?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
This study was designed to examine the total phenolic and flavonoid levels and the antioxidant activities of the essential oil (EO) and various extracts obtained from fruits of Ammodaucus leucotrichus using 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay. According to the results obtained, the odorized extracts exhibited stronger antioxidant activity than the desodorized extracts. The highest radical-scavenging activity was shown by the odorized water soluble (OdMW) of the methanol extract (IC50 = 36.7 µg.ml−1), whereas the EO showed the weakest activity potential with an IC50 = 3326.7 µg.ml−1. As well as the radical-scavenging activity of the extracts, they were evaluated in terms of their total phenolic and flavonoid contents. The subfractions of methanol extracts from odorized material were found to be rich in phenolics and flavonoids, followed by the subfractions of desodorized methanol extracts and the hot water extracts. Finally, a positive correlation was observed between the antioxidant activity and total phenolic and flavonoid levels of the extracts.
GRAPHICAL ABSTRACT
1. Introduction
Free radicals have been claimed to play a key role, affecting human health by causing severe diseases, such as cancer and cardiovascular diseases by cell degeneration. There are increasing suggestions by considerable evidence that these compounds induce oxidative damage to biomolecules (lipids, proteins and nucleic acids) [Citation1]. They also react rapidly with electron acceptors, such as molecular oxygen to become radicals themselves, also referred to as reactive oxygen species (ROS) [Citation2]. Therefore, much attention has been focused on the use of antioxidants to inhibit lipid peroxidation and to protect from damage due to free radicals. There are two basic categories of antioxidants, namely, synthetics and naturals. Synthetic antioxidants such as butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT) have been used as antioxidants since the beginning of this century. However, restrictions on the use of these compounds are being imposed because of their carcinogenicity and other toxic properties [Citation3]. Thus, the interest in natural antioxidants has increased considerably. These compounds exhibit their antioxidant activity by various mechanisms including chain-breaking by donation of hydrogen atoms or electrons that convert free radicals into more stable species and decomposing lipid peroxides into stable final products [Citation4]. In this respect, antioxidative properties of the essential oils and various extracts from many plants are of great interest in both academia and the food industry, since their possible use as natural additives emerged from a growing tendency to replace synthetic antioxidants by natural ones.
Ammodaucus leucotrichus Coss. & Dur. (Apiaceae family) is the only one species of the genus Ammodaucus [Citation5]. It is a small annual plant (10–12 cm. high) with erect, finely striated stems. The leaves are finely dissected and slightly fleshy. The flowers with 5 free petals are grouped in umbels of 2–4 branches. The fruit (8–10 mm) is covered with dense silky white hairs. The plant has a strong smell of anise [Citation5]. A. leucotrichus inhabits the maritime sands in the Saharan and sub-Saharan countries of North Africa, Morocco, Algeria and Tunisia, extending to Egypt and tropical Africa [Citation6]. In Morocco, which locally known as “kammûn es-sofi or akâman”, the fruits are used either by the local population as a powder or in a decoction to treat gastric-intestinal pain, gastralgias and indigestion [Citation7]. It is also frequently used, as an infusion, for diverse infantile diseases of the digestive apparatus: dysentery, nausea, regurgitation, vomiting. Previous studies of aqueous extracts of A. leucotrichus were shown to inhibit the formation of calcium oxalate monohydrate crystals and also found to potently inhibit the nucleation, growth and aggregation phases of calcium oxalate crystallization [Citation8]. It was also reported that the essential oils of A. leucotrichus exhibited the antimicrobial activities against bacteria, yeasts and filamentous fungi [Citation9–11].
As far as our literature survey could ascertain, antioxidant activities of A. leucotrichus fruits have not previously been published. Therefore, the objective of this study was to determine: (1) the antioxidant activities of its essential oil, as well as the various extracts, obtained by using several solvents of varying polarity in a Soxhlet extractor, using DPPH• radical activity assay and (2) the total phenolic and flavonoid content of plant extracts.
2. Materials and methods
2.1. Plant material
The fruits of A. leucotrichus were harvested in March 2012 (full bloom) from Alnif-Errachidia (Morocco). Voucher specimens were deposited in the herbarium of the Faculty of Sciences and Technology of Errachidia (Morocco).
2.2. Preparation of the extracts
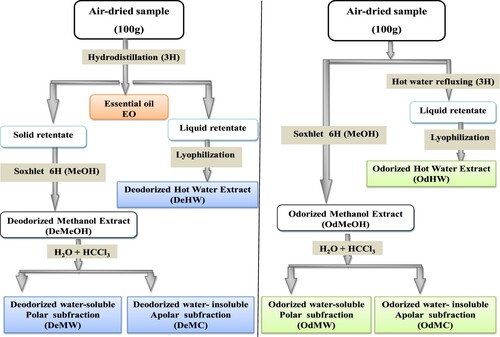
Air-dried fruits of A. leucotrichus were subjected to the different extraction procedures given below (Figure ).
2.2.1. Essential oil isolation (EO)
The EO used in this study was the same we used in our previous study [Citation12]. It was prepared by hydrodistillation for 3 h using a Clevenger type apparatus and analyzed by gas chromatography (GC) and gas chromatography/mass spectroscopy (GC/MS). A total of 10 components, accounting for 94.7% of the total oil, were identified (Table ). It contained peryllaldehyde 7 (73.5%) followed by limonene 5 (12.5%) were the major components.
Table 1. Chemical composition of A. leucotrichus fruits essential oil from Morocco.
2.2.2. Preparation of the Deodorized Hot Water extract (DeHW)
The liquid retentate, obtained after completion of hydrodistillation using a Clevenger-type apparatus to isolate EO, was collected, filtered and centrifuged at 5000 rpm for 30 min. The supernatant was also lyophilized to give finally DeHW in a yield of 5.82% (w/w) [Citation13].
2.2.3. Preparation of the Odorized Hot Water extract (OdHW)
A portion (100 g) of dried plant material was extracted with 1L of water under reflux for 3 h. The liquid retentate was collected, filtered and centrifuged at 5000 rpm for 30 min. The supernatant was also filtered to eliminate any residues and lyophilized to give finally OdHW in a yield of 9.37% (w/w) [Citation13].
2.2.4. Preparation of the Deodorized Methanol extract (DeMeOH)
The solid retentate of the hyrodistillation (100 g) was dried and re-extracted with methanol for 6 h in a Soxhlet extractor. The resulting extract (DeMeOH) (15.23%, w/w) was fractionated with water and chloroform (CHCl3) to obtain Deodorized Water soluble (DeMW) (polar subfraction: 8.22%, w/w) and water-insoluble (Deodorized Chloroformic) (DeMC) (non polar subfraction: 7.74%, w/w) [Citation13].
2.2.5. Preparation of the Odorized Methanol extract (OdMeOH)
A portion (100 g) of dried plant material was extracted with methanol for 6 h in a Soxhlet extractor. The resulting extract (OdMeOH) (10.19%, w/w) was fractionated with water and chloroform (CHCl3) to obtain Odorized Water soluble (OdMW) (polar subfraction: 5.22%, w/w) and water-insoluble (Odorized Chloroformic) (OdMC) (non polar subfraction: 3.97%, w/w). All extracts obtained were concentrated, dried and kept in the dark at 4 °C until tested.
2.3. Antioxidant activity by DPPH• radical scavenging assay
The antioxidant activity of the fruits of A. leucotrichus essential oil and various extracts were assessed by measuring their scavenging abilities to 2,2’-diphenyl-1-picrylhydrazyl stable radicals. The DPPH assay was performed as described [Citation14]. In succinct terms, the aliquots (50 µl) of various concentrations of the test compound in methanol in methanol were added to 5 ml of a 0.004% methanol solution of DPPH. After a 30 min incubation period at room temperature, the absorbance was read against a blank at 517 nm. Inhibition free radical DPPH in percent (I%) was calculated in following way:(I%) = [Ablank-Asample/Ablank] x 100%where Ablank is the absorbance of the control reaction (containing all reagents except the test compound), and Asample is the absorbance of the test compound. Test compound concentration providing 50% inhibition (IC50, expressed in μg.ml−1) was calculated from the graph plotted inhibition percentage against extract concentration. Synthetic antioxidant reagent Ascorbic acid and Trolox are used as the positive control and all tests were carried out in triplicate.
2.4. Assay for total phenolics
Total phenolics constituent of the extracts were determined by using the Folin–Ciocalteu reagent according to the method described by [Citation15] with slight modifications. 0.1 ml of extract solution, containing 1000 µg extract, was added to a volumetric flask. Then, 45 ml distilled water and 1 ml Folin–Ciocalteu reagent was added and the flask was shaken vigorously. After 3 min, 3 ml of Na2CO3 (2%) solution was added and the mixture was allowed to stand for 2 h by intermittent shaking. Absorbance was measured at 760 nm. Total phenols were expressed as caffeic acid equivalents (CAEs), using a calibration curve of a freshly prepared caffeic acid solution used as a standard agent. For the caffeic acid, the curve absorbance versus concentration is described by the equation:
2.5. Assay for total flavonoids
Total flavonoid content was determined by using the method of [Citation15] with slight modifications. Briefly, 1 ml of 2% aluminum trichloride (AlCl3) in methanol was mixed with the same volume of the extract solution (1000 µg). Absorbance values of the samples were determined at 430 nm after 15 min duration against a blank. Total flavonoids were expressed on a dry weight basis as quercetin equivalents (QEs), using a calibration curve of a freshly prepared quercetin solution used as standard agent. For quercetin, the curve absorbance versus concentration is described by the equation:
2.6. Statistical analysis
Each value is the mean of three replications. Values of different parameters were expressed as the mean ± standard deviation (x ± SD). The one-way analysis of variance (ANOVA) followed by Tukey multiple comparisons was performed at the level of p < 0.05 to evaluate the significance of differences between mean values. All statistical analyses were performed using SPSS software (SPSS 10 for Windows).
3. Results and discussion
3.1. Antioxidant activity
Antioxidant properties of the essential oils and various extracts from many plants are of great interest in both academia and the food, cosmetic and pharmaceutical industries, since their possible use as natural additives emerged from a growing tendency to replace synthetic antioxidant by natural ones. The antiradical activities or free radical-scavenging capacities of essential oil and various extracts from fruits of A. leucotrichus were determined by comparing with the activities of known antioxidants, such as trolox and ascorbic acid by 2,2′-diphenyl-1-picrylhydrazyl (DPPH) radical assay. The reduction ability of DPPH radicals’ formation was determined by the decrease in its absorbance at 517 nm induced by antioxidants. The effect of antioxidants on DPPH radical scavenging is thought to be due to their hydrogen donating ability. DPPH is a stable free radical and accepts an electron or hydrogen radical to become a stable diamagnetic molecule [Citation16]. The results of antiradical activities of studied oils were presented in Table .
Table 2. Scavenging effect (%) on DPPH of positive controls, essential oil and various extracts from fruits of A. leucotrichus at different concentrations.a
The scavenging ability of the all samples showed a concentration-dependent activity profile. It increased with an increase in their concentrations. It can be seen that various extracts exhibited a significant difference in free radical scavenging activities (p < 0.05), which reached a higher value in the presence of the highest extract concentration. Figure showed only concentrations of active extracts providing 50% inhibition.
Figure 2. Free radical scavenging capacities of the extracts from fruits of A. leucotrichus and positive controls measured in DPPH assay.
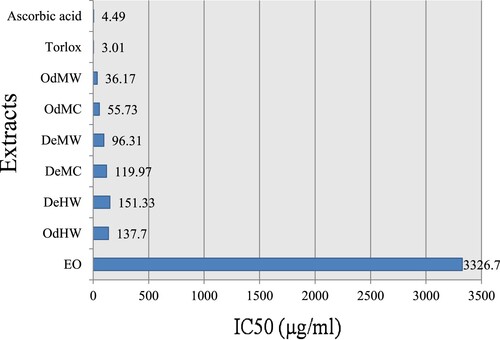
The concentration of antioxidant needed to decrease the initial DPPH concentration by 50% (IC50) is a parameter widely used to measure antioxidant activity. According to the results obtained, the odorized extracts exhibited stronger antioxidant activity than the desodorized extracts, and the free radical scavenging capacities were higher in the polar subfractions of methanol extracts. Indeed, the scavenging ability of the odorized water soluble (OdMW) was found the most active of all other extracts with an IC50 = 36.7 µg.ml−1. This activity was followed by odorized water-insoluble (OdMC) (IC50 = 55.73 µg.ml−1), deodorized water soluble (DeMW) (IC50 = 96.31 µg.ml−1) and deodorized water-insoluble (DeMC) (IC50 = 119.97 µg.ml−1). However, the hot water extracts OdHW and DeHW were found to be less efficient in radical scavenging with an IC50 = 137.7 and 151.33 µg.ml−1, respectively, whereas the essential oil (EO) showed the weakest activity potential with an IC50 = 3326.7 µg.ml−1. IC50 values of the positive control trolox (IC50 = 3.01 µg.ml−1) and ascorbic acid (IC50 = 4.49 µg.ml−1) were also determined in parallel experiments. None of the samples showed activity as strong as the standards. Thus, the DPPH scavenging effect increased in the order of EO < DeHW < OdHW < DeMC < DeMW < OdMC < OdMW < Ascorbic acid < Trolox.
To the best of our knowledge, antioxidant activity of A. leucotrichus has not been previously reported but our findings were in accordance with reports in the literature, indicating that the polar sub-fraction of methanolic extract for other species has the highest radical scavenging activity such Eucalyptus oleosa var. obtuse (IC50 = 39.82 ± 1.55 µg.ml−1) of the polar subfraction of a methanol extract, reported by [Citation17] Rhamnus kurdica (IC50 = 47.16 ± 3.83 and 31.11 ± 3.57 µg.ml−1) of the polar subfraction of methanol extract from Leaves in pre–flowering and leaves in flowering [Citation18], Thymus caramanicus (IC50 = 43.17 ± 0.65 µg.ml−1) of the extract polar subfraction of methanol from aerial parts [Citation19] and Salvia mirzayanii (IC50 = 37.9 ± 0.85 and 40.05 ± 1.4 µg.ml−1) of polar subfractions C and F of ethyl acetate extract, respectively, reported by [Citation20]. Moreover, the antioxidant activity of our sample was superior to other species samples reported previously by various researchers for other species such as polar subfraction of methanolic extract from Teucrium orientale subsp. Taylori (IC50 = 61.45 ± 0.5 µg.ml−1) [Citation21], Mindium laevigatum (IC50 = 165 ± 3.1 µg.ml−1) [Citation22] and Marrubium globosum subsp. Globosum (IC50 = 157.26 ± 1.12 µg ml−1) [Citation23]. The observed antioxidant activity of polar subfractions of a methanol extract could be attributed to the presence of hydrophilic phenolic substances, such as phenolic compounds (flavonoids and phenolic acids). However, the lower antioxidant activity of EO may be partially due to the modest content in bioactive compounds and large content in unsaturated terpenes. Indeed, Lee et al. [Citation24] reported that rosemary EO is not an antioxidant, at variance with rosemary hydroalcoholic extract that has a powerful antioxidant effect, due to the nonvolatile phenols carnosol, carnosic acids, and rosmarinic acid, which are completely removed during EO preparation. Also, Amorati et al. [Citation25] showed that Rosemary EO seems to have some reactivity toward the coloured DPPH radical, although smaller than that of thyme EO, which contains phenols with proven antioxidant power. On the other hand, the antioxidant activity of EO depends on the methodology used for oil evaluations. In fact, the free radical scavenging activity of hydroalcoholic extracts was superior to that of essential oils, when the antioxidant activity was determined by measuring the radical scavenging effect on DPPH. This might be attributed that hydrophilic antioxidants and antiradical activity were mainly attributed to polar secondary metabolites such as phenolics and flavonoids [Citation13,Citation26].
3.2. Assays for total phenolics and flavonoids
Based on the absorbance values of the various extract solutions, reacted with Folin–Ciocalteu reagent and compared with the standard solutions of caffeic acid equivalents as described above, the results of the colorimetric analysis of total phenolics are given in Table .
Table 3. Phenols Total and flavonoid content of essential oil and different extracts of the fruits of A. leucotrichus.
As can be seen from Table , the amount of total phenolics was highest in the subfractions of methanol extracts from odorized material followed by the subfractions of desodorized methanol extracts and the hot water extracts. Indeed, total phenolic was highest in the OdMW (39.84 ± 0.86%), followed by OdMC (30.20 ± 0.99%), DeMW and DeMC extracts (14.14 ± 1.92% and 6.7 ± 0.95%, respectively). The lowest total phenolic was in the OdHW and DeHW (5.72 ± 0.21 and 5.08 ± 0.11%, respectively). The Statistic analysis of phenolic content revealed a highly significant difference (p < 0.05) between all extracts.
For flavonoid, the highest value was also observed in the subfraction of odorized methanol extracts followed by the subfraction of desodorized methanol extracts and the hot water extracts. It varied significantly (p < 0.05) from one another. Moreover, no significant differences were found in the total flavonoid, representing 3.03 ± 0.03 mg QEs/g DeHW for the odorized hot water extract and 4.31 ± 2.17 mg QEs/g DeMC for the desodorized methanol extract.
It is extremely important to point out that; there is a positive correlation between antioxidant activity potential and amount of phenolic compounds of the extracts (R2 = 0.97). The OdMW was found to have high phenolic constituents exhibiting greater radical scavenging activity. This result is in agreement with those previously reported [Citation18,Citation19,Citation23]. It seems clear that the presence of polar phenolics is another fact concerning the evaluation of the activity in free radical scavenging. Besides, the moderate activity seen in the polar subfraction of methanol extract from deodorized retentate (DeMW) and the polar extracts from hot water, which is often spoiled, reflects the thermostable nature of these polar phenolics. The methanolic extract has been shown to have higher total polyphenolic contents and antioxidant capacity than aqueous extract, probably due to the polarity and good solubility for phenolic components in methanol [Citation27]. The key role of phenolic compounds as scavengers of free radicals is emphasized in several reports. Their antioxidant activity is mainly due to their redox properties which make them act as reducing agents, hydrogen donors, and singlet oxygen quenchers. They may have also a metal chelating potential [Citation28,Citation29]. Moreover, radical scavenging activity is one of the various mechanisms to contribute to overall activity, thereby creating a synergistic effect.
4. Conclusion
Our study is the first report of in vitro antioxidant activity of the essential oil and various extracts of A. leucotrichus fruits. It was found that between them, the odorized extracts from methanol extract exhibited stronger antioxidant activity than the desodorized extracts according to the results obtained by the DPPH test. This was attributed to the higher phenolic content of the former. The antioxidant activity of phenolics is mainly due to their redox properties which make them act as reducing agents, hydrogen donors, and singlet oxygen quenchers. The order of the DPPH scavenging effect was EO < DeHW < OdHW < DeMC < DeMW < OdMC < OdMW < Ascorbic acid < Trolox.
In conclusion, results obtained from this study provides evidence that polar sub-fraction of methanol extract concerned here could be used in the field of food industries and the other fields which are processing natural products. But, further studies are needed for the isolation and identification of individual phenolic compounds and also in vivo studies are needed for a better understanding of their mechanism of action as an antioxidant.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Shoib AB, Shahid AM. Determination of total phenolic and flavonoid content, antimicrobial and antioxidant activity of a root extract of Arisaema jacquemontii Blume. J Taibah Univ Sci. 2015;9:449–454. doi: 10.1016/j.jtusci.2014.11.001
- Pincemail JJ. Free radicals and antioxidants in human diseases. In: Favier AE, Cadet J, Kalyanaraman B, Fontecave M, Pierre JL, editor. Analysis of free radicals, in biological systems. Berlin: Birkhauser Verlag; 1995. p. 83.
- Adel FA, Mengjin S, Cunyu L, et al. Comparative analysis of antioxidant activities of essential oils and extracts of fennel (Foeniculum vulgare Mill.) seeds from Egypt and China. Food Sci Human Well. 2019;8:67–72. doi: 10.1016/j.fshw.2019.03.004
- Neelam G, Madhu G, Darshana M, et al. Chemical composition, total phenolic and flavonoid contents, and in vitro antimicrobial and antioxidant activities of crude extracts from red chilli seeds (Capsicum frutescens L). J Taibah Univ Sci. 2016;10:462–470. doi: 10.1016/j.jtusci.2015.06.011
- Ozenda P. Flore et végétation du Sahara. 3ème ed. Paris: CNRS; 1991. p. 360.
- Maberly PL. The plant book. Cambridge: Cambridge University Press; 1998.
- Bellakhdar J. La pharmacopée marocaine traditionnelle. Médecine arabe ancienne et savoirs populaires. Paris: IBIS Press; 1979. p. 764.
- Beghali M, Ghalem MS, Allali H, et al. Screening for anticrystallisation calcium oxalate urolithiasis activity in Algerian plants. J Biochem Molec Biol. 2008;16:11.
- Abu Zarga MH, Al-Jaber HI, Babaamer ZY, et al. Chemical composition, antimicrobial and antitumor activities of essential oil of A. leucotrichus growing in Algeria. TBAP. 2013;3:224–231.
- El-Haci IA, Bekhechi C, Atik-Bekkara F, et al. Antimicrobial activity of A. leucotrichus fruit oil from Algerian Sahara. Nat Prod Commun. 2014;9:711–712.
- Manssouri M, Znini M, El Harrak A, et al. Antifungal activity of essential oil from the fruits of Ammodaucus leucotrichus Coss. and Dur., in liquid and vapour phase against postharvest phytopathogenic fungi in apples. J App Pharm Sci. 2016;6:131–136. doi: 10.7324/JAPS.2016.60520
- Manssouri M, El Ouadi Y, Znini M, et al. Adsorption proprieties and inhibition of mild steel corrosion in HCl solution by the essential oil from fruit of Moroccan A. leucotrichus. Mater Environ Sci. 2015;6:631–646.
- Znini M, Laghchimi A, Ansari A, et al. Total phenolics, flavonoids contents and antioxidant activity of essential oil and aqueous extracts of Salvia aucheri Boiss var. mesatlantica. Chem Sci Rev Lett. 2015;4:1108–1116.
- Bouaziz M, Jemai H, Khabou W, et al. Oil content, phenolic profiling and antioxidant potential of Tunisian olive drupes. J Food Sci Agric. 2010;90:1750–1758. doi: 10.1002/jsfa.4013
- Sarikurkcu C, Sabih Ozer M, Eskici M, et al. Effects of food processing on pesticide residues in fruits and vegetables: A meta-analysis approach. Food Chem Toxicol. 2010;48:1–18. doi: 10.1016/j.fct.2009.10.031
- Pattusamy N, Changa M. Antioxidant activity of 3-arylidene-4-piperidones in the 1,1-diphenyl-2-picrylhydrazyl scavenging assay. J Taibah Univ Sci. 2017;11:40–45. doi: 10.1016/j.jtusci.2014.11.007
- Safaei-Ghomi J, Ghadami M, Batooli H. Bioactivity of Eucalyptus oleosa var. obtuse leaves and flowers. Digest J Nanomat Biost. 2012;7:657.
- Gholivand MB, Piryaei M. Total phenols, flavonoids, anthocyanins, ascorbic acid contents and antioxidant activity of Rhamnus kurdica Boiss for flower and leaves in flowering and pre-flowering stages. Afr J Biotechnol. 2014;13:1131–1135. doi: 10.5897/AJB12.2461
- Safaei-Ghomi J, Ebrahimabadi AH, Djafari-Bidgoli Z, et al. GC/MS analysis and in vitro antioxidant activity of essential oil and methanol extracts of Thymus caramanicus Jalas and its main constituent carvacrol. Food Chem. 2009;115:1524–1528. doi: 10.1016/j.foodchem.2009.01.051
- Moein MR, Moein S, Ahmadizadeh S. Radical scavenging and reducing power of Salvia mirzayanii subfractions. Molecules. 2008;13:2804–2813. doi: 10.3390/molecules13112804
- Amiri H. Antioxidant activity of the essential oil and methanolic extract of Teucrium orientale (L.) subsp. taylori (Boiss.) Rech f. J Pharma Res. 2010;9:417–423.
- Modaressi M, Shahsavari R, Ahmadi F, et al. The evaluation of Antibacterial, Antifungal and antioxidant activity of methanolic extract of Mindium laevigatum (Vent.) Rech. F., from Central Part of Iran. Jundishapur J Nat Pharm Prod. 2013;8:34–40. doi: 10.17795/jjnpp-7730
- Sarikurkcu C, Tepe B, Daferera D, et al. Studies on the antioxidant activity of the essential oil and methanol extract of Marrubium globosum subsp. globosum (lamiaceae) by three different chemical assays. Biores Technol. 2008;99:4239–4246. doi: 10.1016/j.biortech.2007.08.058
- Lee KG, Shibamoto T. Determination of antioxidant potential of Volatile extracts Isolated from various Herbs and Spices. J Agric Food Chem. 2002;50:4947–4952. doi: 10.1021/jf0255681
- Amorati R, Foti MC, Valgimigli L. Antioxidant activity of essential oils. J Agric Food Chem. 2013;61:10835–10847. doi: 10.1021/jf403496k
- Deba F, Xuan TD, Yasuda M, et al. Chemical composition and antioxidant, antibacterial and antifungal activities of the essential oils from Bidens pilosa Linn. var. Radiata. Food Control. 2008;19:346–352. doi: 10.1016/j.foodcont.2007.04.011
- Siddhuraju P, Becker K. Antioxidant properties of various Solvent extracts of total phenolic constituents from three different Agroclimatic Origins of Drumstick Tree (Moringa oleifera Lam.) leaves. J Agric Food Chem. 2003;51:2144–2155. doi: 10.1021/jf020444+
- Gülçin I, Huyut Z, Elmastaş M, et al. Radical scavenging and antioxidant activity of tannic acid. Arab J Chem. 2010;3:43–53. doi: 10.1016/j.arabjc.2009.12.008
- Mradu G, Saumyakanti S, Sohini M, et al. HPLC profiles of standard phenolic compounds present in medicinal plants. Int J Pharmacog Phytochem Res. 2012;4:162–167.