Abstract
The enantiomeric resolution of primaquine, quinacrine and tafenoquine antibacterial medicines is described on Chirobiotc R column (150 ×4.6 mm, 5.0 µm). Two eluents used were methanol–acetonitrile-water-triethyl amine in the ratio of 70:10:20:0.1 (I) and 60:30:10:0.1 II) at 1.0 mL/min flow rate. The retention factors values for first and second enantiomers ranged from 1.0–3.40 and 3.40–5.40. The values of the separation and resolution factors were 1.59–3.40 and 2.10–2.50. In second solvent, only quinacrine could be resolved with 1.30 and 4.60 as a retention factor while the values of separation and resolution factors were 3.54 and 2.08. The supramolecular mechanism of the chiral resolution was established. The limits of detection and quantification ranged from 0.9–1.2 and 9.0–11.0 ng, respectively. The π-π interactions and hydrogen bondings were responsible for enantiomeric resolution. The enantiomeric method is useful for the chiral separation of primaquine, quinacrine, and tafenoquine antibacterial medicines.
1. Introduction
The bacterial infection is a common disease in many countries. It may due to lack of sanitation, and proper medication. Many antibiotics are available to control various bacterial infections but some have become resistant. Some antibiotics are racemic, for example, some clinicians are not aware that one of the enantiomers is active although the other is useless or sometimes, create some problems and side effects. In this way, the racemic dose of such medication is not sufficient to kill bacteria but rather made resistant to the bacterial species. Consequently, a great demand for optically active pure forms of anti-bacterial agents is raising [Citation1–6]. Quinolones are very important anti-bacterial agents as they react with bacterial DNA; through the attack on topoisomerase enzymes, and check their growth. But unfortunately, these quinolones are being used frequently for controlling bacterial infections.
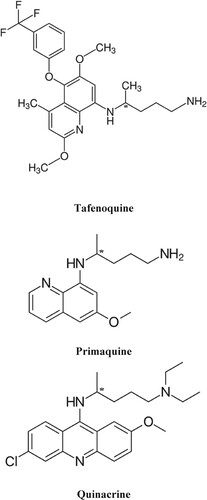
Among various primaquine, quinacrine and tafenoquine (Figure ) are very important anti-bacterial agents. Primaquine is a very important medicine used to cure Pneumocystis pneumonia and malaria [Citation7]. It is used due to treating wide-spread malaria because of Plasmodium vivax and Plasmodium ovale; when other medicines fail to control malaria. It is also different management for Pneumocystis pneumonia with clindamycin [Citation8]. Quinacrine is a broad-spectrum bactericidal and used to cure bacterial infections in humans and animals. It is effective for both Gram-negative and positive bacteria. Tafenoquine is used to treated malaria, as well as for malaria because of the Plasmodium vivax and Plasmodium ovale. The chief benefit of this medicine is its long half-life (15–21 days) and, hence, a one-time treatment may be enough to clear hypnozoites. In this way, these anti-bacterial agents are very important and need their availability in optically active pure forms.
A thorough examination of literature was performed but only a few publications are accessible on the chiral separation of these anti-bacterial agents. Among dissimilar techniques, HPLC is well-known as the best one. Several chiral selectors such as cyclodextrins, polysaccharides, macrocyclic glycopeptide antibiotics, proteins, Pirkl’s phases chiral crown ethers [Citation9–16] are being used to archive the chiral separation task but macrocyclic glycopeptide antibiotics are gaining importance due to their wide range of chiral recognition capabilities. Most popularly used macrocyclic glycopeptide antibiotics are vancomycin, teicoplanin, ristocetin, rifamycin, kanamycin, streptomycin, etc. [Citation17–21]. Despite good chiral recognition capabilities, ristocetin is not studied widely in the chiral separation of different racemates. Hence, in this paper, the efforts are made to resolve enantiomers of primaquine, quinacrine, and tafenoquine using ristocetin chiral column.
2. Experiment
2.1. Chemicals
Primaquine, quinacrine, and tafenoquine were supplied by Sigma Aldrich Chem. Co., USA. MeOH and ACN LiChrosolve and TEA of AR obtained from Merck, Mumbai, India. The solutions (1.0 mg/mL) of primaquine, quinacrine and tafenoquine were made in methanol.
2.2. Equipment
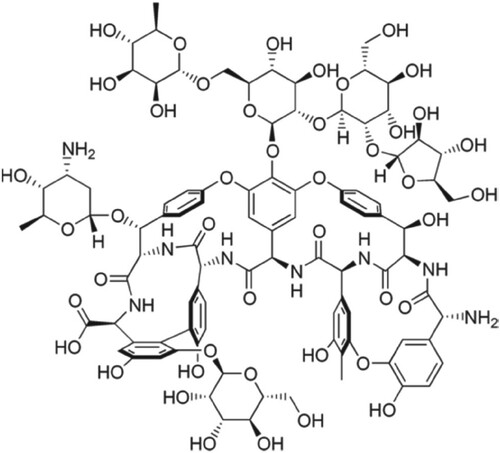
The experiments were performed with HPLC equipment (Shimadzu, Japan) as described elsewhere [Citation22]. The chiral columns used were Chirabiotic R (Figure ) and supplied by Sigma Aldrich Chem. Co., USA.
2.3. HPLC appropriateness test
HPLC appropriateness test was carried out with five replicate experiments (n = 5) of the solutions of the reported molecules in the HPLC equipment. The tailing factors, resolution, %RSD of chromatograms area and retention times were determining. The results indicated a small <1.0% for peak area and <1.0% for retention time and RSD values. The tailing factors of primaquine, quinacrine and tafenoquine were 0.89, 0.84 and 0.82.
2.4. Specificity
HPLC specificity study was measured for the interfering of any other chromatogram of the main peaks in blank solution. The diluents were loaded as blank solutions. No chromatogram was seen from blank solutions with the same retention time as of the main peaks.
2.5. Linearity
HPLC linearity plots were drawn with chromatogram area against dissimilar amounts for the reported compounds. The linearity was drawn and the y-intercepts, slopes, regression coefficient (r2) and correlation coefficient (r) were estimated. The explanations of regression curves are tabulated. Worthy linearity was seen for all the reported racemates. The regression coefficient (r2) varied from 0.9953–0.9994.
2.6. HPLC conditions
The experiments were performed with HPLC equipment as described above. 20.0 µL of primaquine, quinacrine, and tafenoquine solutions were loaded onto HPLC equipment. The eluents used were methanol–acetonitrile-water-diethyl amine (70:10:20:0.1%) and (60:30:10:0.1%) with 1.0 mL flow rate. The detection was achieved at 351, 450 and 530 nm for primaquine, quinacrine and tafenoquine, respectively. The column used was Chirobiotic R (150 × 4.6 mm, 5.0 μm). The experimental temperature was 26 ± 2 °C for all the experiments. The eluents were filtered and degassed each day before use. The eluents were filtered using 0.45 µm pore size and 25 mm diameter nylon membrane.
2.7. Limit of detection (LOD) and quantification (LOQ)
Limits of detection (LOD) and limits of quantitation (LOQ) were estimated for the described racemates. These were performed using the signal to noise ratio of 3.0 and 10.0, correspondingly. The limits of detection and quantification altered from 0.9–1.2 and 9.0–11.0 ng, respectively. The values were tabulated.
2.8. Robustness
HPLC robustness was estimated through small variation in one factor at a time while making the other factors fixed. The variations were analyzed in the alteration in peaks, that may affect the working of the method. The differences in retention time (Rt) and peak area were <1.0, that assured the method robustness.
3. Results and discussion
3.1. Chromatography
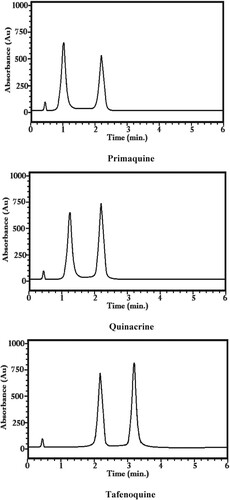
The HPLC parameters i.e. retention (k), separation (α), and resolution (Rs) factors were estimated for the resolved enantiomers of the reported racemates. These values are calculated for two eluents i.e. MeOH:ACN:Water:TEA (70:10:20:0.1%, v/v) [I] and (60:30:10:0.1%, v/v) [II]. The determined values of these racemates are given in Table . It is evident from the table that all the three racemates got enantiomeric resolved in eluent I while the only quinacrine could be resolved in eluent II. The values of retention factors for the first and second enantiomers ranged from 1.0–3.40 and 3.40–5.40. The degrees of the separation and resolution factors ranged from 1.59–3.40 and 2.10–2.50. In the case of the second solvent, only quinacrine could be resolved with 1.30 and 4.60 as a retention factor while the values of separation and resolution factors were 3.54 and 2.08. These detail evaluations of these values may be performed in Table , which indicated that all values are greater than 1.0; an indication of thorough resolution of all the racemates. The chromatograms of the separated enantiomers are given in in Figure . An examination of this figure depicts the clear cut baseline separation of the reported racemates. The optically active pure enantiomers were used to identify the enantiomers. It was seen that S-enantiomers of all the reported racemates eluted first following by R-enantiomers.
Table 1. HPLC values for enantiomeric resolution of primaquine, quinacrine and tafenoquine.
Experimental conditions:
Chirobiotc R column (150 × 4.6 mm, 5.0 μm); Flow rate: 1.0 mL/min and detection: 351, 450 and 530 nm for primaquine, quinacrine and tafenoquine, respectively.
Experimental conditions: As in Table .
3.2. HPLC method optimization
The enantiomeric resolution of primaquine, quinacrine and tafenoquine antibacterial medicines was optimized by using different experimental variables. The dissimilar combinations of the mobile phases including methanol and acetonitrile were tested. Besides, eluent modifiers such as diethylamine, triethylamine, trifluoroacetic acid, etc. were added to the mobile phases. The eluent flow rate was also diversed from 0.5–2.0 mL/min. It was observed that at a low flow rate the peaks were broad while it was the partial resolution at a high flow rate. Additionally, the wavelength of detections was also varied from 220 to 500 nm. The other conditions optimized were amounts injected (5–25 µL) and temperature (10–50 °C). The extensive experiments were carried out and after all the best chromatographic conditions were achieved and reported in Table .
3.3. Mechanics of separation at supramolecular level
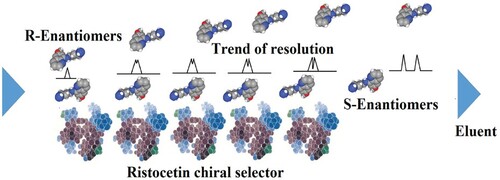
We are working in the area of chiral resolution for more than 25 years. To the best of our knowledge, the chiral environment is needed for the enantiomeric resolution. The enantiomers fitted differently on the chiral sites of the chiral selectors and stabilize at different extents by a number of forces. In the case of ristocetin the molecules have several chiral cavities and various functional groups including hydroxyl and amine. This chiral selector also has seven benzene rings. A perusal of the structures of primaquine, quinacrine, and tafenoquine indicate that these molecules also have amino and methyl groups with benzene rings. The order of elution of the enantiomers of these racemates was primaquine > quinacrine > and tafenoquine. It may be explained on the basis that primaquine has one primary and one secondary amine group along with two aromatic rings while quinacrine has one primary amine with three aromatic rings. Besides, quinacrine has a number of electronegative atoms than primaquine. These facts depict that the number of hydrogen bonds among ristocetin and the enantiomers of quinacrine are greater than in the case of primaquine. Besides, the number of π-π interactions among ristocetin and the enantiomers of quinacrine (3 aromatic rings) are greater than in the case of primaquine (2 aromatic rings). Therefore, quinacrine is retained more than primaquine. Similarly, the elution order of quinacrine and tafenoquine can be compared. As mentioned above, quinacrine has one secondary amine with three aromatic rings and five electronegative atoms (oxygen, nitrogen and chlorine). On the other hand, tafenoquine has one primary and one secondary amine group along with three aromatic rings. It also has nine electronegative atoms (oxygen, nitrogen and fluorine). Since both quinacrine and tafenoquine have three aromatic rings and the magnitude of π- π interactions are almost the same but the magnitude of hydrogen bonding in tafenoquine is greater than quinacrine due to nine electronegative atoms in tafenoquine in comparison to quinacrine. Besides, the presence of the fluorine atom in tafenoquine augmented the hydrogen bonding magnitude in comparison to quinacrine. In this way, tafenoquine is strongly bonded to ristocetin than to quinacrine and, consequently, eluted later than quinacrine. Based on these observations, it was realized that the chiral resolution of the reported racemates is because of the different magnitudes of π- π interactions and hydrogen bondings. Also, other forces such as van der Waal’s forces, steric effect, dipole induced dipole interactions are contributory to the chiral resolution. As a schematic representation, the chiral recognition mechanism is shown in Figure .
4. Conclusion
The enantiomeric resolution of primaquine, quinacrine and tafenoquine antibacterial medicines is achieved successfully. The Chirobiotc R column along with two mobile phases results in successful and base-lined resolution. The values of retention, separation and resolution factors for all the enantiomers were superior to 1.0; showing complete enantiomeric resolution of primaquine, quinacrine, and tafenoquine antibacterial medicines. The supramolecular mechanism suggested that π-π interactions and hydrogen bondings were responsible for enantiomeric resolution. The described enantiomeric method is useful for the enantiomeric separation of primaquine, quinacrine, and tafenoquine antibacterial medicines.
Acknowledgment
The authors extend their appreciation to the Deanship of Scientific Research, King Saud University for funding this work through Research Group no RGP-043.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Aboul-Enein HY, Ali I. Determination of tadalafil in pharmaceutical preparation by HPLC using monolithic silica column. Talanta. 2004;65:276–280. doi: 10.1016/j.talanta.2004.06.012
- Ali I, Gaitonde VD, Aboul-Enein HY, Hussain A. Chiral separation of β-adrenergic blockers on CelluCoat column by HPLC. Talanta. 2009;78:458–463. doi: 10.1016/j.talanta.2008.11.043
- Aboul-Enein HY, Ali I. HPLC enantiomeric resolution of nebivolol on normal and amylose based chiral phases. Pharmazie. 2001;56:214–216.
- Gupta VK, Aboul-Enein HY. Role of racemization in optically active drugs development. Chirality. 2007;6:453–463.
- Ali I, Sanagi MM, Aboul-Enein HY. Advances in chiral separations by non-aqueous capillary in pharmaceutical and biomedical analysis. Electrophoresis. 2014;35:926–936. doi: 10.1002/elps.201300222
- Ali I. Nano anti-cancer drugs: pros and cons and future perspectives. Curr Can DrugsTrarget. 2011;11:131–134. doi: 10.2174/156800911794328457
- The American Society of Health-System Pharmacists. Archived from the original on 20 December 2016. Retrieved 2 December, 2016.
- Vale N, Moreira R, Gomes P. Primaquine revisited six decades after its discovery. Eur J Med Chem. 2009;44:937–953. doi: 10.1016/j.ejmech.2008.08.011
- Ali I, Aboul-Enein HY. Impact of immobilized polysaccharide chiral stationary phases on enantiomeric separations. J Sep Sci. 2006;29:762–769. doi: 10.1002/jssc.200500372
- Ali I, Aboul-Enein HY. Enantioseparation of some clinically used drugs by HPLC using cellulose Tris (3, 5-dichlorophenylcarbamate) chiral stationary phase. Biomed Chromatogr. 2003;17:113–117. doi: 10.1002/bmc.220
- Aboul-Enein HY, Ali I. Comparative study of the enantiomeric resolution of chiral antifungal drugs econazole, miconazole and sulconazole by HPLC on various cellulose chiral columns in normal phase mode. J Pharm Biomed Analysis. 2002;27:441–446. doi: 10.1016/S0731-7085(01)00575-1
- Naim L, Ghanem A, Aboul-Enein HY. Chiral separations of piperidine-2, 6-dione analogues on Chiralpak IA and Chiralpak IB columns by using HPLC. Talanta. 2006;69:1013–1017. doi: 10.1016/j.talanta.2005.12.004
- Aboul-Enein HY, Ali I. Studies on the effect of alcohols on the chiral discrimination mechanisms of amylose stationary phase on the enantioseparation of nebivolol by HPLC. J Biochem Biophys Methods. 2001;48:175–188. doi: 10.1016/S0165-022X(01)00148-8
- Aboul-Enein HY, Ali I. Optimization strategies for HPLC enantioseparation of racemic drugs using polysaccharides and macrocyclic glycopeptide antibiotic chiral stationary phases. IL Farmaco. 2002;57:513–529. doi: 10.1016/S0014-827X(02)01242-9
- Al-Othman ZA, Alwarthan A, Ali I. Advances in enantiomeric resolution on chiral monolithic phases in liquid chromatography and electrochromatography. J Sep Sci. 2014;37:1033–1057. doi: 10.1002/jssc.201301326
- Gupta VK, Aboul-Enein HY. Chirality: a challenge to the environmental scientists. Curr Sci. 2003;84:152–156.
- Ali I, Aboul-Enein HY, Ghanem A. Enantioselective toxicity and carcinogenesis. Curr Pharm Anal. 2005;1:109–125. doi: 10.2174/1573412052953328
- Aboul-Enein HY, Simons C, Gubitz G. Enantiomeric resolution of the novel aromatase inhibitors by HPLC on cellulose and amylose based reversed and chiral stationary phases. Chirality. 2000;12:727–733. doi: 10.1002/1520-636X(2000)12:10<727::AID-CHIR5>3.0.CO;2-T
- Al-Othman ZA, Hussain A, Saleem K, et al. Chiral separation of β-adrenergic blockers in human plasma by SPE-HPLC. Chromatographia. 2011;73:251–256. doi: 10.1007/s10337-010-1891-4
- Ali I, Singh P, Aboul-Enein HY, Sharma B. Chiral analysis of ibuprofen residues in water and sediment. Anal Lett. 2009;42:1747–1760. doi: 10.1080/00032710903060768
- Aboul-Enein HY, Ali I. Comparison of the chiral resolution of econazole, miconazole, and sulconazole by HPLC using normal-phase amylose CSPs. Fresenius J Anal Chem. 2001;370:951–955. doi: 10.1007/s002160100884
- Al-Shaalan NH, Ali I, ALOthman ZA, et al. Enantioselective degradation of dufulin pesticide in water: Uptake, thermodynamics and kinetics studies. Chirality. 2019;31:1060–1069. doi: 10.1002/chir.23150