Acid degradation studies
To 1 ml of stock solution Emtricitabine, Bictegravir and Tenofovir alafenamide, 1 ml of 2N Hydrochloric acid was added and refluxed for 30 mins at 60°C. The resultant solution was diluted to obtain 200 µg/ml, 50 µg/ml and 25 µg/ml of all components and 10 µl solutions were injected into the system and the chromatograms were recorded to assess the stability of sample.
3.7.3. Alkali degradation studies
To 1 ml of stock solution Emtricitabine, Bictegravir and Tenofovir alafenamide, 1 ml of 2N sodium hydroxide was added and refluxed for 30 mins at 60°C. The resultant solution was diluted to obtain 200 µg/ml, 50 µg/ml and 25 µg/ml of all components and 10 µl were injected into the system and the chromatograms were recorded to assess the stability of sample.
3.7.4. Dry heat degradation studies
The standard drug solution was placed in oven at 105°C for 6 h to study dry heat degradation. For HPLC [reverse phase high performance liquid chromatography] study, the resultant solution was diluted obtain 200 µg/ml, 50 µg/ml and 25 µg/ml of all components and 10 µl were injected into the system and the chromatograms were recorded to assess the stability of the sample.
3.7.5. Photo stability studies
The photochemical stability of the drug was also studied by exposing the 2000 µg/ml, 500 µg/ml and 250 µg/ml solution to UV Light by keeping the beaker in UV Chamber for 7days or 200 Watt hours/m2 in photo stability chamber. For HPLC [reverse phase high performance liquid chromatography] study, the resultant solution was diluted to obtain 200 µg/ml, 50 µg/ml and 25 µg/ml of all components and 10 µl were injected into the system and the chromatograms were recorded to assess the stability of sample.
3.7.6. Neutral degradation studies
Stress testing under neutral conditions was studied by refluxing the drug in water for 6 h at a temperature of 60°C. For HPLC [reverse phase high performance liquid chromatography] study, the resultant solution was diluted to obtain 200 µg/ml, 50 µg/ml and 25 µg/ml of all components and 10 µl were injected into the system and the chromatograms were recorded to assess the stability of the sample.
4. Results and discussions
4.1. Development and optimization of HPLC [reverse phase high performance liquid chromatography] method
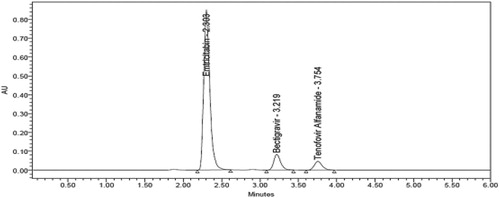
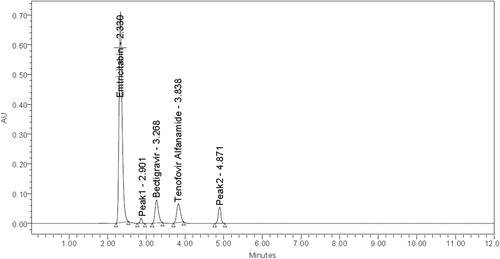
The present work was focused to develop a stability indicating RP-HPLC [reverse phase high performance liquid chromatography] method for the simultaneous estimation of Emtricitabine, Bictegravir and Tenofovir alafenamide in pharmaceutical dosage form. The solubility of the active pharmaceutical ingredient was checked in different solvents like methanol, water, acetonitrile and in different ratios but finally the standard is soluble in water and Acetonitrile 1:1 ratio so it was chosen as a diluent. The different mobile phases like acetonitrile and potassium dihydrogen phosphate buffer, Methanol and OPA buffer and acetonitrile and OPA buffer were used in compositions with a flow rate of 1 ml/min but the peak resolution, retention time and tailing factor were not satisfactory, so at last 0.1% OPA and acetonitrile was selected as a buffer at flow rate of 1 ml/min. Initially Symmetry®"(150 mm × 4.6 mm × 5µ), “Discovery®” (250 mm × 4.6 mm × 5 µm), “Kromosil®” (250 mm × 4.6 mm × 5 µm) and “Denali®” (150 mm × 4.6 mm × 5 µm) columns with different temperatures like 30, 35, 40, 45°C were used but the retention time, run time and peak resolution were not exact and the problem was get rid by using Denali C18 column (150 mm × 4.6 mm × 5 µm) kept at 30°C with a run time of 6 min. Finally the method was optimized by altering the various mobile phase composition / ratio and the optimized wavelength of three drugs Emtricitabine, Bictegravir and Tenofovir alafenamide was found to be at 272 nm [Figure ].
4.2. System suitability parameters
The system suitability tests were conducted before performing the validation and the parameters were within the acceptable criteria like retention times were 2.303, 3.219 and 3.754 min for Emtricitabine, Bictegravir and Tenofovir alafenamide, plate count was >2000, peak tailing was < 2 and the %RSD of peak areas of six injections were ≤ 2% (Table ). Hence the proposed method was successfully applied to routine analysis without any problems.
Table 1. System suitability parameters for Emtricitabine, Bictegravir, and Tenofovir alafenamide.
4.3. Linearity range
The linearity range was in the interval of Emtricitabine (50–300 µg/ml), Bictegravir (12.5–75 µg/ml) and Tenofovir alafenamide (6.25–37.5 µg/ml), respectively. These were represented by a linear regression equation as follows: y (Emtricitabine) = 22754x + 17915. (r2 = 0.999), y (Bictegravir) = 9802.x + 4237 (r2 = 0.999) and y (Tenofovir alafenamide) = 12575x + 7065. Regression line was established by least squares method and correlation coefficient (r2) for Emtricitabine, Bictegravir and Tenofovir alafenamide were found to be greater than 0.999. Hence the curves established were linear. (Table )
Table 2. Linearity table for Emtricitabine, Bictegravir and Tenofovir alafenamide.
4.4. Precision
Six replicate injections at the same concentration were analysed on the same day and also in two different days for verifying the variation in the precision and the % RSD for Emtricitabine, Bictegravir and Tenofovir alafenamide were within acceptable limit of ≤ 2. Hence the method is reproducible on different days with different analyst and column. This indicates that the method is precise (Table ).
Table 3. Determination of repeatability and intermediate precision.
4.5. Accuracy
The percentage recoveries for Emtricitabine, Bictegravir and Tenofovir alafenamide were found to be 99.89%, 100.13% and 100.38% respectively (Table ). The results of the recovery studies undoubtedly demonstrate accuracy of the proposed method.
Table 4. Accuracy results of Emtricitabine, Bictegravir and Tenofovir alafenamide.
4.6. Limit of detection (LOD) and limit of quantification (LOQ)
The determined values of LOD and LOQ were calculated by using slope and Y-intercept. The LOD and LOQ values for Emtricitabine were found to be 1.04 and 3.14 µg/ml and Bictegravir were found to be 0.62 and 1.89 µg/ml, Tenofovir alafenamide were found to be 0.70 and 2.13 µg/ml respectively (Table ).
Table 5. Sensitivity table of Emtricitabine, Bictegravir and Tenofovir alafenamide.
4.7. Robustness
Robustness of the proposed method demonstrated a non-significant alteration through analysis of the sample and standard Emtricitabine, Bictegravir and Tenofovir alafenamide solution (Table ). After this the results obtained were compared with that of optimized method. It was confirmed that by the deliberate changes in the parameters there were no significant changes in standard deviation, relative standard deviation, theoretical plates, retention time and USP tailing factor.
Table 6. Robustness data for Emtricitabine, Bictegravir and Tenofovir alafenamide.
4.8. Assay
The Content of Emtricitabine, Bictegravir and Tenofovir alafenamide in the pharmaceutical dosage form was found by using the developed method. The percentage purity of Emtricitabine, Bictegravir and Tenofovir alafenamide were found to be 99.80%, 99.89% and 100.35% and %RSD values for Emtricitabine, Bictegravir and Tenofovir alafenamide were within limit of ≤2 (Table ).
Table 7. Assay results of Emtricitabine, Bictegravir and Tenofovir alfanamide.
4.9. Forced degradation studies
The forced degradation studies were conducted on Emtricitabine, Bictegravir and Tenofovir alafenamide drug products and all the parameters were within the acceptable limits. Emtricitabine, Bictegravir and Tenofovir alafenamide have shown significant sensitivity towards the treatment of acidic, basic and peroxide solutions. The drugs gradually undergone degradation with time and prominent degradation was observed.
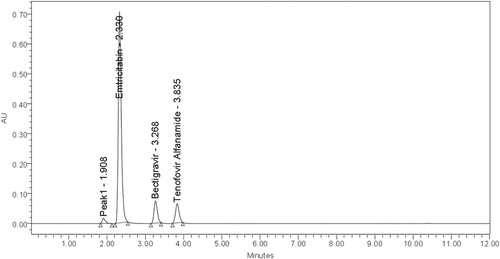
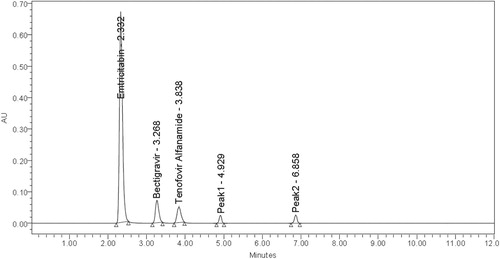
Degradation peaks in acidic media at RT 1.908, in basic media at RT 4.929; 6.858 and in oxidation Medium at RT 2.901; 4.871 were observed (Figures ).
Forced thermal degradation, photolytic degradation and neutral degradation studies showed that all the drug molecules did not undergo any degradation and were stable under these conditions. There was no change observed in retention times of all the drugs.
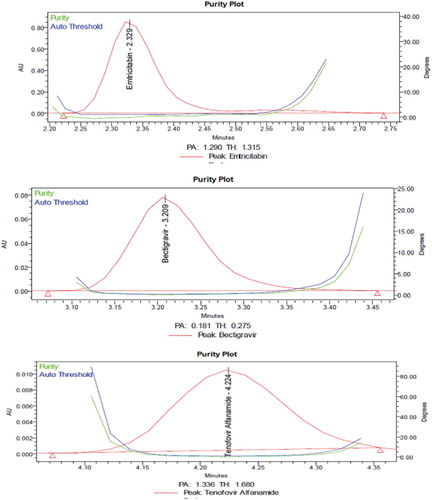
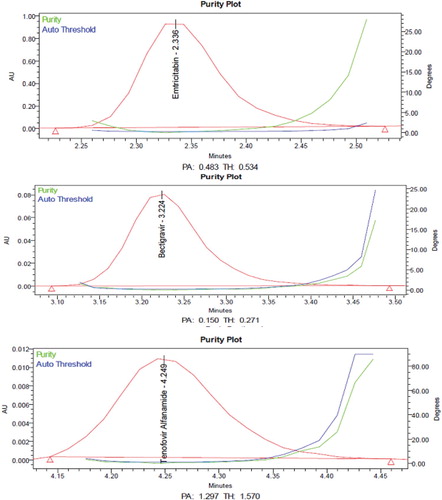
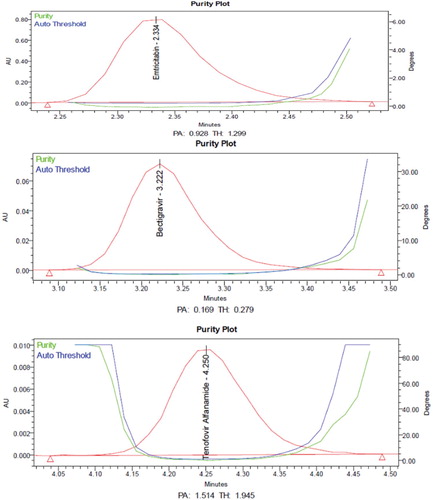
From the chromatograms of the degradation products, the peak of the degradation products was not interfering with the peaks of drugs products and these degradation studies showed that the developed stability indicating method is specific.
From the degradation studies, Peak purity test results derived from PDA detector, confirmed that the Emtricitabine, Bictegravir and Tenofovir alafenamide peaks were homogeneous and pure in all the analysed stress samples. The mass balance of stressed samples was close to 99.5% (Table and Figures ).
Table 8. Degradation data of Emtricitabine, Bictegravir and Tenofovir alfanamide
5. Conclusion
In this paper a novel, simple, efficient, rapid and precise stability indicating Reverse Phase High Performance Liquid Chromatographic method was effectively developed and validated for the simultaneous estimation of Emtricitabine, Bictegravir and Tenofovir alafenamide in pharmaceutical drug product. The current method was validated according to ICH guidelines in terms of Linearity, Accuracy, Precision, Limit of detection, Limit of quantification, Robustness and Stability indicating capability.
We have conducted the stability studies by exposing the drug product to different stress conditions like acid, alkali, peroxide, thermal, photolytic, hydrolytic conditions and observed the degradation of drug product in acidic medium, basic medium and in oxidation medium. Based on the chromatograms of the degradation studies, we have detected that the peak of the degradation products was not interfering with the peaks of drugs products and so we conclude that the current developed Reverse Phase High Performance Liquid Chromatographic method is specific with respect to the pharmaceutical drug product containing Emtricitabine, Bictegravir and Tenofovir alafenamide.
Hence the current developed method can be fruitfully applied for the estimation of Emtricitabine, Bictegravir and Tenofovir alafenamide in drug testing laboratories and pharmaceutical industries.
Acknowledgements
The authors were thankful for Spectra pharma research solutions, Hyderabad, for providing Emtricitabine, Bictegravir and Tenofovir alafenamide reference standards as a gift sample to carry out the research work.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Duvvuri Suryakala http://orcid.org/0000-0001-7964-1764
Tej Kumar Kokkirala http://orcid.org/0000-0002-1655-0045
References
- https://www.drugbank.ca/drugs/DB00879.
- https://www.drugbank.ca/drugs/DB11799.
- https://www.drugbank.ca/drugs/DB09299.
- http://static.medicine.iupui.edu/divisions/infdresearch/GS1489.pdf.
- Akram NMD, Umamahesh M. A New Validated RP-HPLC Method for the Determination of Emtricitabine and Tenofovir AF in its Bulk and Pharmaceutical Dosage Forms. JCHPS. 2017;10(4):54–59.
- Badgujar BP, Mahajan MP, Sawant SD. Development and Validation of RP-HPLC Method for the Simultaneous Estimation of Tenofovir Alafenamide and Emtricitabine in Bulk and Tablet Dosage Form. Int J ChemTech Res. 2017;10(5):731–739.
- Mastanamma S, Venkata Reddy D, Saidulu P, et al. Development and Validation of Stability Indicating RP-HPLC Method for the Simultaneous Estimation of Emtricitabine Tenofovir Alafenamide Bulk and their Combined Dosage Form. J. Chem. Pharm. 2017;9(9):70–80.
- Joshi M, Nikalje AP, Shahed M, et al. HPTLC method for the simultaneous estimation of emtricitabine and tenofovir in tablet dosage form. Indian J Pharm Sci. 2009;71:95–97. doi: 10.4103/0250-474X.51951
- Venkateswara Rao B, Vidyadhara S, Nagaraju B, et al. A Novel Stability Indicating RP-HPLC Method Development And Validation For The Determination Of Tenofovir Disoproxil Fumarate And Emtricitabine In Bulk And Pharmaceutical Formulations. IJPSR. 2017;8(5):2168–2176.
- Yenumula BRR, Singampalli MR, Challa BSR. Simultaneous Estimation of Emtricitabine and Tenofovir Disoproxil Fumarate in Tablet Dosage Form by Reverse Phase High-performance Liquid Chromatography. SOJ Chromatograph Sci. 2015;1:6), doi:10.15226/2471-3627/1/1/00105.
- Sharma R, Gupta P. A Validated RP - HPLC Method for Simulataneous Estimation of Emtricitabine and Tenofovir Disoproxil Fumarate in a Tablet Dosage Form. Eurasian J Anal Chem. 2009;4(3):276–284.
- Shelke A, Shinde M, Mogal R, et al. Application of UV Spectrophotometric methods for simultaneous estimation of Emtricitabine and Tenofovir alafenamide fumarate in bulk. Asian J Pharm Techn. 2018;8:2231–5713.
- Snyder RL, kirklannd JJ, Glaijch LJ. Practical HPLC [reverse phase high performance liquid chromatography] method development. 2nd ed. New York: Wiley; 1997. p. 30–100.
- Satinder A, Dong MW. Method development and validation.pharmaceutical analysis by HPLC [reverse phase high performance liquid chromatography]. Vol. 6. Newyork: Elsevier; 2005. p. 16–70.
- Swartz ME, krull I. Analytical method development and validation. 1st ed. New York: Marcel Dekker; 2009. p. 17–80.