ABSTRACT
Salvadora persica L. is a medicinal plant distributed in desert and subtropical regions. In Rabigh, the few persisting populations of this plant are exposed to overcutting, overgrazing and, recently, human habitation. The conservation of S. persica requires an assessment of their genetic diversity and genetic differentiation. We applied 40 simple sequence repeat (SSR) primers, with which 22 polymorphic loci were identified. The PIC values were varied between 0.858 and 0.967. 240 plant accessions were sampled from four sites in Rabigh: Wadi EL Khaneg, Wadi Al Johfa, Wadi Al Hakak, and Wadi Khurieba. The percentage of polymorphic loci PPL% were ranged between 60% and 32% and a considerable inbreeding value (F = 0.942). Elevated genetic differentiation among the populations and a low gene migration suggest isolation among S. persica populations. Several recommendations for the protection of the existing populations, including seed preservation, restoration of highly endangered sites, and management of cutting and grazing activities.
1. Introduction
Salvadora persica L. (Salvadoraceae) is one of the most valuable medicinal and economic shrubs grown in xeric habitats of Africa and Asia [Citation1]. Its stems and branches are historically known as miswak or siwak (tooth cleaning sticks), and are used for brushing teeth, a daily dental care routine for oral hygiene undertaken by different cultures in many countries of Asia, Africa [Citation2]. Moreover, the outstanding medicinal application as toothbrush has a high value in subsistence the gums and in healing toothache [Citation3].
The growth form of S. persica was characterized by Evenari and Gutterman [Citation4] as prostrate that 37 accumulate up to 2–3 m high sandhills. The trunk is buried by sand and its branches and leaves 38 grow out and covering the sand [Citation4,Citation5]. S. persica have an edible fruit with high contents of sugars and calcium [Citation5]. The fruit with leaves considered as a soft and appealing meal for camels and sheep folks owned by the inhabitants.
S. persica grow along valleys slopes associated with high humic content, its growth area distinguished by frequent, sudden and fast dense rains [Citation6]. S. persica favours sites along drainage lines where groundwater is readily accessible in xeric regions. The tree endure a highly arid habitats with average 100 mm/ year rainfall. It proved a high salt tolerance enable it to grow in coastal sites and inland salines, it withstand 6.23 dSm−1 salinity [Citation7]. It proves a high salt tolerance enable it to grow in coastal sites and inland salines [Citation8].
Rabigh is historically known as a flood watershed area, which is composed of a number of surrounding valleys: Johfa, Khaneg, Hakak and Khurieba that encouraged agriculture activities, because of this unique geographical location, Rabigh took its name which means in Arabic: the region of Prosperous or flourishing living.
The distribution of S. persica is restricted to valleys and moderate hills surrounding Rabigh, western Saudi Arabia. The apparently small populations of S. persica could be attributed due to the growing aridity of this environment [Citation9]. Anthropogenic factors as well as increasing changes due to climate change [Citation10,Citation11] are likely to lead to further decline in the population sizes of S. persica and other associated species.
Individuals of the existing S. persica populations may suffer low genetic diversity because of co-occurring genetic drift, which is a leading factor in the low fitness and the capability of populations to acclimatize to environmental dynamics [Citation12–14].
Therefore, elucidating the genetics and structure of the populations of S. persica is necessary to conserve and recover this threatened plant species [Citation15].
For Salvadora persica, an additional aspect of its floral biology makes it a compelling study species: its tiny flowers arranged in loose, slender-branched axillary or terminal panicles [Citation16], is thought to promote outcrossing [Citation17]. In highly rare species with many small populations, outcrossing is considered an additional limitation to the persistence and productivity of these populations. However, this breeding system might neutralize the impacts of genetic drift and reduce the loss of useful alleles.
Because of global climate change and human overutilization, many plants of the Rabigh valleys, in addition to Salvadora persica, are threatened with extinction, owing to decrease of population size and the subsequent erosion of genetic variation.
For exploring genetic diversity and genetic structure, microsatellites DNA offer greater probability of detecting genetic variation than any other PCR marker system, because they target highly variable repeat regions of the genome. Automated PCR makes microsatellites the markers of choice for screening genotypes, especially because they can be interpreted as co-dominant genetic markers (contrary to other marker types, for example, AFLPs) [Citation18].
In the present work, we determined the distribution pattern of genetic diversity and genetic structure among and within the sampled populations of S. persica using microsatellite loci.
This research provided information on the levels of inbreeding within S. persica populations prior to the development of conservation strategies.
2. Materials and methods
2.1. Plant material
Twenty populations of S. persica were sampled from four sites in the valleys of the Rabigh region, western Saudi Arabia (Table , Figures and ). Five populations were selected for sampling from each site. The largest population sampled, Wadi (Valley) Khurieba (Wkhb5), with 32 observed individuals, was located in a mountainous region near Bany Ayoub Mountain (2000 ft). This site is located in the northern part of the Rabigh region and is far (80 km) from the three other sites. The sites in Wadi Al Johfa, Wadi Al Hakak, and Wadi EL Khaneg were found in eastern Rabigh city, at a distance of at least 3 kilometres from each other. Twelve plants from each population were genotyped using 40 microsatellite loci. One to three leaves per individual were wrapped in filter paper and kept in silica gel to maintain complete dryness until DNA extraction. Plant identification based on morphological characteristics was confirmed according to Orwa et al [Citation16].
Figure 1. The main sites of the studied populations of S. persica in Rapigh province, A: Wadi Al Hakak; B: Wadi EL Khaneg; C: Wadi Al Johfa; D: Wadi Khurieba.
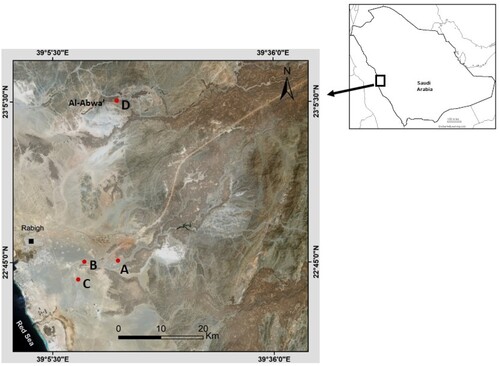
Figure 2. (A) S. persica shrub on low level hill in Gohfa valley, Rabigh. (B) Fruits of S. persica during fruiting season.
Table 1. Sites and population information of S. persica in region of Rabigh.
2.2. Genomic DNA isolation and PCR amplification
DNA isolation was performed on the dried leaf samples using a DNeasy Plant Mini Kit (Qiagen, Switzerland). Microsatellite markers were chosen because they exhibited successful amplification in other closely related species, which in accordance with Primmer et al [Citation19]. For each individual, 40 loci were tested for polymorphisms using published primers (Table ); 22 loci exhibited polymorphisms.
Table 2. Status of SSR loci used.
PCR reactions were conducted with a master mix of 25 µl containing 2.5 µl of 10× reaction buffer, 1 µl of MgCl2 (50 mM), 0.5 µl of a dNTP mix, 0.2 µl of a forward primer, 0.5 µl of a reverse primer (10 µM), 0.5 µl of the universal M13 primer (10 µM) tagged with four fluorescent dyes (FAM, NED, VIC, or PET), 0.1 µl of Taq DNA polymerase (Dream Tag, Fermentas; 50 U/µl), 1.0 µl of bovine serum albumin (20 mg/ml), 1.0 µl of 10 ng/µl genomic DNA, and ultrapure sterile water to the total volume. All the PCRs were performed as singleplex assays following protocol of Schuelke [Citation23], using a C1000 Thermal Cycler (BioRad, USA) under the following modifications: initial denaturation at 94°C for 5 min, 50 cycles at 94°C for 30 s, 55°C for 45 s and 72°C for 1 min, 8 cycles at 94°C for 30 s, 53°C for 45 s, 72°C for 1 min, and a final extension step at 72°C for 5 min. The PCR products were analysed as multiplexes on a 3130xl Genetic Analyzer (Applied Biosystems, Foster City, CA, USA) using size standard LIZ500 (Applied Biosystems, USA). The amplified fragments were scored by GeneMapper 4.0 (Applied Biosystems, USA), and the lengths of the amplified fragments ranged from 50 to 1050 bp according to Arif et al [Citation24].
2.3. Population genetic analysis
The determination of the parameters of genetic diversity, genetic structure and inbreeding was performed using GenAlEx 6.1 [Citation25].
The genetic differentiation among the populations was measured with RST, an equivalent to FST developed for microsatellite loci [Citation26]. Analysis of molecular variance AMOVA (999 permutations) was applied to evaluate the genetic structure in populations of the S. persica [Citation27,Citation28]. Gene flow (Nm) was estimated (Barton and Slatkin, 1986). The established heterozygosity (Ho), the expected heterozygosity (He), and Wright’s [Citation29] fixation index (F = 1 − Ho/He) were assessed to determines inbreeding.
3. Results
A total of 22 out of 40 loci exhibited polymorphisms (Table ). The highest value for polymorphic information content (PIC value) was exhibited by EMS9 is 0.858 whereas the lowest value was 0.967 calculated for MO8 EMS9 loci.
Table 3. List of polymorphic loci shows total number of alleles and value of polymorphic information content.
Among the populations of Khurieba site, the percentage of polymorphic loci PPL% (Table ) was the highest (52%) in the Wkhb5 population, while the lowest PPL% (25%) was found in the Whak3 population in Wadi Al Hakak.
Table 4. %P (the percentage of polymorphic loci) and F (fixation index) in the twenty studied populations of S. persica.
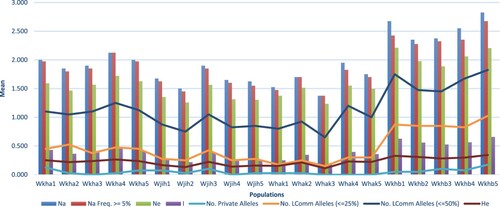
The mean number of alleles per locus (Na) varied between 3.08 (Wkhb1 population) and 1.480 (Whak3 population) (Figure ). The means of the effective number of alleles per locus (Ne), Shannon’s Index (I), and expected heterozygosity (He) ranged between 2.21, 0.66, and 0.41 in the Wkhb1 population and 1.236, 0.226, and 0.138 in the Whak3 population, respectively (Figure ).
Figure 3. The measured parameters of genetic variation showing the mean allelic pattern across the studied twenty populations of S. persica.
The average total heterozygosity (HT) was 0.451 of all loci across the studied populations. High selfing is suggested by our results for S. persica, as the average fixation index (F) was equal to 0.942, confirming an explicit deficit of heterozygotes.
The AMOVA revealed considerable genetic differentiation for S. persica populations (FST = 0.373, RST = 0.781). The highest genetic differentiation existed among the populations (78%, P = 0.010), while the lowest value (19%, P = 0.010) was detected among individuals within the populations.
4. Discussion
The analysis of fifteen polymorphic loci, considering the mean number of alleles per locus (Na), means of the effective number of alleles per locus (Ne), Shannon’s Index (I), expected heterozygosity (He), and total genetic diversity of S. persica, showed moderate to low genetic diversity in all the populations of S. persica. Likewise, other studies have pointed out that low genetic diversity has been measured for other species with isolated and small populations (e.g. [Citation30–34]).
Low polymorphism rates can be a consequence of a reduction in population size; therefore, genetic drift and inbreeding will exacerbate the danger of losing genetic diversity in species grown in extreme environmental conditions [Citation35–37]. The random allele deficiency and thus the moderate-low genetic variation found in the current research could be due to the substantial recent decline in the population size of S. persica; the count of individuals ranged between 14 and 32. These results were consistent with the studies on other small plant populations in fragmented arid habitats [Citation38,Citation39].
The moderate percentage of polymorphic loci which were exhibited by populations of Wadi EL Khaneg and Wadi Khurieba could be used as an argument for the availability of more water in these two sites. The Wadi EL Khaneg site is a narrow valley linking the two neighbouring valleys, Eljohfa and Alhakak; therefore, floods aggregate in the Wadi EL Khaneg valley. Wadi Khurieba is located close to a mountainous region, where more water from frequent rains is accessible; this is reflected in the populations of S. persica and its genetic diversity. Similar maintenance of genetic diversity in other rare plant populations grown in relation to water availability and altitude of its sites in desert, was reported by Al-Gharaibeh et al [Citation40].
The leading factors behind the noticeable reduction in the population size of S. persica include severe cutting and root removal by natives for resources such as firewood and dental sticks for tooth care. Overgrazing by camel herds is the main cause of the decline in the taxon. Last, overutilization of underground water resources by local populations and for recent growing industrial activities in the Rabigh region are severely depleting local water reserves [Citation9,Citation41].
Because of these anthropogenic factors, together with the predictions for warmer and drier conditions as a result of global climate change [Citation10,Citation11], S. persica is threatened with extinction owing to continuous decline of population size and the subsequent loss of genetic diversity.
In addition to the direct consequence of scarce water on plant existence, the upturn in temperatures imposed a harmful effect on the flowering prospective of the species and subsequently inhibit the pollination capability [Citation42]. Eventually, the decrease in genetic diversity and gene migration among the populations might enhance the negative effects of aridification.
The high genetic differentiation among the populations were in accordance with the extremely low value calculated for gene flow among the populations (Nm = 0.070).
These results agree with the high value of genetic differentiation between populations of some other rare species, including other plant species grown in arid habitats [Citation34,Citation43].
The scarce gene migration among the studied populations could be attributed to the inability of S. persica to disperse its seeds over long distances due to the size and shape of its fruits, which restrict its dispersal distance to be very close to the parental plants (Mansour, personal observation).
The means for long-distance seed dispersal are absent due to the depletion of seed adaptations for wind dispersal in desert habitats in the Rabigh region. Low seed dispersal was found in other rare plant species [Citation43]. Therefore, the sole source of gene migration is pollen transfer, as reported by Hylmö and Fryer [Citation44] in other plant species that reproduce sexually. However, the negative influences of aridity on pollinator potential which led to reduction of flight behaviour of pollinators to populations [Citation45], thus, it could suggest the presence of many unviable seeds. The measured value of gene flow was lower than the limit required to prevent genetic drift [Citation46]. The combined effect of genetic drift and gene flow could exacerbate the potential for severe future decline in genetic diversity of the existing populations of S. persica.
5. Conclusions and recommendations
Our research represents a first attempt to provide insight into the population genetics and genetic structure of S. persica in one of the most important areas in the western region of the Arabian Peninsula.
Our results indicated a mild to severe loss of polymorphic genes coupled with noticeable genetic differentiation and high inbreeding.
Strict measures should be taken to achieve sustainable management and conservation of the existing populations of S. persica in Rabigh. The protection and restoration of habitats are elementary actions for long-term conservation plans, and they can be summarized as follows. First, the grazing of camel and sheep flocks should be monitored and managed in areas with highly endangered populations with low genetic diversity parameters, e.g. Johfa and Hakak valleys. Second, comprehensive programmes to reduce water consumption will be established, and these programmes will be announced to the public through media and educational institutes. Their aim will be to effectively manage underground water.
Third, the noticeable reduction in the genetic diversity and increased genetic differentiation supported the idea that the populations of S. persica in Rabigh could be recovered mainly by inclusive seed collection from the S. persica trees of all the existing populations [Citation47].
The collected seeds would primarily be incorporated into S. persica rehabilitation programmes, in which the seeds will be germinated in nurseries, and then the seedlings will be planted in highly endangered populations. The new S. persica plants will be reintroduced into habitats similar to those of its original populations in order to prevent consequences including imminent inbreeding and a severe decline in gene flow.
Some of the collected seeds should be saved using proper seed preservation procedures in special reservoirs; these will be helpful to pursue future efforts to conserve S. persica in its original environments.
Acknowledgements
This Project was funded by the Deanship of Scientific Research (DSR), at King Abdulaziz University, Jeddah, under grant no. G-304-662-1439. The authors, therefore, acknowledge with thanks DSR for technical and financial support.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Lyengar ERR, Patolia JS, Chikara J. A useful plant for coastal saline soil. Wastelands News. 1992. 50–51.
- Hyson JM. History of toothbrush. J Hist Dent. 2003;51:73–80.
- Abhary M, Al-Hazmi A. Antibacterial activity of Miswak (Salvadora persica L.) extracts on oral hygiene. J Taibah Univ Sci. 2016;10(4):513–520. doi: 10.1016/j.jtusci.2015.09.007
- Evenari M, Gutterman Y. Some Notes on Salvadora persica L. in Sinai and its use as a toothbrush. Flora Bd. 1973;162:118–112. doi: 10.1016/S0367-2530(17)31695-X
- Von Maydell HJ. (1990). Trees and shrubs of the Sahel their characteristics and uses. Eschborn 370 GTZ. 371.
- Moeyersons J, Vermeersch PM. Dry cave deposits and their Palaeoenvironmental significance during the last 115 ka, Sodmein cave, Red Sea Mountains, Egypt. Quat Sci Rev. 2002;21:837–851. doi: 10.1016/S0277-3791(01)00132-9
- Reddy MP, Shah MT, Patolia J S. Salvadora persica, a potential species for industrial oil production in semiarid saline and alkali soils. Ind Crops Prod. 2008;28:273–278. doi: 10.1016/j.indcrop.2008.03.001
- Kassas M, Girgis W A. Habitat and plant communities in the Egyptian desert: VII. Geographical facies of plant communities. J Ecol. 1970;58(2):335–350. doi: 10.2307/2258275
- Tarawneh QY, Chowdhury S. Trends of climate change in Saudi Arabia: implications on water resources. Climate. 2018;6(8):1–19.
- Issar AS. The impact of global warming on the water resources of the Middle East: past, present and future. In: Zereini F, Hötzl H, editors. Climate changes and water resources in the Middle East and North Africa. Heidelberg: Springer; 2008. p. 145–164.
- Soultan A, Wikelski M, Safi K. Risk of biodiversity collapse under climate change in the Afro-Arabian region. Sci Rep. 2019;955:1–12.
- Bastiaan S, Hamish GS. Effects of genetic drift and gene flow on the selective maintenance of genetic variation. Genetics. 2013;194(1):235–244. doi: 10.1534/genetics.113.149781
- Hansson B, Westerberg L. On the correlation between heterozygosity and fitness in natural populations. Mol Ecol. 2002;11:2467–2474. doi: 10.1046/j.1365-294X.2002.01644.x
- Luijten SH, Dierick A, Gerard J, et al. Population size, genetic variation, and reproductive success in a rapidly declining, self incompatible perennial (Arnica montana) in the Netherlands. Conserv Biol. 2000;14:1776–1787.
- Hatmaker EA, Staton ME, Dattilo AJ, et al. Population structure and genetic diversity within the endangered species Pityopsis ruthii (Asteraceae). Front Plant Sci. 2018;9(943):1–15.
- Orwa C, Mutua A, Kindt R, et al. Agroforestree Database: a tree reference and selection guide version 4.0. Kenya: World Agroforestry Centre; 2009.
- Upendra JM, Rao SR, Dagla R. Genetic diversity analysis of Salvadora persica: an evergreen halo-xeric species of semi-arid and sub-humid regions of Rajasthan, India. Ecol Genet Genom. 2017;2:35–41.
- Kim KS, Sappington TW. Microsatellite data analysis for population genetics. In: Kantartzi S, editor. Microsatellites. Methods in molecular biology (methods and protocols). Totowa, NJ: Humana Press; 2013. p. 271–295.
- Primmer CR, Moller AP, Ellegren H. A wide-range survey of cross-species microsatellite amplification in birds. Mol Ecol. 1996;5:365–378. doi: 10.1111/j.1365-294X.1996.tb00327.x
- Ul Haq S, Jain R, Sharma M, et al. Identification and characterization of microsatellites in expressed sequence tags and their cross transferability in different plants. Int J Genom. 2014;2014:1–12. doi: 10.1155/2014/863948
- Wu J, Yang J, Gu Z, et al. Isolation and characterization of twenty polymorphic microsatellite loci for Moringa oleifera (Moringaceae). Hortscience. 2010;45(4):690–692. doi: 10.21273/HORTSCI.45.4.690
- Li Y, Sun XQ, Yan QQ, et al. Isolation and characterization of microsatellite DNA loci for wild Brassica juncea (Brassicaceae). Genet Mol Res. 2013;12(4):5392–5395. doi: 10.4238/2013.November.8.1
- Schuelke M. An economic method for the fluorescent labeling of PCR fragments. Nat Biotechnol. 2000;18:233–234. doi: 10.1038/72708
- Arif IA, Khan HA, Shobrak M, et al. Interpretation of electrophoretograms of seven microsatellite loci to determine the genetic diversity of the Arabian Oryx. Genet Mol Res. 2010;9:259–265. doi: 10.4238/vol9-1gmr714
- Peakall R, Smouse PE. Genalex v.6.5: genetic analysis in excel. population genetic software for teaching and research. Bioinformatics. 2012;28:2537–2539. doi: 10.1093/bioinformatics/bts460
- Slatkin M. A measure of population subdivision based on microsatellite allele frequencies. Genetics. 1995;139:457–462.
- Excoffier L, Smouse PE, Quattro JM. Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics. 1992;131:479–491.
- Michalakis Y, Excoffier L. A generic estimation of population subdivision using distances between alleles with special reference for microsatellite loci. Genetics. 1996;142:1061–1064.
- Wright S. Isolation by distance. Genetics. 1943;28:114–138.
- Crowford DJ, Ruiz E, Stuessy T F, et al. Allozyme diversity in endemic flowering plant species of the Juan Fernandez Archipelago, Chile: ecological and historical factors with implications for conservation. Am J Bot. 2011;88:2195–2203. doi: 10.2307/3558381
- Hamrick JL, Godt MJW, Sherman-Broyles SL. Factors influencing levels of genetic diversity in woody plant species. New For. 1992;6:95–124. doi: 10.1007/BF00120641
- Hirai M, Kubo N, Ohsako T, et al. Genetic diversity in the endangered coastal violet Viola grayi Franchet et Savatier (Violaceae) and its genetic relationship to the species in subsection Rostratae. Cons Genet. 2012;13:837–848. doi: 10.1007/s10592-012-0333-2
- Mansour H, Bryngelsson T, Garkava-Gustavsson L. Development, characterization and transferability of 10 novel microsatellite markers in Cotoneaster orbicularis Schltdl. (Rosaceae). J Genet. 2016;95:e9–e12. doi: 10.1007/s12041-016-0687-1
- Mansour H, Sliwinska E. Genetic diversity and inbreeding level of Cotoneaster orbicularis Schltdl. in the Sinai Mountains revealed by microsatellite markers and flow cytometry. Egypt J Bot. 2017;57(2):351–361. doi: 10.21608/ejbo.2017.694.1037
- Blomqvist D, Pauliny A, Larsson M, et al. Trapped in the extinction vortex? Strong genetic effects in a declining vertebrate population. BMC Evol Biol. 2010;10(33):1–9.
- Jacquemyn H, Roldán-Ruiz I, Honnay O. Evidence for demographic bottlenecks and limited gene flow leading to low genetic diversity in a rare thistle. Cons Genet. 2010;11:1979–1987. doi: 10.1007/s10592-010-0089-5
- Smyser TJ, Duchamp JE, Johnson SA, et al. Consequences of metapopulation collapse: comparison of genetic attributes between two Allegheny woodrat metapopulations. Cons Genet. 2012;13:849–858. doi: 10.1007/s10592-012-0334-1
- Al Salameen F, Habibi N, Kumar V, et al. Genetic diversity and population structure of Haloxylon salicornicum moq. in Kuwait by ISSR markers. PLos One. 2018;13:11. doi: 10.1371/journal.pone.0207369
- Su Z, Richardson BA, Zhuo L, et al. Genetic diversity and structure of an endangered desert shrub and the implications for conservation. AoB Plants. 2017;9:3. doi: 10.1093/aobpla/plx016
- Al-Gharaibeh MM, Hamasha HR, Rosche C, et al. Environmental gradients shape the genetic structure of two medicinal Salvia species in Jordan. Plant Biol. 2017;19:227–238. doi: 10.1111/plb.12512
- Harter T, Davis H, Mathews M, et al. Shallow ground water quality on dairy farms with irrigated forage crops. J Contam Hydrol. 2002;55:287–315. doi: 10.1016/S0169-7722(01)00189-9
- Root TL, Price JT, Hall KR, et al. Fingerprints of global warming on wild animals and plants. Nature. 2003;421:57–60. doi: 10.1038/nature01333
- Jimenez A, Mansour H, Keller B, et al. Low genetic diversity and a high level of inbreeding in the Sinai primrose (Primula boveana), a species on the brink of extinction. Plant Syst Evol. 2014;300:1199–1208. doi: 10.1007/s00606-013-0955-y
- Hylmö B, Fryer J. Cotoneasters in Europe. Acta Bot Fenn. 1999;162:179–184.
- Van Rossum F, Stiers I, Van Geert A, et al. Fluorescent dye particles as pollen analogues for measuring pollen dispersal in an insect-pollinated forest herb. Oecologia. 2011;165:663–674. doi: 10.1007/s00442-010-1745-7
- Spieth PT. Gene flow and genetic differentiation. Genetics. 1974;78:961–965.
- Holsinger KE, Gottlieb LD. Conservation of rare and endangered plants: principles and prospects. In: Falk DA, Holsinger KE, editor. Genetics and conservation of rare plants. New York: Oxford University Press; 1991.
