?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The leaf and twig aqueous extracts of Arabian balsam tree (Commiphora gileadensis) were tested to protect against alloxan-induced hyperglycaemia in hypercholesterolemic male rats. The antioxidants, high density lipoproteins, IgE and α-Amylase were decreased, whereas kidney function indices, liver function enzymes, triglycerides, cholesterol, low density lipoproteins and all immunoglobulins except IgE were increased as a result of alloxan administration. Moreover, the renal, hepatic and pancreatic tissue showed a drastic pathological damage. In G3 and G4, treating the diabetic rats with C. gileadensis leaf and twig extracts, respectively greatly ameliorated all biochemical parameters and the injured tissues and restored them nearly to the normal. In G4, the aqueous extract of C. gileadensis twig was more efficient in treating the diabetic rats than the leaf aqueous extract in G3. Both C. gileadensis leaf and twig aqueous extracts succeeded in adjusting the glucose levels of diabetic rats under hypercholesterolemic condition due to their antioxidant activity.
1. Introduction
Diabetes mellitus (DM) is a complex disorder that disturbs the metabolism of carbohydrates, protein and fat leading to increased blood glucose due to either low insulin levels or insensitivity of organs to insulin [Citation1,Citation2]. The antidiabetic drugs always aim to reduce insulin resistance and to stimulate insulin secretion [Citation3]. The current synthetic antidiabetic drugs have several limitations, so searching for herbal non-synthetic chemical drugs continues [Citation2].
Hypercholesterolemia is a disorder between cholesterol secretion and uptake in the blood leading to numerous diseases e.g. renal failure, type 2 diabetes, atherosclerosis, inflammation and ageing [Citation4,Citation5]. It is also a main cause of non-alcoholic fatty liver diseases which results from the accumulation of triglyceride inside liver cells [Citation6]. In spite of the presence of numerous drugs for lowering blood cholesterol levels, about 80% or more of people in developing countries prefer natural compounds (traditional medicinal plants) in controlling and treating diseases rather than synthetic ones because of their side effects [Citation7]. Recently, patients are looking for safer alternatives, and are searching for new drugs from natural products for regulating cholesterol levels [Citation8].
Nowadays, therapeutic dietary nutrition and numerous plant natural products can assist the mechanisms of body homeostasis and protect or ameliorate metabolic syndrome [Citation9]. Several herbal extracts and natural products have proved highly effective in the treatment of both diabetes and hyperlipidaemia [Citation9–12]. In addition, Lachin and Reza [Citation13] reported that cherries protect against alloxan-induced diabetes. El Rabey et al. [Citation14] used Nigella sativa and propolis in controlling STZ-induced diabetes. Balasubramanian et al. [Citation15] also reported that Stereospermum suavelolens ameliorates diabetic nephropathy. El Rabey et al. [Citation16,Citation17], Al-Seeni et al. [Citation18] and Abulnaja and El Rabey [Citation19] used natural products in lowering hyperlipidaemia. Al-Malki and El Rabey [Citation20] used Moringa oleifera in controlling streptozotocin-induced diabetes, whereas Qusti et al. [Citation21] stated that cinnamon and cress seed can control diabetes in male rats.
The Arabian balsam tree (Commiphora gileadensis) belongs to the genus Commiphora that grows in the Arabian Peninsula and in southeast Egypt that has exceptional medicinal properties in the sap, wood, bark and seeds [Citation22,Citation23]. It has also some other economic and scientific importance such as the production of a tasty resinous gum [Citation22] and presence of a novel peroxidase [Citation24]. Al-Sieni [Citation23] reported that C. gileadensis aqueous extract of 0.04 mg/ml revealed an antimicrobial activity against Fusobacterium nucleatum, Streptococcus salivarius, Streptococcus mutans, Lactobacillus casei and Staphylococcus epidermidis.
The aim of the current study was evaluating the antioxidant, antidiabetic and hypolepidaemic activity of the leaf and twig aqueous extracts of C. gileadensis in alloxan-induced diabetic male rat under hypercholesterolemic condition.
2. Materials and methods
2.1. The fat-rich diet
The fat-rich diet consisted of the following constituents according to Knapka and Judge [Citation25]: corn starch (64.6%), casein (16%), corn oil (10%), salt mixture (4%), cellulose “N.N” (4%), mixture of vitamins (1%), choline chloride (0.2%) and methionine “DL.” (0.2%).
2.2. Plant materials
Fresh materials of the C. gileadensis (leaves and twigs) were directly collected from C. gileadensis trees in Jeddah, Saudi Arabia. These plant materials were washed with distilled water, dried and kept at 4°C until use.
2.3. Preparation of the aqueous extracts
The leaf and twig aqueous extracts of C. gileadensis were prepared according to the method of Sharma et al. [Citation26]. In brief, a total of 100 g of each of the leaf and twig powder was macerated in a litter of distilled water and incubated in dark for 48 h at room temperature. 1% chloroform was added to the extract to prevent microbial growth, filtered by Whatman I filter paper and a powdery extract was obtained by drying in a rotary evaporator. A 12.5% of this powder extract was obtained by adding 12.5 g of the powder to 100 ml of distilled water.
2.4. Animals, housing and design of the experiment
A 180–200 g twenty-four adult male albino rats (Rattus norvegicus) were purchased from the animal experimental unit of The Faculty of Pharmacy, King Abdulaziz University. In addition, the protocol of this study was approved by The Institutional Animal House, University of King Abdulaziz at Jeddah, Saudi Arabia. The animals were housed 6 per cage, received fat-rich diet and water ad libitum during the experiment. The animals were kept under observation for acclimatization to the lab condition for one week before the start of the experiment. Four groups of rats (n = 6) were randomly divided; the first negative control group (G1) received only the fat-rich diet. The other 18 rats were intraperitonially injected with 150 mg/kg b.w alloxan monohydrate to induce diabetes according to the protocol of Dash et al. [Citation27]. Rats of fasting blood glucose higher than 200 mg/dl were considered diabetic that were divided into 3 groups as follows: the second positive alloxan-treated diabetic control group (G2), the third group (G3) was treated with 1 ml/kg bw of the 12.5% of C. gileadensis leaf aqueous extract for 28 days and the fourth group (G4) was treated with 1 ml/Kg bw of the 12.5% C. gileadensis twig aqueous extract. The experiment was conducted for 4 weeks after treatment with C. gileadensis leaf and twig aqueous extracts.
2.5. Dissection, collection of blood samples and liver tissue homogenate preparation
Blood samples (12–15 ml from each rat) were collected from the heart of 12 h fasted rats under anaesthesia with diethyl ether. Blood sera were separated and stored at −80°C for analysis. One kidney, a piece of the pancreas and a piece of the liver were washed and saved in saline buffer for histopathological preparations. In addition, the liver tissue homogenate was prepared from an ice cold liver tissue as described in El Rabey et al. [Citation28] as follows: the ice cold liver tissues were rinsed in cold phosphate-buffer, cut into small pieces, homogenized, sonicated for 15 s in an ice bath and then centrifuged for 5 min at 12,000 rpm under cold condition (4°C). The resulting supernatant was used for determination of antioxidants and lipid peroxidation.
2.6. Determination of blood glucose, glycated haemoglobin Alc and serum α-Amylase
Serum glucose concentration was estimated colorimetrically using Human (Germany) as described by Barham and Trinder [Citation29], whereas serum haemoglobin Alc was determined by Glycohaemoglobin kit, POINTE Scientific Inc. (USA). In addition, serum α-Amylase was determined calorimetrically using EnzyChrom™ α-Amylase Assay Kit, BioAssay Systems (California, USA) according to the method described by YingFoo and Bais [Citation30]. All analyses were done according to the instruction of the kit supplier.
2.7. Determination of serum immunoglobulins (IgA, IgM, IgE and IgG)
Serum immunoglobulins (IgA, IgM, IgE and IgG) were estimated using Genway Biotech Kit (USA) according the instruction of the kit supplier.
2.8. Estimation of antioxidants and lipid peroxidation
The activity of superoxide dismutase (SOD) was determined according to the method of Nishikimi et al. [Citation31], the activity of catalase (CAT) was estimated according to Aebi et al. [Citation32] and the activity of glutathione-S-transferase (GST) was determined according to Habig et al. [Citation33]. All analyses were estimated in the liver tissue homogenate using Biodiagnostic kit (Egypt). Moreover, the lipid peroxidation was determined in the liver tissue homogenate by estimating the concentration of malondialdehyde (MDA) according to Ohkawa et al. [Citation34] using Biodiagnostic kit (Egypt). All analyses were done according to the instruction of the kit supplier.
2.9. Determination of lipid profile in the serum
The triglycerides (TG) and total cholesterol (TC) were estimated according to Young [Citation35] using Spinreact Kit (Spain), whereas the high density lipoprotein (HDL) was estimated as described by Naito [Citation36] using Spinreact kit (Spain). All analyses were done according to the instruction of the kit supplier. In addition, the low density lipoprotein (LDL) and the very low density lipoprotein (VLDL) were calculated according to Srivastava et al. [Citation37] equations:
2.10. Estimation of liver enzymes in the serum
The activity of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) was estimated according to Thefeld et al. [Citation38] using Human Kit (Germany) and Swemed Diagnostics kit (India), respectively. The activity of alkaline phosphatase (ALP) was estimated using Human kit (Germany) according to Schlebusch et al. [Citation39]. In addition, the activity of gamma-glutamyl transferase (GGT) was assayed according to the method of Thomas [Citation40] using ARCHITECT c Systems (USA). All analyses were done according to the instruction of the kit supplier.
2.11. Determination of kidney function indices in the serum
The level of urea was estimated using an enzymatic colorimetric kit from Human (Germany) as described by Fawcett and Scott [Citation41], whereas the level of creatinine (CR) was estimated as described by Bartels et al. [Citation42] using Human kit (Germany). In addition, the level of uric acid (UA) was estimated as described by Barham and Trinder [Citation29] using Human kit (Germany). All analyses were done according to the instruction of the kit supplier.
2.12. Histopathological examinations
For microscopic investigation of injured tissues, sections of the kidney, liver and pancreas were prepared as described by Drury et al. [Citation43] by fixing these tissues in 10% formalin, dehydration in ethyl alcohol series and embedding in paraffin. A 5-µ thick section was prepared from each tissue and stained with haematoxylin and eosin (H&E) dye for microscopic investigation and photography.
2.13. Statistical analysis
Data were analyzed using SPSS program version 16 and they appear as mean ± SE. The t-test was run to determine the significant difference between the means of two groups; so G1 (the negative control) was compared with G2 (the positive control) and then G2 (the positive control) was compared with G3 and G4. The one way analysis of variance (ANOVA, p < 0.05) was calculated using SAS software by a protected least significant difference (LSD) test. Means of all groups in the same raw were compared simultaneously, means with different superscript (a, b, c or d) within the same raw are significantly different at P < 0.05, whereas means with the same superscripts are non-significantly different at P < 0.05.
3. Results
3.1. Biochemical and immunoglobulin analysis in the serum
The serum fasting blood sugar (FBS) and HbA1C were significantly increased as a result of induced hyperglycaemia in G2 compared to the negative control, as shown in Table . Treating the hyperglycaemic rats in G3 and G4 with the aqueous extract of leaf and twig of C. gileadensis, respectively significantly decreased serum fasting blood sugar and HbA1C levels. The aqueous extract of C. gileadensis twig in G4 was more efficient in ameliorating FBS and HbA1C than the aqueous extract of leaf in G3.
Table 1. Effect of treating diabetic rats with C. gileadensis on blood glucose, haemoglobin A1, α-amylase and Immunoglobulins (IgE, IgG, IgA and IgM).
Table shows also that the alpha amylase was non-significantly decreased as a result of the alloxan-induced hyperglycaemia in G2 compared to the negative control. On the other hand, treating the hyperglycaemic rats with the C. gileadensis in G3 and G4, non-significantly increased alpha amylase levels.
Table also shows that serum immunoglobulins IgA, IgG and IgM were significantly (at P < 0.001) increased in G2, whereas IgE was significantly (at P < 0.001) decreased compared to G1 (Table ). However, treating the hyperglycaemic rats with the aqueous extract of C. gileadensis in G3 and G4 significantly restored the immunuglobulins IgA, IgE, IgG and IgM levels nearly to the normal values as in G1. The aqueous extract of C. gileadensis twig in G4 was more efficient than leaf aqueous extract in G3 in restoring the interrupted immunoglobulin levels.
3.2. Antioxidant enzymes and lipid peroxidation
Table shows that the enzyme activity of superoxide dismutase, catalase and glutathione-s-transferase in the kidney homogenate was significantly (at P < 0.001) decreased, whereas lipid peroxidation, as indicated by malondialdehyde (MDA), was significantly (at P < 0.001) increased as a result of the alloxan-induced hyperglycaemia in the second group (G2) compared to the negative control. However, treating the hyperglycaemic rats with the aqueous extract of C. gileadensis in G3 and G4 significantly (at P < 0.001) increased Cat, SOD and GST activity in the liver homogenate and significantly (at P < 0.001) decreased the MDA levels compared to the G2 rats (the positive control). Similar to other analysis of this study, the aqueous extract of C. gileadensis twig in G4 was more efficient than leaf aqueous extract in G3 in restoring the antioxidant enzymes approaching the normal values.
Table 2. Effect of treating diabetic rats with C. gileadensis on antioxidant enzymes and lipid peroxidation (MDA) in the liver tissue homogenate.
3.3. Serum lipids
The TG, TC, LDL and VLDL levels were significantly (at P < 0.001) increased, whereas the HDL was significantly decreased as a result of the alloxan-induced hyperglycaemia in G2 compared to G1 as shown in Table . Treating the hyperglycaemic rats with the aqueous extract of C. gileadensis in G3 and G4 significantly (at P < 0.001) restored all the serum lipid profile index levels by the significant (at P < 0.001) decrease of the TC, TG, LDL, VLDL and the significant increase of the HDL compared to G2 rats (the positive control). Similar to the above-mentioned results, the aqueous extract of C. gileadensis twig in G4 was more efficient than leaf aqueous extract in G3 in restoring the lipid profile approaching the normal levels.
Table 3. Effect of treating diabetic rats with C. gileadensis leaf and twig aqueous extract on lipid profile.
3.4. Liver function enzymes
The activities of ALT, AST, GGT, ALP were non-significantly increased as a result of the alloxan-induced hyperglycaemia in G2 compared to G1 as shown in Table , whereas treating the hyperglycaemic rats with the aqueous extract of C. gileadensis in G3 and G4 restored the levels of liver function enzymes nearly to the normal. It is also worthy to mention that the aqueous extract of C. gileadensis twig in G4 was more efficient than leaf aqueous extract in G3 in ameliorating the levels of liver function parameters nearly to the normal as in G1.
Table 4. Effect of treating diabetic rats with the aqueous extracts of C. gileadensis on serum liver enzymes.
3.5. Kidney function indices in the serum
Creatinine and urea were significantly (at P < 0.001) increased, whereas uric acid levels were nonsignificantly increased as a result of the alloxan-induced hyperglycaemia in G2 compared to G1 as shown in Table . However, treating the hyperglycaemic rats with the aqueous extract of C. gileadensis in G3 and G4 significantly ameliorated all the serum kidney index levels. Similar to the other analysis of this study, the aqueous extract of C. gileadensis twig in G4 was more efficient than leaf aqueous extract in G3 in ameliorating the kidney function indices to their normal levels as in G1.
Table 5. Effect of treating diabetic rats with the aqueous extracts of C. gileadensis on kidney function indices.
3.6. Histopathology
Figure (A) shows the normal histology of the normal renal tissues of G1, whereas Figure (B) shows the renal tissues of the alloxan-treated group (G2) showing atrophy of glomerular tuft and vacuolation of epithelial lining renal tubules. Figure (C) shows nearly restored normal renal tissues of G3 treated with the leaf aqueous extract of C. gileadensis with no histopathological changes. Figure (D) shows also nearly normal renal tissues of G4 treated the twig aqueous extract of C. gileadensis.
Figure 1. A. Kidney section of G1 showing the normal renal tissue, B: Kidney section of G2 with vacuolated epithelial lining and atrophy of glomerular tufts, C: Kidney section of G3 showing no histopathological changes, D: Kidney section of G4 showing normal tissues (H & E ×400).
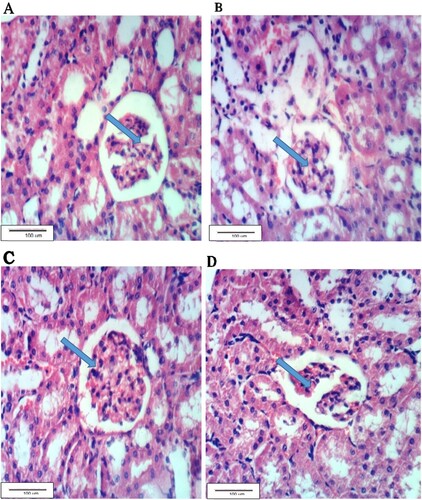
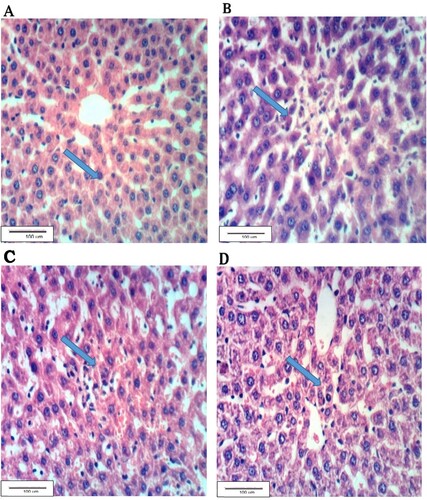
Figure (A) shows the hepatic tissues of G1 group with a normal histological structure. Figure (B) shows the hepatic tissue of the alloxan-treated G2 rats with hepatic necrosis accompanied with cell infiltration. Near-normal hepatic tissues are shown in Figure (C) of G3 rats treated with the leaf aqueous extract of C. gileadensis. Figure (D) shows the hepatic tissues of G4 rats treated with the twig aqueous extract of C. gileadensis showing a normal hepatic tissue.
Figure 2. Liver section of G1 showing the normal histological structure, B: hepatic tissues of G2 with necrosis and inflammatory cell infiltration, C: Liver section of G3 showing nearly normal hepatic tissue, D: Liver section of G4 showing normal hepatic tissue (H & E ×400).
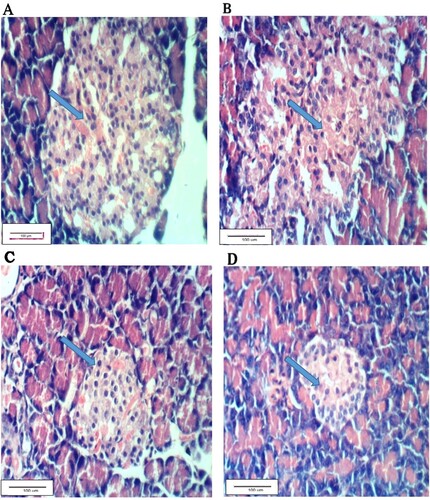
The normal pancreatic tissues of G1 are shown in Figure (A), whereas Figure (B) shows pancreatic tissues of the alloxan-treated G2 rats with vacuolation of Langerhan’s islets. Figure (C) shows pancreatic tissues of G3 treated with the leaf aqueous extract of C. gileadensis showing Langerhan’s islets with slight hyperplasia. Figure (D) shows pancreas tissues of G4 rats treated with the twig aqueous extract of C. gileadensis showing restored normal pancreatic tissues.
4. Discussion
In the present study, the alloxan-induced diabetes in the hypercholesterolemic rats occurs via a series of correlated events in the pancreatic beta cells, started with the injury of beta cells by the accumulation of alloxan followed by generation ROS with its reduction product, dialuric acid and finally the autoxidation of the dialuric acid that generates superoxide radicals, hydrogen peroxide and hydroxyl radicals that lead to the death of these beta cells [Citation12,Citation27,Citation44,Citation45]. The pancreatic beta cells are characterized by their low antioxidative defence capacity that leads to their death and the insulin-dependent alloxan diabetes [Citation44,Citation45].
The oxidative stress resulted from alloxan-induced diabetes in the present study increased blood glucose levels due to the low insulin level that increased glycated proteins, immunoglobulins (IgA, IgG and IgM), lipid peroxidation, cholesterol, triglycerides, LDL,VLDL, liver function parameters, kidney function indices, and decreased antioxidant activity, α-Amylase, high density lipoproteins and IgE immunoglobulin compared with the negative control group. Moreover, renal, hepatic and pancreatic tissues were also severely affected by the induction of diabetes due to the oxidative stress resulted from the alloxan-induced diabetes [Citation44,Citation45].
The blood sugar is increased in the alloxan-induced diabetic animals in the alloxan-treated positive control group because of the reduction in endogenous insulin release due to the damage of insulin-secreting β-pancreatic cells that leads to lowered intake of glucose by the cells because of the glucose-induced insulin secretion caused by alloxan inhibits the ability of alloxan to inhibit glucokinase which is the beta cell glucose sensor [Citation14,Citation21,Citation27,Citation44,Citation45]. The increase in blood sugar enhances the oxidative stress that increases the reactive oxygen species reflected by the low activity of antioxidant enzymes and the higher level of lipid peroxidation as revealed by the increase in MDA as a result of diabetes in the positive alloxan-treated control group [Citation14,Citation18,Citation20,Citation46].
The increase in immunoglobulins (IgA, IgG and IgM) may be as a result of an immune response to advanced glycosylation end products caused by the presence of high blood glucose levels [Citation14,Citation20,Citation45], whereas the decrease in IgE is consistent with Attia et al. [Citation45] and Al-Seeni [Citation46]. Similarly, induction of diabetes in the alloxan-treated positive control group increased the oxidative stress and the reactive oxygen species of the free radicals that increased cholesterol, triglycerides, LDL, VLDL and decreased HDL due to the oxidative stress of the free radicals resulted from the increased blood sugar [Citation11,Citation14,Citation15,Citation45].
Restoring blood glucose, glycated haemoglobin (HbA1c), antioxidant enzymes, lipid profile and lipid peroxidation nearly their normal levels after the administration of C. gileadensis leaf and twig extracts in G3 and G4 may be attributed to their content of the free radical scavengers such as saponins, flavonoids, volatile oils, sterols and triterpenes [Citation46], who reported that C. gileadensis has a hepatoprotective and remarkable anti- platelet effect against diethyl nitrosamine-induced hepatic injury in albino rats.
In addition, similar to other tested parameters, liver and kidney functions were increased as a result of alloxan administration in the positive control group due to the low insulin level that increased blood glucose, and consequently increased lipid peroxidation, lipids and inflammation that all increased oxidative stress and affected the vital organs [Citation20,Citation47]. C. gileadensis leaf and twig extracts ameliorated all altered liver and kidney functions due to their content of phenols and other antioxidants that scavenged the free radicals and restored the liver parameters nearly to their normal levels in the control negative group [Citation46].
The damage of the liver, kidney and pancreas tissue in the positive control group as a result of alloxan toxicity is due to the increase of the reactive oxygen species correlated with the increased oxidative stress and lipid peroxidation due to the increase in blood glucose [Citation11,Citation44,Citation47,Citation48]. The antioxidant activity of C. gileadensis leaf and twig aqueous extract nearly restored the damaged tissues nearly to the normal, due to their content of the antioxidant compounds that adjusted the oxidative stress by scavenging the free radicals [Citation46,Citation49,Citation50].
The present study is supported by previous investigations emphasized that the therapeutic and anti-inflammatory activity of C. gileadensis aqueous extract may be due to the presence of glycosylated substances known as lectin-binding sugars that function in restoring the pancreas to its active normal state [Citation50,Citation51].
5. Conclusion
C. gileadensis leaf and twig aqueous extracts succeeded in treating alloxan-induced diabetes in hypercholesterolemic male rats. They nearly restored all altered biochemical and histopathological parameters nearly to their normal levels of the negative control. The twig aqueous extract of C. gileadensis was more efficient in alleviating hyperglycaemia and hyperlipidaemia than the leaf aqueous extract in G3.
List of abbreviations
| ALP: | = | Alkaline phosphatase |
| ALT: | = | Alanine aminotransferase |
| AST: | = | Aspartate aminotransferase |
| Bw: | = | Body weight |
| CAT: | = | Catalase |
| CRE: | = | Creatinine |
| DM: | = | Diabetes mellitus |
| FBS: | = | Fasting Blood Sugar |
| G1: | = | Negative control group fed fat-rich diet |
| G2: | = | Hyperglycaemic positive control group intraperitonially injected with 150 mg/kg b.w alloxan monohydrate |
| G3: | = | Hyperglycaemic rats as in G2 and treated with 12.5% leaf aqueous extract of C. gileadensis |
| G4: | = | Hyperglycaemic rats as in G2 and treated with 12.5% leaf aqueous extract of C. gileadensis |
| GGT: | = | Gamma-glutamyl transferase |
| GST: | = | Glutathione S-transferase |
| HbA1C: | = | glycated haemoglobin Alc |
| HDL: | = | High density lipoprotein |
| IgA: | = | Immunoglobulin A |
| IgE: | = | Immunoglobulin E |
| IgG: | = | Immunoglobulin G |
| IgM: | = | Immunoglobulin M |
| LDL: | = | Low density lipoprotein (LDL) |
| MDA: | = | Malondialdehyde |
| MODY: | = | Maturity-onset diabetes of the young |
| ROS: | = | Reactive oxygen species |
| SOD: | = | Superoxide dismutase |
| TC: | = | The total cholesterol |
| TG: | = | Triglycerides |
| U/g: | = | Unit per gram |
| U/l: | = | Unit per litre |
| U/ml: | = | Unit per millilitre |
| UA: | = | Uric acid |
| VLDL: | = | Very low density lipoprotein |
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Maiti R, Jansa D, Das UK, et al. Antidiabetic effect of aqueous extract of seed of Tamarindus indica in streptozotocin induced diabetic rats. J Ethnopharmacol. 2004;92:85–91. DOI:10.1016/j.jep.2004.02.002.
- Arumugam G, Manjula P, Paari N. A review: anti diabetic medicinal plants used for diabetes mellitus. J Acute Dis. 2013;2(3):196–200. DOI:10.1016/S2221-6189(13)60126-2.
- Lehninger AL, Nelson DL, Cox MM. Principle of biochemistry. New York (NY): Worth Publishers; 2010.
- Orešič M, Hänninen VA, Vidal-Puig A. Lipidomics: a new window to biomedical frontiers. Trends Biotechnol. 2008;26(12):647–652. DOI:10.1016/j.tibtech.2008.09.001 Epub 2008 Oct 23.
- Van der Wulp MYM, Verkade HJ, Groen AK. Regulation of cholesterol homeostasis. Mol Cell Endocrinol. 2013;368(1–2):1–16. DOI:10.1016/j.mce.2012.06.007.
- Kim EJ, Kim BH, Seo HS, et al. Cholesterol-induced non-alcoholic fatty liver disease and atherosclerosis aggravated by systemic inflammation. PLoS One. 2014;9(6):1–11. DOI:10.1371/journal.pone.0097841.
- Neto CC, Owens CW, Langfield RD, et al. Antibacterial activity of some Peruvian medicinal plants from the callejon de Huaylas. J Ethnopharmacol. 2002;79:133–138. DOI:10.1016/S0378-8741(01)00398-1.
- Saravanan M, Ignacimuthu S. Hypocholesterolemic effect of Indian medicinal plants – a review. Med Chem. 2015;5(1):40–49. DOI:10.4172/2161-0444.1000241.
- Mohamed S. Functional foods against metabolic syndrome (obesity, diabetes, hypertension and dyslipidemia) and cardiovasular disease. Trends Food Sci Technol. 2014;35:114–128. DOI:10.1016/j.tifs.2013.11.001.
- Eliza J, Daisya P, Ignacimuthub S, et al. Antidiabetic and antilipidemic effect of eremanthin from Costus speciosus (Koen.) Sm., in STZ-induced diabetic rats. Chem-Biol Interact. 2009;182:67–72. DOI:10.1016/j.cbi.2009.08.012.
- Rabey HA E, Attia ES, Al- Seeni MN, et al. The hypolipidemic and antioxidant activity of Christ’s thorn (Ziziphus spina- Christi) leaves powder in hypercholesterolemic male rats. Life Sci J. 2014;11(10):1010–1021.
- Rabey HA E, Almutairi FM, Al-Sieni AI, et al. The antioxidant, antidiabetic and antilipidemic activity of Salvadora persica twig in alloxan diabetic male rats. Ind J Biochem Biophy. 2017;54(6):314–322. DOI:10.4066/biomedicalresearch.64-18-549.
- Lachin T, Reza H. Antidiabetic effect of cherries in alloxan induced diabetic rats. Recent Pat Endocr Metab Immune Drug Discov. 2012;6(1):67–72. doi: 10.2174/187221412799015308
- Rabey HA E, Al-Seeni MN, Bakhashwain A. The antidiabetic activity of Nigella sativa and propolis on streptozotocin-induced diabetes and diabetic nephropathy in male rats. Evid Based Complement Alternat Med. 2017; DOI:10.1155/2017/5439645.
- Balasubramanian T, Senthilkumar G, Karthikeyan M, et al. Therapeutic effect of Stereospermum suavelolens on diabetic nephropathy. Clin Exp Pharmacol. 2014;4(5):162–168. doi: 10.4172/2161-1459.1000162
- Rabey HAE, Al-Seeni MN, Amer HM. Efficiency of barley bran and oat bran in ameliorating blood lipid profile and the adverse histological changes in hypercholesterolemic male rats. Bio Med Res Int. 2013; DOI:10.1155/2013/263594. Epub 2013 Aug 1.
- Rabey HAE, Almutairi FM, Al-Sieni AI, et al. Salvadora persica leaf aqueous extract attenuates hyperglycemia and hyperlipidemia in alloxan induced diabetic male rats. Biomed Res. 2018;29(11):2424–2434. DOI:10.4066/biomedicalresearch.64-18-549.
- Al-Seeni MN, El Rabey HA, Al-Ghamdi H, et al. Assessment of the antioxidant activity of parsley and carob in hypercholesterolemic male rats. Biomed Res. 2018;29(17):3370–3377. DOI:10.4066/biomedicalresearch.29-18-1036.
- Abulnaja KO, El Rabey HA. The efficiency of barley (Hordeum vulgare) bran in ameliorating blood and treating fatty heart and liver of male rats. Evid-Based Compl Alt Med. 2015. DOI:10.1155/2015/740716.
- Al-Malki AL, El Rabey HA. The antidiabetic effect of low doses of Moringa oleifera Lam. seeds on streptozotocin induced diabetes and diabetic nephropathy in male rats. BioMed Res Int. 2015; DOI:10.1155/2015/381040.
- Qusti S, El Rabey HA, Balashram SS. The hypoglycemic and antioxidant activity of cress seed and cinnamon on streptozotocin induced diabetes in male rats. Evid Based Complement Altern Med. 2016; DOI:10.1155/2016/5614564.
- Groom N. Frankincense and myrrh: a study of the Arabian incense trade. London: Longman, Librairie de Liban; ISBN 0-582-76476-9, 1981.
- Abdulbasit AI. The antibacterial activity of traditionally used Salvadora Persica L. (miswak) and Commiphora Gileadensis (palsam) in Saudi Arabia. Afr J Tradit Complement Altern Med. 2014;2(11-1):23–27.
- Almulaiky YQ, Al-Harbi SA. A novel peroxidase from Arabian balsam (Commiphora gileadensis) stems: its purification, characterization and immobilization on a carboxymethylcellulose/Fe3O4 magnetic hybrid material. Int J Biol Macromol. 2019;133(15):767–774. DOI: 10.1016/j.ijbiomac.2019.04.119.
- Knapka JJ, Judge FJ. The effects of various levels of dietary fat and apple supplements on growth of golden hamster. Lab Anim. Sci. 1974;24:318–325.
- Sharma D, Dey YN, Sikarwar I, et al. In vitro study of aqueous leaf extract of Chenopodium album for inhibition of calcium oxalate and brushite crystallization. Egy J Basic Appl Sci. 2016;3(2):164–171. DOI:10.1016/j.ejbas.2016.02.001.
- Dash GK, Suresh P, Ganapaty S. Studies on hypoglycaemic and wound healing activities of Lantana camara Linn. J Nat Remed. 2001;1(2):105–110.
- Rabey HA E, Al-Seeni MN, Al-Sieni AI, et al. Honey attenuates the toxic effects of the low dose of tartrazine in male rats. J Food Biochem. 2019;43(4):e12780, DOI:10.1111/jfbc.12780.
- Barham D, Trinder P. GOD-PAP enzymatic colorimetric method of glucose estimation without deproteinization. Analyst. 1972;97:312–322. doi: 10.1039/an9729700142
- YingFoo A, Bais R. Amylase measurement with 2-chloro-4-nitrophenyl maltotrioside as substrate. Clin Acta. 1998;272:137–147. DOI:10.1016/s0009-8981(98)00009-6.
- Nishikimi M, Roa NA, Yogi K. Measurement of superoxide dismutase. Biochem Bioph Res Common. 1972;46:849–854. DOI:10.1016/S0006-291X(72)80218-3.
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/S0076-6879(84)05016-3
- Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases, the first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139.
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxidation in animal tissues by thiobarbituric acid reaction. Annals Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3
- Young DS. Effects of drugs on clinical laboratory tests. 4th ed. Washington (DC): AACC Press; 1995.
- Naito HK. (1984). High-density lipoprotein (HDL) cholesterol. Kaplan A. Clin. Chem. The C. V. Mosby Co St. Louis, Toronto Princeton. pp. 437, 1207–1213.
- Srivastava LM, Das N, Sinha S. Essentials of practical biochemistry. New Delhi: CBC Publishers and Distributors; 2002.
- Thefeld W, Hffmiester H, Busch EW, et al. Reference value for determination of GOT (glutamic opal acetic transaminase), GPT (glutamic pyruvic transaminase) and alkaline phosphatase in serum with optimal standard methods. Deut Med Wochenchr. 1974;99(8):343–351. DOI:10.1055/s-0028-1107760.
- Schlebusch H, Rick W, Lang H, et al. Standards in the activities of clinically important enzymes. Dtsch Med Wochenschr. 1974;99:765–766. doi: 10.1055/s-0028-1107840
- Thomas L. Clinical laboratory diagnostics: use and assessment of clinical laboratory results. Frankfurt/Main. Germany: TH-Books Verlagsgesellschaft mbH; 1998. pp. 80–86
- Fawcett J, Scott J. A rapid and precise method for the determination of urea. J Clin Pathol. 1960;13:156–159. DOI:10.1136/jcp.13.2.156.
- Bartels H, Bohmer M, Heierli C. Serum Kreatininbestimmung ohne Enteiweissen. Clin Chim Acta. 1972;37:193–197. DOI:10.1016/0009-8981(72)90432-9.
- Drury R, Wallington E, Cancerson R. . Carleton’s histological technique. 4th ed. Oxford: Oxford University Press; 1976.
- Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia. 2008;51(2):216–226. DOI:10.1007/s00125-007-0886-7.
- Attia ES, Amer AH, Hasanein MA. The hypoglycemic and antioxidant activities of garden cress (Lepidium sativum L.) seed on alloxan-induced diabetic male rats. Nat Prod Res. 2017;33(6):901–905. DOI:10.1080/14786419.2017.1413564.
- Al-Seeni MN. The hypoglycemic and hypolipidemic activity of wolfberry (Lycium Barbarum) in alloxan induced diabetic male rats. IOSR J Pharm Biol Sci (IOSR-JPBS). 2017;12(6):55–64. DOI:10.9790/3008-1206045564.
- Anwar D, Amani S, Alzoreqy A, et al. Therapeutic and preventive effects of Commiphora gileadensis against diethylnitrosamine-induced hepatic injury in albino rats. Afrc J Pharmacy Pharmacol. 2015;10(16):356–363. DOI:10.5897/AJPP2015.4374.
- Ahmed D, Kumar V, Verma A, et al. Antidiabetic, renal/hepatic/pancreas/cardiac protective and antioxidant potential of methanol/dichloromethane extract of Albizzia Lebbeck Benth. stem bark (ALEx) on streptozotocin induced diabetic rats. BMC Complement Alternat Med. 2014;14:243–260. DOI:10.1186/1472-6882-14-243.
- Ramudu S, Korivi M, Kesireddy N, et al. Nephro-protective effects of a ginger extract on cytosolic and mitochondrial enzymes against streptozotocin (STZ)-induced diabetic complications in rats. Chin J Physiol. 2011;54(2):79–86. doi: 10.4077/CJP.2011.AMM006
- Iluz D, Hoffman M, Gilboa-Garber N, et al. Medicinal properties of Commiphora gileadensis. Afric J Pharm Pharmacol. 2010;4(8):516–520. Available from: http://www.academicjournals.org/ajpp.
- Wineman E, Douglas L, Wineman V, et al. Commiphora gileadensis sap extract induces cell cycle-dependent death in immortalized keratinocytes and human dermoid carcinoma cells. J Herbal Med. 2015;5(4):199–206. DOI:10.1016/j.hermed.2015.08.001.