Abstract
Reaction of imidazolidineiminothiones with some amino acids methyl ester afforded imidazopyrazine and imidazolidine derivatives. Some of obtained products were synthesized by nano technology; where this method reduces the reaction time significantly. The evaluation of biological activity of some selected compounds was carried out and some of synthesized compounds displayed anticancer activity.
1. Introduction
Many of natural and synthetic heterocyclic compounds are identified as potential drug candidates and they have wide range of biological activities [Citation1–3]. Therefore, the organic chemistry was interested in the synthetic medicinal chemistry that oriented to develop new and efficient synthetic methods [Citation4,Citation5].
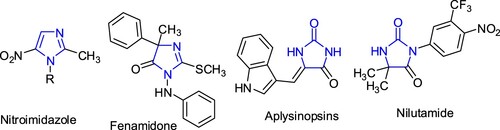
Imidazole moiety is present in many bioactive natural and synthetic compounds such as histidines [Citation6]. As shown in Figure , some of imidazole derivatives such as nitroimidazoles are well-known antibacterial and antiprozoal agents [Citation7], fenamidone has fungicidal activity [Citation8]. Imidazolidin-2-one moiety found in variety of biologically active compounds, for example, aplysinopsin is exhibiting cytotoxicity towards cancer cells [Citation9], nilutamide use for the treatment of advanced prostate cancer [Citation10]. Moreover, imidazolidineiminothione derivatives exhibited wide range of biological properties such as antibacterial, antifungal, antiviral, antitumour and antiinflammatory activities [Citation11–14].
Recently, there are interesting for the development of efficient methodologies that are environmentally benign. After the discovery of carbon nanotube (CNT) [Citation15], the attention was directed to this unique nanostructure material. Large numbers of various nanotubular materials have been reported [Citation16–18]. Among them, TiO2 nanotube (TNT) is one of the promising tubular structures. TNT is inexpensive and chemically stable. TNT has photochemical properties such as high photocatalytic activity. Many researchers studied TNT as a photocatalyst [Citation19,Citation20]. They make reaction processes more economic, more convenient and environmentally benign.
In view of these facts and to develop of synthetic method by investigating new, convenient and efficient method for imidazole derivatives through the reaction of imidazolidineiminothiones with amino acids was the aim of this investigation.
2. Results and discussion
2.1. Chemistry
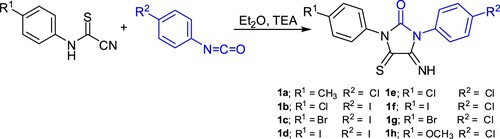
Initially, the imidazolidineiminothione derivatives 1a-h were prepared according to reported method [Citation21] by reaction of cyanothioformamide derivatives with an equimolar amount of isocyanate derivatives in ether followed by the addition of triethylamine as catalyst (Scheme 1).
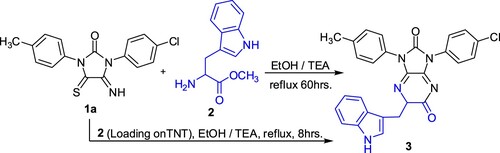
In the initial study, as shown in Scheme 2, when imidazolidineiminothione1a was left to react with (S)-tryptophan methyl ester 2 in ethanol under reflux in the presence few drops of triethylamine, imidazopyrazine derivative 3 was obtained after prolonged reaction time (60 h). The application of nanotechnology in the organic synthesis has considered in this study. The titanium oxide nanotube (TNT) was chosen. In this investigation the amino acid methyl ester was loaded on TNT. When the above reaction was carried out by using (S)-Tryptophan methyl ester 2 (loaded on TNT) significant decrease in reaction time (60→8 h) was observed. The spectral data and elemental analysis supported the formation of imidazopyrazine 3. IR spectrum showed absorption bands at: 3500 (NH), 1720, 1785 (C=O) and 1690 (C=N) cm−1. 1H NMR spectrum of 3 showed three aliphatic signals at δ = 2.51, 2.98 and 5.65 ppm for CH3, CH2 and CH protons, respectively. Beside the latter three aliphatic signals, the 1H NMR spectrum exhibited at δ = 7.21–7.91 ppm multiplet signals, which were assigned to aromatic protons. The broad exchangeable signal at δ = 9.81 ppm was assigned for the imine proton. The 13C NMR revealed three signals at δ = 20.73, 30.03 and 50.56 ppm for the aliphatic carbons (CH3, CH2 and CH, respectively).
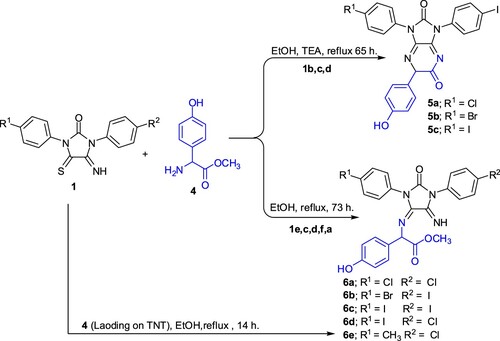
When imidazolidineiminothione derivatives 1b,c,d were reacted with 4-hydroxy-R-phenylglycine methyl ester 4 in ethanol under reflux in the presence of few drops of triethylamine, the imidazopyrazine derivatives 5a-c were obtained (Scheme 3). As further addition to our search, we decided to change the condition reaction between imidazolidineiminothione 1 and 4-hydroxy-R-phenylglycine methyl ester 4. Thus, when imidazolidineiminothione derivatives 1e, c, d, f, a were reacted with 4-hydroxy-R-phenylglycine methyl ester 4 in ethanol only without triethylamine as a catalyst under reflux, the diimino derivatives 6a-e were produced.
4-Hydroxy-R-phenylglycine methyl ester 4 was loaded on TNT. The structure of product 4 has been assigned as a loading product on the basis of X-Ray powder diffraction. From the XRD spectrum of amino acid 4 loading on TNT, it was clear that TiO2 was anatase phase with the main peaks located at 2θ = 25.3°, 37.8°, 48.0° and 55.1° TiO2 (JCPD#21-1272), respectively [Citation22]. This indicates that TNT has still anatase structure after loading amino acid 4.
When the reaction of 1a with 4 was repeated by using 4-hydroxy-R-phenylglycine methyl ester 4 (loaded on TNT) remarkable decrease in reaction time (73→14 h) was occurred. IR spectrum of 5a showed absorption bands at ν = 1722–1780 (2C=O), 1630–1623 (2C=N) cm−1. 1H NMR spectrum of 5a showed singlet signal at δ = 4.61 ppm for CH proton. Beside the latter aliphatic signal, the 1H NMR spectrum exhibited at 7.21–7.90 ppm multiplet signals for aromatic protons. The broad exchangeable signal at 9.55 ppm was assigned for the OH proton. IR spectrum of 6b showed absorption band of at 3483 and 3230 cm−1 for NH and OH functional groups, two absorption bands at 1780 and1720cm−1 for two C=O functional groups. 1H NMR spectrum of 6b showed two singlet signals at δ = 3.37 and 4.61 ppm for CH and OCH3 protons, respectively. Beside the latter two aliphatic signals, the 1H NMR spectrum exhibited at δ = 7.24-7.91 ppm multiplet signals for aromatic protons. The two broad exchangeable signals at δ = 9.81 and 11.29 ppm were assigned for the NH & OH protons. Mass spectrum of 6b showed a molecular ion peak at m/z = 632.84.
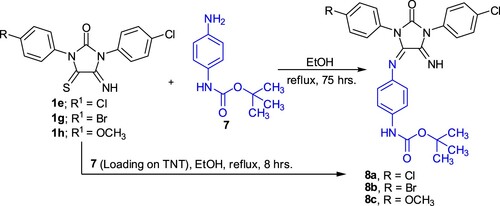
As shown in Scheme 4, reaction of imidazolidineiminothione derivatives 1e,g,h with (4-aminophenyl)methylcarbamic acid tert-butyl ester 7 in ethanol under reflux 75 h afforded carbamate derivatives 8a-c. (4-Amino-phenyl)methylcarbamic acid tert-butyl ester 7 was loaded on titanium oxide nanotube. The structure of product 8 has been assigned as a loading product on the basis of X- Ray powder diffraction. From the XRD pattern of 7/TiO2,the peaks at 2θ = 25.5°, 37.83°, 48.03° and 55.08° were found. This indicates that TNT has still anatase structure after loading tert-butyl 4-aminophenylcarbamate 7. When the reaction of 1e with 7 was carried out by using (4-amino-phenyl) methyl carbamic acid tert-butyl ester 7 (loaded on TNT) significant decrease in reaction time (75→8 h) was observed. The structure of 8a has been assigned as a reaction product on the basis of analytical and spectral data. IR spectrum showed absorption bands of at 3350 and 3300 cm−1 for NH functional group, two absorption bands at 1750 and 1725cm−1 for two C=O functional groups. The 1H NMR showed singlet signal at δ = 1.52 ppm for tert-butyl protons.
2.2. Cytotoxic activity evaluation
Sulforhodamine B colorimetric assay [Citation23] was used for evaluation the cytotoxic activity on three cancer cell lines, human hepatocellular carcinoma (HEPG-2), colon carcinoma (HCT-116) and mammary gland breast cancer (MCF-7) cell lines. Doxorubicin was used as standard drug. The IC50 values for the tested compounds were reported in Table .
Table 1. Cytotoxic activity of the synthesized imidazole derivatives against the cancerous cell lines.
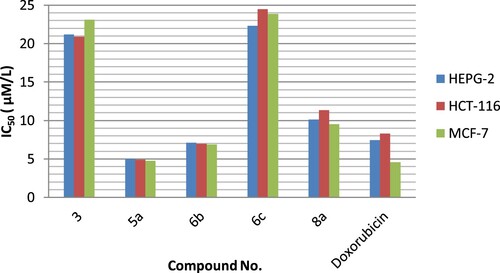
The mean IC50 values suggested that most of the imidazole derivatives have remarkable cytotoxic activity to all of the cancerous cell lines. Certain aspects for the structure activity relationships (SAR) of the imidazole derivatives were highlighted. The comparison between the anticancer activity of our potent compounds and doxorubicin is represented graphically in Figure .
The highest cytotoxic activity was observed with 5a, Structure 5a has a imidazo[4,5-b]pyrazine-2,5-dione moiety substituted at positions 1, 3 and 6 with 4-chlorophenyl, 4-iodophenyl and 4-hydroxyphenyl moieties. Its IC50 values were less than the reference drug against HEPG-2, HCT-116 and MCF-7 cell lines. IC50 values of 5a were 5.02, 4.91 and 4.78 μM for HEPG-2, HCT-116 and MCF-7, respectively; while for doxorubicin were 7.46, 8.29 and 4.56 μM, respectively.
Compound 6b has imidazole scaffold bear 4-bromophenyl, 4-iodophenyl, 4-hydroxyphenyl and acetate moieties. The imidazole derivative 6b showed activity greater than the reference drug against most of the tested cell lines. Its IC50 values were 7.12, 6.98 and 6.88 μM for HEPG-2, HCT-116 and MCF-7, respectively.
Imidazole derivative 8a with tert-butyl carbamate moiety had activity near to the doxorubicin against most of the tested cell lines.
Structure 3 has a imidazo[4,5-b]pyrazine-2,5-dione moiety substituted at positions 1, 3 and 6with p-tolyl, 4-chlorophenyl and 1H-indol-3-yl-methyl moieties. Compound 3 exhibited moderate cytotoxic activity against all cell lines with IC50 value less than the reference drug by about three folds. Nearly, the same result was obtained by compound 6c.
3. Conclusion
In conclusion, imidazopyrazine and imidazolidine derivatives were synthesized from the reaction of imidazolidineiminothiones with some amino acids methyl ester afforded. In the present investigation, we modify the reaction protocol and successfully synthesized some of the latter imidazopyrazine and imidazolidine derivatives through nano technology. This method reduces the reaction time significantly. The anticancer activity of some of our compounds was carryout and the most of the imidazole derivatives showed remarkable cytotoxic activity to all of the cancerous cell lines.
4. Experimental section
Melting points were determined on a digital Gallen-Kamp MFB-595 instrument and are uncorrected. IR spectra were recorded (KBr) on a Perkin Elmer 1650 spectrophotometer. NMR spectra were recorded on Avance II Bruker FT-NMR spectrometer using and TMS as an internal standard. Chemical shifts are expressed as δ ppm units. The mass spectra were recorded on Finnegan MAT 212 instrument, the ionizing voltage was 70 ev. Elemental analyses were carried out by the micro-analytical unit of Faculty of Science, Cairo University. Antitumour screening was carried out in National Cancer Institute, Cairo, Egypt. TEM imaging was conducted on The JEOL JEM-1400. X-ray diffraction (XRD) analysis was carried out on a Bruker D8−Advance X-ray diffractometer.
4.1. Synthesis of titanium oxide nanotube
Hydrothermal method was used to prepare TiO2 nanotubes. Nanotubes were obtained by treating a mixture of 0.5 g TiO2 nanoparticles (P25-Sigma Aldrich) and 30 mL of 10 M NaOH (Sigma Aldrich) aqueous solution that stirred at room temperature for 30 min. The mixture was transferred to a 50 mL. Teflon-lined stainless steel autoclave and hydrothermal treatment was performed at 150°C for 24 h. After hydrothermal treatment, the white precipitate was separated and washed with dilute HCl until pH was equal 2 and deionization DI water until the pH value of the rinsing solution reached 6. The sample was dried at 90°C for 10 h followed by annealing at 400 °C for 2 h [Citation24].
4.2. Method of loading amino acids methyl ester on titanium oxide nanotube
To a solution of TiO2 nanotube (0.1 g in 50 mL of EtOH) CTAB (0.0182 g) as a surfactant compound was added and the reaction mixture was ultrasanofication at rt for 2 h. After that an equimolar amounts of amino acids methyl ester was added and stirred for 24 h. The structure of product has been assigned as a loading product on the basis of X-Ray Diffraction.
4.3. Synthesis of 6-((1H-indol-3-yl)methyl)-3-(4-chlorophenyl)-1-(4-methylphenyl)-1h-imidazo [4,5-bpyrazine-2,5(3H,6h)-dione (3)
Traditional method: A mixture of equimolar amounts of the respective imidazolidineiminothione 1a and (S)-tryptophan methyl ester 2 in ethanol (30 mL) and 4 drops from TEA as a catalyst was heated under reflux for 60 h. The reaction mixture was concentrated to give a product, which crystallized from ethanol to give compound 3 in 95% yield.
Nanotechnology method: A mixture of equimolar amounts of imidazolidineiminothione 1a and (S)-tryptophan methyl ester 2 (loaded on titanium oxide nanotube) in ethanol (30 mL) and four drops from TEA was heated under reflux for 8 h. The reaction mixture was filtered off, then concentrated to give a product which crystallized from ethanol to give compound 3. Yield 81%; m.p. 201 °C; IR: ν/cm−1 = 3500 (NH), 1720, 1785 (C=O), 1690 (C=N); 1H NMR (400 MHz, DMSO): δ/ppm = 2.51 (s, 3H, CH3), 2.98 (dd, 2H, J = 7.2 Hz, CH2), 5.65 (t, 1H, J = 7.8 Hz, CH), 7.21–7.91 (m, 13H, Ar-H), 9.81 (br, 1H, NH); 13C NMR (101 MHz, DMSO): 20.73 (CH3), 30.03 (CH2), 50.56 (CH), 116.26, 120.62, 120.86, 129.69, 131.99, 132.40, 137.76, 138.22, 153.74, 168.39; MS, m/z (%): 480.5 (M+; 81%); Anal. Calcd for C27H20N5O2Cl (481.93): C, 67.29; H, 4.18; N, 14.53; Found: C, 67.33; H, 4.15; N, 14.49%.
4.4. Synthesis of 1,6-(diaryl)-3-(4-iodophenyl)-1h-imidazo[4,5-bpyrazine-2,5(3H,6h)-diones 5a-c
A mixture of equimolar amounts of the respective imidazolidineiminothiones 1b,c,d and 4-hydroxy-R-phenylglycine methyl ester 4 in ethanol (30 mL) and 4 drops from TEA was heated under reflux for 65 h, then concentrated to give a product which was filtered off and crystallized from ethanol to give imidazo[4,5-b]pyrazine-2,5-diones 5a-c.
4.4.1. 1-(4-Chlorophenyl)-6-(4-hydroxyphenyl)-3-(4-iodophenyl)-1H-imidazo[4,5-b]pyrazine-2,5(3H,6H)-dione (5a)
Yield 90%; m.p. 152 °C; IR: ν/cm−1 = 3480 (OH), 1722–1780 (2C=O), 1630–1623 (C=N); 1H NMR (400 MHz, DMSO): δ/ppm = 4.61 (s, 1H, CH), 7.21-7.90 (m, 12H, Ar-H), 9.55 (br, 1H, OH); 13C NMR (101 MHz, DMSO): 50.51, 94.70, 120.56, 128.23, 129.42, 129.58, 132.00, 137.29, 138.23, 153.49, 168.29; MS, m/z (%): 556.31 (M+; 30.11%); Anal. Calcd for C23H14ClIN4O3 (556.74): C, 49.62; H, 2.53; N, 10.06; Found: C, 49.54; H, 2.51; N, 9.94%.
4.4.2. 1-(4-Bromophenyl)-6-(4-hydroxyphenyl)-3-(4-iodophenyl)-1H-imidazo[4,5-b]pyrazine-2,5(3H,6H)-dione (5b)
Yield 85%; m.p. 173 °C; 1H NMR (400 MHz, DMSO): δ/ppm = 4.58 (s, 1H, CH), 7.18-8.00 (m, 12H, Ar-H), 9.51 (br, 1H, OH); MS, m/z (%): 601.40 (M+; 62%); Anal. Calcd for C23H14BrIN4O3 (601.19): C, 45.95; H, 2.35; N, 9.32; Found: C, 45.91; H, 2.32; N, 9.28%.
4.4.3. 6-(4-Hydroxyphenyl)-1,3-bis(4-iodophenyl)-1H-imidazo[4,5-b]pyrazine-2,5(3H,6H)-dione (5c)
Yield 80%; m.p. 188 °C; 1H NMR (400 MHz, DMSO): δ/ppm = 4.55 (s, 1H, CH), 7.10-7.85 (m, 12H, Ar-H), 9.42 (br, 1H, OH); MS, m/z (%): 648.12 (M+; 38%); Anal. Calcd for C23H14I2N4O3 (648.19): C, 42.62; H, 2.18; N, 8.64; Found: C, 42.58; H, 2.21; N, 8.53%.
4.5. Synthesis of methyl 2-(1,3-diarnyl-5-imino-2-oxoimidazolidin-4-ylideneamino)-2-(4-hydroxyphenyl)acetate 6a-e
Traditional method: A mixture of equimolar amounts of the imidazolidineiminothiones 1e,c,d,f,a and 4-hydroxy-R-phenylglycine methyl ester 4 in ethanol (30 mL) was heated under reflux for 73 h, The reaction mixture was concentrated, left to cool then filtered off to give products 6a-e which crystallized from ethanol.
Nanotechnology method: A mixture of equimolar amounts of the respective imidazolidineiminothione 1a and 4-hydroxy-R-phenylglycinemethyl ester 4 (loaded on titanium oxide nanotube) in ethanol (30 mL) and four drops from TEA was heated under reflux for 14 h. The reaction mixture was filtered off, then concentrated to give compound 6e in 75% yield.
4.5.1. Methyl 2-(1,3-bis(4-chlorophenyl)-5-imino-2-oxoimidazolidin-4-ylideneamino)-2-(4-hydroxyphenyl)acetate (6a)
Yield 80%; m.p. 155°C; 1H NMR (400 MHz, DMSO): δ/ppm = 3.33 (s, 3H, OCH3), 4.58 (s, 1H, CH), 7.20 - 7.80 (m, 12H, Ar-H), 9.77, 11.31 (2br, 2H, NH & OH); MS, m/z (%): 497.52 (M+; 21%); Anal. Calcd for C24H18Cl2N4O4 (497.33): C, 57.96; H, 3.65; N, 11.27; Found: C, 58.11; H, 3.63; N, 11.31%.
4.5.2. Methyl 2-(3-(4-bromophenyl)-5-imino-1-(4-iodophenyl)-2-oxoimidazolidin-4-ylideneamino)-2-(4-hydroxyphenyl)acetate (6b)
Yield 70%; m.p. 157 °C; IR: ν/cm−1 = 3483 (OH), 3230 (NH), 1621 (C=N), 1780 (COO), 1720 (C=O); 1H NMR (400 MHz, DMSO): δ/ppm = 3.37 (s, 3H, OCH3), 4.61 (s, 1H, CH), 7.24 - 7.91 (m, 12H, Ar-H), 9.81, 11.29 (2br, 2H, NH & OH); 13C NMR (101 MHz, DMSO): 45.95, 50.48, 94.73, 116.26, 120.86, 129.57, 132.02, 132.32, 137.76, 138.22, 153.48, 168.26; MS, m/z (%): 632.84 (M+; 5.79); Anal. Calcd for C24H18BrIN4O4 (633.23): C, 45.52; H, 2.87; N, 8.85; Found: C, 45.48; H, 2.90; N, 8.79%.
4.5.3. Methyl 2-(4-hydroxyphenyl)-2-(5-imino-1,3-bis(4-iodophenyl)-2-oxoimidazolidin-4-ylideneamino)acetate (6c)
Yield 85%; m.p. 168 °C; 1H NMR (400 MHz, DMSO): δ/ppm = 3.53 (s, 3H, OCH3), 4.60 (s, 1H, CH), 7.18 - 7.95 (m, 12H, Ar-H), 9.64, 11.33 (2br, 2H, NH & OH);MS, m/z (%): 680.78 (M+; 42%); Anal. Calcd for C24H18I2N4O4 (680.23): C, 42.38; H, 2.67; N, 8.24; C, Found: C, 42.43; H, 2.65; N, 8.21%.
4.5.4. Methyl 2-(1-(4-chlorophenyl)-5-imino-3-(4-iodophenyl)-2-oxoimidazolidin-4-ylideneamino)-2-(4-hydroxyphenyl)acetate (6d)
Yield 90%; m.p. 170 °C; 1H NMR (400 MHz, DMSO): δ/ppm = 1H NMR (400 MHz, DMSO): δ/ppm = 3.48 (s, 3H, OCH3), 4.59 (s, 1H, CH), 7.30–8.00 (m, 12H, Ar-H), 9.80, 11.40 (2br, 2H, NH & OH); MS, m/z (%): 588.20 (M+; 35%); Anal. Calcd for C24H18ClIN4O4 (588.78): C, 48.96; H, 3.08; N, 9.52; Found: C, 49.06; H, 3.04; N, 9.47%.
4.5.5. Methyl 2-(1-(4-chlorophenyl)-5-imino-2-oxo-3-(4-methylphenyl)-imidazolidin-4-ylideneamino)-2-(4-hydroxyphenyl)acetate (6e)
Yield 90%; m.p. 179 °C; 1H NMR (400 MHz, DMSO): δ/ppm = 2.32 (s, 3H, CH3), 3.44 (s, 3H, OCH3), 4.60 (s, 1H, CH), 7.10-7.90 (m, 12H, Ar-H), 9.70, 11.85 (2br, 2H, NH & OH); MS, m/z (%): 476.31 (M+; 28%); Anal. Calcd for C25H21ClN4O4 (476.91): C, 62.96; H, 4.44; N, 11.75; Found: C, 63.11; H, 4.41; N, 11.69%.
4.6. Synthesis of tert-butyl 4-(1,3-diaryl-5-imino-2-oxoimidazolidin-4-ylideneamino)phenylcarbamate 8a-c
Traditional method: A mixture of equimolar amounts of the respective imidazolidineiminothione 1e,g,h and tert-butyl(4-aminophenyl) carbamatein ethanol (30 mL) was heated under reflux for 75 h. The reaction mixture was then concentrated left to cool then filtered off to give a product which crystallized from ethanol to give compound 8a-c.
Nanotechnology method: A mixture of equimolar amounts of imidazolidineiminothione 1e and tert-butyl(4-aminophenyl) carbamate (loaded on titanium oxide nanotube) in ethanol (30 mL) was heated under reflux for 8 h. The reaction mixture was filtered off, and then concentrated to give 8a in 80% yield.
4.6.1. Tert-Butyl 4-(1,3-bis(4-chlorophenyl)-5-imino-2-oxoimidazolidin-4-ylideneamino)phenylcarbamate (8a)
Yield 93%; m.p. 193 °C; IR: ν/cm−1 = 3350, 3300 (NH), 2920 (CH3), 1750 (COO), 1725 (C=O), 1690 (C=N); 1H NMR (400 MHz, DMSO): δ/ppm = 1.52 (s, 9H, 3CH3), 5.77 (s, 1H, NH), 7.27–7.45 (m, 13H, 12Ar-H & NH); 13C NMR(101 MHz, DMSO): 24.23 (3CH3), 81.71, 120.62, 128.07, 129.26, 129.49, 132.00, 137.33,138.43, 153.52; MS, m/z (%): 524.40 (M+; 90%); Anal. Calcd for C26H23Cl2N5O3 (524.40): C, 59.55; H, 4.42; N, 13.36; Found: C, 59.46; H, 4.39; N, 13.41%.
4.6.2. Tert-Butyl 4-(3-(4-bromophenyl)-1-(4-chlorophenyl)-5-imino-2-oxoimidazolidin-4-ylideneamino)phenylcarbamate (8b)
Yield 70%; m.p. 208 °C; 1H NMR (400 MHz, DMSO): δ/ppm = 1.50 (s, 9H, 3CH3), 5.81 (s, 1H, NH), 7.20-7.50 (m, 13H, 12Ar-H & NH); MS, m/z (%): 458.21 (M+; 18%); Anal. Calcd for C26H23BrClN5O3 (568.85): C, 54.90; H, 4.08; N, 12.31; Found: C, 54.87; H, 4.11; N, 12.26%.
4.6.3. Tert-Butyl 4-(1-(4-chlorophenyl)-5-imino-3-(4-methoxyphenyl)-2-oxoimidazolidin-4-ylideneamino)phenylcarbamate (8c)
Yield 83%; m.p. 184 °C; 1H NMR (400 MHz, DMSO): δ/ppm = 1.51 (s, 9H, 3CH3), 2.32 (s, 3H, CH3), 5.71 (s, 1H, NH), 7.20-7.45 (m, 13H, 12Ar-H & NH); MS, m/z (%): 519.31 (M+; 25%); Anal. Calcd for C27H26ClN5O4 (519.98): C, 62.37; H, 5.04; N, 13.47; Found: C, 62.29; H, 5.01; N, 13.41%.
4.7. Anticancer screening
The cancer cell lines were cultured in RPMI-1640 medium with 10% fetal bovine serum. Antibiotics (penicillin 100 units/mL and streptomycin 100 µg/mL) were added at 37°C in a 5% CO2 incubator. The cells were seeded in a 96-well plate at a density of 1.0 × 104 cells/well at 37°C for 48 h under 5% CO2. After incubation, the cells were treated with different concentrations of the tested compounds and incubated for 24 h. Then the medium was discarded. Fixation was carried out by 10% trichloroacetic acid (TCA) 150 μL/well for 1 h at 4 °C, then wash by water three times (TCA reduce SRB protein binding). Wells were stained by SRB 70 μL/well for 10 min at room temperature with 0.4% 70 μL/well (keep in dark place). Then washed with acetic acid 1% to remove unbound dye (end point: colorless drainage). The plates were subjected to air drying for 24 h. The dye were solubilized with 50 μL/well of 10 mMtris base (PH 7.4) for 5 min on a shaker at 1600 rpm. The optical density (OD) of each well was measured at 570 nm with an ELISA microplate reader (EXL 800 USA). The inhibitory concentration at 50% (IC50) was determined from the exponential curve of viability versus concentration.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Al-Harbi RAK, El-Sharief MAMS, Abbas SY. Synthesis and anticancer activity of bis-benzo[d][1,3]dioxol-5-yl thiourea derivatives with molecular docking study. Bioorg Chem. 2019;90:103088. doi: 10.1016/j.bioorg.2019.103088
- Ammar YA, El-Sharief AMS, Belal A, et al. Design, synthesis, antiproliferative activity, molecular docking and cell cycle analysis of some novel (morpholinosulfonyl) isatins with potential EGFR inhibitory activity. Eur J Med Chem. 2018;156:918–932. doi: 10.1016/j.ejmech.2018.06.061
- Shehry MF E, Ghorab MM, Abbas SY, et al. Quinoline derivatives bearing pyrazole moiety: synthesis and biological evaluation as possible antibacterial and antifungal agents. Eur J Med Chem. 2018;143:1463–1473. doi: 10.1016/j.ejmech.2017.10.046
- Abbas SY, Zhao P, Overman L. 1,6-Addition of tertiary carbon radicals generated from alcohols or carboxylic acids by visible-light photoredox catalysis. Org Lett. 2018;20(3):868–871. doi: 10.1021/acs.orglett.7b04034
- Abbas SY, El–Bayouki KAM, Basyouni WM. Utilization of isatoic anhydride in the syntheses of various types of quinazoline and quinazolinone derivatives. Synthetic Commun. 2016;46(12):993–1035. doi: 10.1080/00397911.2016.1177087
- Gao Z, Duhl DD, Harrison SD. Chapter 20. Beyond kinases: Purine-binding enzymes as cancer targets. Ann Rep Med Chem. 2003;38:193–202.
- Trunz BB, Jedrysiak R, Tweats D, et al. 1- Aryl-4-Nitro-1H-imidazoles, a new promising series for the treatment of human African Trypanosomiasis. Eur J Med Chem. 2011;46:1524–1535. doi: 10.1016/j.ejmech.2011.01.071
- Bascou JP, Lacroix G, Gadras A, et al. Derives optiquement actifs de 2-imidazoline-5-one et 2-imidazoline-5-thiones fungicide. E P Patent 1994; 0 629616 A2.
- Odake S, Morikava T, Tsuchiya M, et al. Inhibition of helicobacter pylori urease activity by hydroxamicp acid derivatives. Biol Pharm Bull. 1994;17:1329–1332. doi: 10.1248/bpb.17.1329
- Kassouf W, Tanguay S, Aprikian A. Nilutamide as second line hormone therapy for prostate cancer after androgen ablation fails. G J Urol. 2003;169:1742–1744. doi: 10.1097/01.ju.0000057795.97626.66
- Abbas SY, El-Sharief MAMS, Al-Harbi RAK, et al. Synthesis and evaluation of 5-imino-4-thioxoimidazolidin-2-one derivatives as antibacterial and antifungal agents. Med Chem. 2020; doi:10.2174/1573406416666191227112648.
- El-Sharief MAMS, Abbas SY, El-Sharief AMS, et al. 5-Thioxoimidazolidine-2- one derivatives: synthesis, anti-Inflammatory activity, Analgesic activity, COX Inhibition assay and molecular Modelling study. Bioorg Chem. 2019;87:679–687. doi: 10.1016/j.bioorg.2019.03.075
- Moussa Z, El-Sharief MAMS, Abbas SY. New imidazolidineiminothione derivatives: synthesis, spectral Characterization and evaluation of antitumor, antiviral, antibacterial and antifungal activities. Eur J Med Chem. 2016;122:419–428. doi: 10.1016/j.ejmech.2016.06.051
- El-Sharief MMS, Abbas SY, Moussa Z, et al. Synthesis and evaluation of antibacterial and antifungal activities of 1,3-Disubstituted-4- Thioxoimidazolidin-2-One derivatives. Croat Chem Acta. 2018;91(3):335–340. doi: 10.5562/cca3354
- Iijima S. Helical microtubules of graphitic carbon. Nature. 1991;354:56–58. doi: 10.1038/354056a0
- Kijima T., (Ed.) Inorganic and Metallic nanotubular materials. Top Appl Phys. 2010;117:17–32. doi: 10.1007/978-3-642-03622-4_2
- Gong D, Grimes CA, Varghese OK, et al. Titanium oxide nanotube arrays prepared by anodic oxidation. J Mater Res. 2001;16:3331–3334. doi: 10.1557/JMR.2001.0457
- Nakamura H, Matsui Y. Silica Gel nanotubes obtained by the Sol-Gel method. J Am Chem Soc. 1995;117(9):2651–2652. doi: 10.1021/ja00114a031
- Linsebigler AL, Lu G, Yates JT. Photocatalysis on TiO2 surfaces: Principles, mechanisms, and selected Results. Chem Rev. 1995;95:735–758. doi: 10.1021/cr00035a013
- Fujishima A, Hondam K. Electrochemical photolysis of water at a semiconductor electrode. Nature. 1972;238:37–38. doi: 10.1038/238037a0
- Abbas SY, El-Sharief MAMS, Salem MA, et al. Utilization of cyanothioformamides in the syntheses of various types of imidazole derivatives. Synthetic Commun. 2020;50:621–648. doi: 10.1080/00397911.2019.1700524
- Qu XF, Yuan J, Deng X, et al. An efficient method to Form TiO2/CdS nanotube Arrays using Anodic Aluminum oxide (AAO) templates. Key Eng Mater. 2016;727:374–380. doi: 10.4028/www.scientific.net/KEM.727.374
- Skehan P, Storeng R, Scudiero D, et al. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107
- Kustiningsih I, Purwanto WW. Synthesis of Titania nanotubes and Titania nanowires by combination sonication-hydrothermal treatment and their photocatalytic activity for hydrogen production. Int J Technol. 2014;5(2):133. doi: 10.14716/ijtech.v5i2.400