 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The antiplasmodial and safety profile of alkaloid, flavonoid and phenol extracts of Sida acuta (300 and 600 mg/kgbw) on hepatic and renal integrity of rats were investigated. Alkaloid, flavonoid and phenol extracts produce parasitaemia suppression of 50.83%, 33.50% and 64.64%, respectively. Sub-chronic administration of the phytochemicals caused marked (p < 0.05) dose-dependent increase in erythrocytic and leucocytic indices while serum ALP, AST, ALT, albumin, urea and creatinine concentrations compared well (p > 0.05) with the controls. Total proteins, sodium and chloride levels were altered in rats treated with 300 mg/kgbw of the phytochemicals. The integrity of hepatocytes and renal cells increases with increase extract concentrations and no degenerative changes were observed in all treatment groups. However, flavonoid extract caused severe renal vacuolation at 300 mg/kgbw which cleared out at 600 mg/kgbw. Conclusively, phenol exhibited higher antiplasmodial activities and safety profile and thus could be considered a potential candidate for the development of a new drug.
1. Introduction
Malaria is a public health and life-threatening infectious disease caused by a parasitic protozoan of the Plasmodium genus and transmitted via the bite of Anopheles mosquito [Citation1]. Malaria is one of the major tropical diseases with a global estimate of 219 million incidence and ∼445,000 malarial deaths in 2017 [Citation2]. The burden of malaria is the greatest in Africa, representing 90% of the estimated malaria-associated death [Citation3]. It is the greatest cause of mortality and hospitalization among children <5 years of age in Sub-sahara Africa [Citation4]. The malaria incidence or burden is increasing rapidly, due to the increase in parasite resistance to conventional drugs [Citation5] and side effects associated with conventional antimalarial drugs [Citation6]. This global concern has, therefore, called for an urgent need to search for new antimalarial agents particularly from medicinal plant extracts which are the potential source of new affordable and effective antimalarial agents. This idea originated from the historical use of medicinal plants in malaria treatment by traditional healers. Moreover, artemisinin and quinine, standardized antimalarial drugs, were derived from Cinchona species and Artemisia annua, respectively, thus suggesting that other medicinal plant could also serve as reservoir for new effective antimalarial agents [Citation7]. A number of plants have been reportedly used in the traditional management of malaria and have received ample scientific validation in both in vivo and in vitro models [Citation8]. One of such plants is Sida acuta.
Sida acuta Burm.f (Malvaceae) is one of those plants commonly used by traditional healers for the management of some health problems [Citation9]. This plant is a branched, erect, perennial herb of about 1.5 m height [Citation10]. S. acuta has been reportedly used as an antipyretic, stomachic, antipyretic and diaphoretic. It is used as a tonic and astringent and for treatments of urinary, bile, hepatic and nervous disorders [Citation11,Citation12]. Pharmacologically, S. acuta has been reported for inhibitory activities against Anopheles stephensi [Citation13], antioxidants [Citation14], antiulcer [Citation15], wound healing [Citation16] cardioprotective [Citation17], antidiabetic [Citation18] and antibacterial activities [Citation19,Citation20]. Previous studies on the crude extract of S. acuta have shown markedly antimalarial activities in both in vitro (IC50 0.05 µg/ml compared to <0.042 µg/ml for chloroquine) and in vivo models [Citation21,Citation22].
Phytoconstituents are natural secondary metabolites in plants, responsible for the plant odour, colour and its therapeutic potencies. Most of the phytoconstituents have antioxidant, antimicrobial and antimalarial properties and they protect cells against oxidative stress damage [Citation23,Citation24]. Screening of phytochemicals from medicinal plants is, therefore, an important tool in identifying active metabolites of medicinal and industrial application. Previous phytochemical studies of S. acuta have reported the presence of alkaloids (vasicine, cryptolepine and ephedrine), phenolic compounds (scopoletin, evofolin-A and B, 4-ketopinoresinol and loliolide), polyphenol, flavonoids, coumarins, saponosides, steroids (ecdysterone, β-sitosterol, stigmasterol, ampesterol) and tannins as the major bioactive components of the plant [Citation14,Citation19,Citation25]. The present study investigated the antimalarial potencies of alkaloid, flavonoid and phenol extracts from Sida acuta against P. bergehi--infected mice and also evaluated their safety profile of on hepatic and renal integrity in albino rats.
2. Materials and methods
2.1. Chemicals and reagents
Organic solvents (analytical grade) used for the extraction of the plant material (methanol, ethanol and n-hexane) were products of Sigma Chemical Co St. Louis M.O (USA). All analyses were performed with commercial kits from Randox Laboratories Limited, United Kingdom and Quimica Clinica Applicada, Spain. All other chemicals used were also of analytical grade and obtained from the Department of Biochemistry, Federal University of Technology, Minna.
2.2. Plant materials
Sida acuta (broom weed) plant was randomly collected in the environment of Zumba, Shiroro, Niger State. The leaf was air-dried (36.5°C) for six days and was ground into fine powder after which it was sieved and packaged in air-tight containers.
2.3. Plasmodium parasite
P. berghei NK 65 chloroquine-sensitive strain was obtained from the Institute for Medical Research and Training, University of Ibadan, Nigeria and was maintained in the laboratory by serial passage in mice [Citation3].
2.4. Experimental animals
Healthy albino animals (mice and rats) were procured from Animal Breeding unit of Ahmadu Bello University Zaria, Nigeria. The animals were maintained under standard laboratory conditions with access to commercial feed pellets (growers) and water ad libitum. The principles, governing the use of laboratory animals as laid out by the Federal University of Technology, Minna Committee on Ethics for Medical and Scientific Research and also existing internationally accepted principles for laboratory animal use and care as contained in the Canadian Council on Animal Care Guidelines and Protocol Review and international standard (NIH Publication No. 85-23, 1985) were duly observed. The animals were fasted 12 h before the commencement of any study.
2.5. Sample extraction
2.5.1. Alkaloid extraction
The extraction of the alkaloid was done by the continuous extraction method using the Soxhlet apparatus, as described by Gonzales and Tolentino [Citation26]. Sida acuta-powdered leaf (350 g) was moistened with 650 mL of 95% ethanol and alkalinified with 525 mL ammonia. Following the overnight maceration, the sample was extracted with ethanol; the extract was filtered and concentrated in a water bath at 60°C. The crude alkaloid was acidified with 1.0 N hydrochloric acid (30 mL), filtered and the filtrate was alkalinified with ammonia (40 mL), followed by chloroform (600 mL) partitioning. Extraction was continued with chloroform until the last chloroform extract tested negative to Dragendorff’s reagent. The extract was weighed and the percentage yield (0.56%) was documented.
2.5.2. Flavonoid extraction
Flavonoid extraction was carried out according to the method of Yahaya [Citation27]. Sida acuta (175 g)-powdered leaf sample was defatted with n-Hexane (250 mL) using a Soxhlet extractor. The extraction was carried out for six hours at 65°C. After the extraction, the thimbles were dried in an oven at 50°C. The extracted marc was further extracted with methanol (250 mL). The extract obtained was then evaporated (40°C) using water bath to yield concentrated flavonoids (6.78%).
2.5.3. Phenolic extraction
Dried and powdered leaves of Sida acuta (100 g) were solubilized in methanol:water 240:60 mL (80:20, v/v) and homogenized at room temperature (36.5°C) for 1 h 30 min. The solution was filtered with Whatman filter paper, using a separatory funnel under vacuum. The filtrate was then evaporated using a water bath at 40°C to obtain the free phenol (5.24%) extract [Citation28].
2.6. Acute toxicity test
The acute toxicity studies were conducted as per the Organization for Economic Cooperation and Development (OECD) guidelines 425 [Citation29]. Five female mice (6–8 weeks) were fasted for three hours and orally given a dose of 5000 mg/kg bw of the phytochemical extract. The mice were observed for physical signs first for three hours and further for 72 h. The experiment was terminated after 2 weeks of observations.
2.7. Antiplasmodial screening (curative test)
Evaluation of the curative potential of the extract was done using the method described by Ryley and Peters [Citation30]. On the first day (D0), standard inoculums of about 1 × 107 P. berghei-infected red blood cells were injected intraperitoneally into the mice. Seventy-two hours later, eight groups (consisting of three mice each) were set up. Groups A and B were treated with 300 mg/kg bw and 600 mg/kg bw of the alkaloid extract, groups C and D were treated with 300 mg/kg bw and 600 mg/kg bw of the phenol extract, while groups E and F were treated with 300 mg/kg bw and 600 mg/kg bw of the flavonoid extract, respectively. Chloroquine (25 mg/kg bw) was given to the positive control group (group G) and distilled water to the negative control group (group H). The drugs/extracts were given once daily for 5 days. Each day of treatment, blood samples were collected from the tail prick of each mouse and thin smears were prepared and stained with 10% Giemsa solution. The dried slides were examined under the microscope at x100 magnification and parasitaemia level was determined by counting the parasites in a field [Citation23]. Percentage parasitaemia and percentage suppression were calculated using the following formula.
Calculation
2.8. Sub-acute toxicity
Sub-acute toxicity was carried out according to OECD guideline 410 [Citation31]. Both sexes of the albino rats (100–170 g) were divided into seven groups (5 animals per group). 300 mg/kg bw and 600 mg/kg bw of each phytochemical extract were administered orally to six groups and the seventh group (positive control) was given distilled water. All treatments were administered orally with the aid of intubation cannula once daily for 21 days. All the animals were observed for clinical signs at the time of onset, duration of these symptoms, if any, were recorded. Body weights of the rats in all groups were recorded once before the start of dosing and once weekly.
2.8.1. Collection of blood, preparation of serum and tissue homogenate
The methods described by Yusuf et al. [Citation32] were used for blood sample and liver collections. At the end of the experiment (on the 21st day), the blood samples were collected from overnight fasted rats (only water allowed) by retro-orbital bleeding into heparinized and non-heparinized tubes for haematological analysis and biochemical analyses. Blood samples for biochemical study were centrifuged at 3000 r.p.m. for 15 min, after which the serum was transferred into a plain sample bottle. The liver and kidney were carefully harvested and stored in formalin for histological analysis.
2.8.2. Biochemical parameters
Spectrophotometric methods were used for the determination of aspartate transaminase and alanine transaminase activities, as described by Reitman and Frankel [Citation33]. Urea in serum/plasma is hydrolyzed to ammonia in the presence of urease. The ammonia is measurable photometrically by Berthelot’s reaction [Citation34]. Creatinine was analyzed as the amount of coloured complex formed when alkaline solution reacts with picric acid [Citation35]. Determination of total protein was done, as described by Weichselbaum [Citation36]. Albumin measurement was done with Bromocresol Green according to the methods of Doumas et al. [Citation37]. Sodium concentration was estimated, as described by Maruna [Citation38], while serum chloride concentration was determined, as described by Tietz [Citation39].
2.8.3. Determination of haematological parameters
The haematological components including haemoglobin (Hb), packed cell volume (PCV), red blood cells (RBC), mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH), mean corpuscular haemoglobin concentration (MCHC), white blood cells (WBC), platelate count (PLC), differential count (lymphocytes and neutrophils) and RBC Distribution Width Count (RDWC) were determined using the automated haematologic analyzer SYSMEX KX 21, a product of SYSMEX Corporation, Japan employing the methods described by Dacie and Lewis [Citation40].
2.8.4. Histopathological studies
Ten percent formalin solution was used to fix the kidney and liver tissues. Staining was done using Harri’s haematoxylin and eosin method. The dried slides were mounted with Distyrene Plasticizer Xylene (DPX) mountant and cover slips and examined under the microscope to verify histological details [Citation41].
2.9. Statistical analysis
The analysis was performed using SPSS statistical package for WINDOWS (version 21.0; SPSS Inc, Chicago). Data were expressed as the Mean ± SEM of three determinations. Results were subjected to ANOVA followed by DMRT. Statistically significance was considered at p < 0.05.
3. Results
3.1. Acute toxicity
There was no death recorded in animals dosed with alkaloid, flavonoid and phenolic extracts of S. acuta leaf at 5000 mg/kg bw. No toxic symptom was observed in any of the experimental animals. All animals in the extract-treated groups were normal and did not display any observable signs of toxicity on the skin, breathing, food intake, water consumption, postural patterns and hair loss.
3.2. In vivo antiplasmodial effect of the phytochemicals
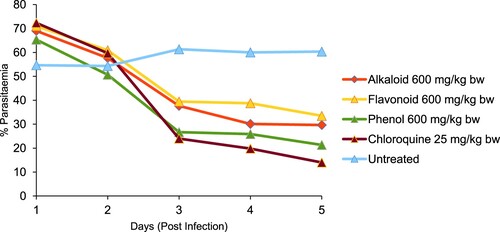
The % parasitaemia of P. bergei-infected mice treated with alkaloid, flavonoid and phenolic extracts of S. acuta are presented in Figures and . The negative control (infected untreated mice) had the highest % parasitaemia of 60.33 ± 4.05. Alkaloid extract (300 mg/kg bw) gave the lowest % parasitaemia and highest % suppression of 25.00 ± 7.00 and 58.56%, respectively, while phenolic extract (600 mg/kg bw) gave the lowest % parasitaemia and highest % suppression of 21.33 ± 2.84 and 64.64%, respectively. However, chloroquine (25 mg/kg bw) had the loweR % parasitaemia and higheR % suppression of 14.00 ± 1.00 and 76.79%, respectively when compared with the extracts-treated groups.
Figure 1. In vivo antiplasmodial effect of alkaloid, flavonoid and phenolic extracts (300 mg/kg bw) of S. acuta leaf on Plasmodium berghei-infected mice.
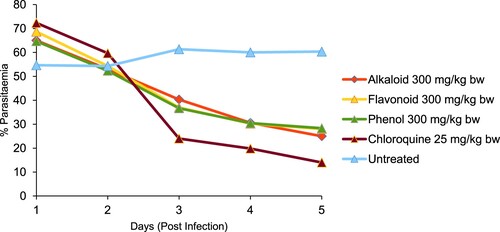
Figure 2. In vivo antiplasmodial effect of alkaloid, flavonoid and phenolic extracts (600 mg/kg bw) of S. acuta leaf on Plasmodium berghei-infected mice.
There was loss of body weight in all the treated and untreated mice (Table ). The PCV of untreated mice as well as those treated with the chloroquine (25 mg/kg bw) and phytochemicals at 300 mg/kg bw had a loss of PCV after treatments. Only mice treated with the flavonoids and phenols at 600 mg/kg bw gain PCV of 1.74 and 6.61%, respectively, after treatments (Table ).
Table 1. Effect of alkaloid, flavonoid and phenolic extracts of S. acuta leaf on the bodyweight of Plasmodium berghei-infected mice.
Table 2. Effect of alkaloid, flavonoid and phenolic extracts of S. acuta leaf on packed cell volume of Plasmodium berghei-infected mice.
3.3. Sub-chronic effect of flavonoid, alkaloid and phenolic extracts of S. acuta leaf
3.3.1. Haematological parameters
Red Blood Cell was significantly increased (p < 0.05) in rats treated with 600 mg/kg bw of alkaloids and phenol extracts when compared with other treatment groups and the control group. The result also showed a significant (p < 0.05) increase in Hb, MCH, MCHC, PCV and WBC in all the treatment groups (alkaloid, flavonoid and phenol extracts of S. acuta leaf) when compared with the control group. No significant difference (p > 0.05) was noted in MCV, LY, PLC and RDWC when compared with the control group (Table ).
Table 3. Effects of alkaloid, flavonoid and phenolic extracts of S. acuta leaf on haematological parameters of albino rats.
3.3.2. Biochemical parameters
Serum aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), albumin, urea and creatinine concentrations were not significantly different (p > 0.05) in all the treatment groups (alkaloid, flavonoid and phenol extracts of S. acuta) when compared with the control group, while sodium was significantly lowerd (p < 0.05) in all treatment groups when compared with the control group. Total protein concentration was significantly (p < 0.05) lower in rats treated with 300 mg/kg bw of flavonoid extract, while chloride was significantly (p < 0.05) higher in rats treated with 300 mg/kg bw of alkaloid and phenol extracts than other treatment groups and the control group (Table ).
Table 4. Effects of alkaloid, flavonoid and phenolic extracts of S. acuta leaf on biochemical parameters of albino rats.
3.3.3. Bodyweight
The effects of the administration of alkaloid, flavonoid and phenolic extracts of S. acuta on the bodyweight of albino rats are shown in Table . The bodyweight gains of the rats administered with alkaloid, flavonoid and phenol extracts of S. acuta were not significantly (p > 0.05) different from each other but were significantly (p < 0.05) lower than those of the control group.
Table 5. Effect of alkaloid, flavonoid and phenolic extracts of S. acuta leaf on the bodyweight of albino rats.
3.4. Histopathology
3.4.1. Effect of phytochemical extracts of S. acuta leaf on the kidney histopathology of albino rats
The histological features as regards to renal vacuolation, integrity of renal corpuscles, degenerative changes and general appearances of kidney in the albino rats treated with alkaloid, flavonoid and phenol extracts of S. acuta are presented in Tables and . There were no renal vacuolation in rats treated with phenol and alkaloid extracts of Sida acuta when compared with the control group. However, rat treated with flavonoid extract had severe renal vacoulation at 300 mg/kg bw (Table ) which clears out at 600 mg/kg bw (Table ) when compared with the control group. The integrity of renal corpuscles is better as the administered doses increases. There were also mild degenerative changes in rats treated with 300 mg/kg bw phenol and flavonoids; however, normal cellular kidney appearance was observed in all treatment groups.
Table 6. Effect of alkaloid, flavonoid and phenolic extracts of S. acuta (300 mg/kg bw) Leaf on the kidney histopathology of albino rats.
Table 7. Effect of alkaloid, flavonoid and phenolic extracts of S. acuta (600 mg/kg bw) leaf on the kidney histopathology of albino rats.
3.4.2. Effect of phytochemical extract of S. acuta leaf on the liver histopathology of albino rats
The histological features, as regards to central vein, integrity of hepatocyte, degenerative changes and general appearances of liver in albino rats treated with alkaloid, flavonoid and phenol extracts of S. acuta, are presented in Tables and . Severe congestion with flavonoid at 300 mg/kg bw (Table ) which cleared out at 600 mg/kg bw (Table ) when compared with the control group was observed. The integrity of hepatocytes is better as the administered doses increase; no any degenerative changes were observed in all treatment groups. Also, normal hepatocellular appearance was observed in all treatment groups (Figures and ).
Figure 3. Histomicrogram of the kidney of alkaloid, flavonoid and phenolic extracts of S. acuta leaf in albino rats. (Arrow indicates renal vacuolation at 300 mg/kg bw which was corrected at 600 mg/kg bw).
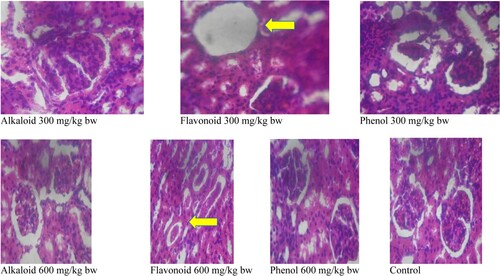
Figure 4. Histomicrogram of the liver of alkaloid, flavonoid and phenol extracts of S. acuta leaf in albino rats. (Arrow indicates central vein congestion at 300 mg/kg bw which was corrected at 600 mg/kg bw).
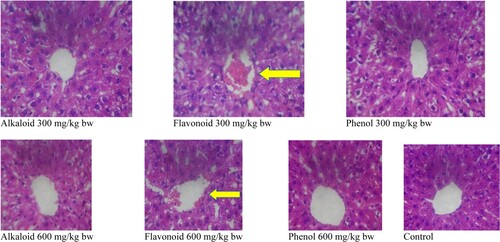
Table 8. Effect of alkaloid, flavonoid and phenolic extracts of S. acuta (300 mg/kg bw)leaf on the liver histopathology of albino rats.
Table 9. Effect of alkaloid, flavonoid and phenolic extracts of S. acuta (600 mg/kg bw) leaf on the liver histopathology of albino rats.
4. Discussion
Phytochemicals are secondary metabolites of plants known to exhibit diverse pharmacological and biochemical effects on living organisms [Citation42]. The acute toxicity of the alkaloids, flavonoids and phenols was measured to obtain information regarding the oral safety of the secondary metabolites. Interestingly, it was discovered that all phytochemicals tested had LD50 > than 5000 mg/kg bw, thus exhibiting a wide safety margin upon acute oral administration. However, Konaté et al. [Citation43] reported LD50 values of 3.2 g/kg for crude extract of S. acuta in mice, thus suggesting negligible level of toxicity of the crude extract. Results of the present study indicated that alkaloids, flavonoids and phenols exhibited active antiplasmodial activity in accordance with the reports of Carvalho [Citation44] who stated that a compound with reduction in parasitaemia ≥ 30% is considered active. This suggests that alkaloid, flavonoid and phenolic extracts of S. acuta leaf may be considered as a bioactive metabolite with potential for the development of a novel antimalarial drug.
However, the higher % suppression of the parasite produce by phenol extract (64.64% at 600 mg/kg bw) compared to alkaloid and flavonoid extracts indicated that the phenol extracts would be better antimalarial than the other phytochemicals. Chloroquine has been reported for the disruption of the parasite’s cell membrane and induction of auto-parasite digestion via the formation of FP-chloroquine complex which impaired the haeme polymerization [Citation45]. The lower percentage suppression observed in the extract-treated group than that of the chloroquine-treated group may be because the phytochemicals in its current form and at the doses administered had not accumulated sufficiently to bring about considerable suppression [Citation46] or it has a lower speed of action than that of chloroquine. This is similar to the study of Builder [Citation47] who reported 83% inhibition of parasitaemia for chloroquine. Although the activities demonstrated by each phytochemical extract were lower than those of a standard drug (chloroquine) (76.79%), it is possible that a combination of 2 or 3 of these phytochemicals would act synergistically to exhibit higher antimalarial activities comparable to or better than the chloroquine.
Anaemia is one of the recognized effects of malaria that occurs due to the destruction of red blood cell. Therefore, effective antimalariaL agents are expected to reverse or prevent the parasite-induced anaemic condition. The increases in PCV (1.74 and 6.61%) of mice treated with the flavonoid and phenol extracts of S. acuta (Table ) are an indication of enhanced resistance to erythrocyte haemolysis [Citation48]. The flavonoids and phenols, therefore, prevented the parasite-induced anaemic condition in mice. The high loss of body weight in mice treated with high dose (600 mg/kg bw) of each phytochemical extract could be attributed to the depressing effect of the phytochemical extracts on feed uptake/appetite [Citation49] with consequent effects on the phytochemicals.
Hematological parameters are widely used markers in assessing the toxicity or safety of a drug/extract as well as assessing the health status of an animal [Citation50]. The significant increase in the WBC count in the rats treated with alkaloid, flavonoid and phenolic extracts could be an indication of leucopoetic potentials and possible immunomodulatory properties of the phytochemical extract which enhances the production of more WBC [Citation51]. This will enhance the antibody-generating potential of the animals via phagocytosis and will have high resistance to infection and diseases [Citation52] Similarly, the significant (p < 0.05) increase in erythrocytic indices, including HGB, PCV, MCH, MCHC, in all the treatment groups (alkaloid, flavonoid and phenolic extracts of S. acuta) when compared with the control is an indication of stimulation of erythropoiesis by the phytochemicals. The phytochemicals must have enhanced the release of erythropoietin in the kidney, a humoral mediator of RBC production [Citation53].
Analyses of biochemical parameters, such as transaminases, phosphatase, albumins and total proteins, have been identified as a reliable indicator of organ function and healthy or disease status of animals during subacute administration of extracts or phytochemicals to animals [Citation54,Citation55,Citation56]. Alterations in serum levels of these markers are indicators of hepatocellular impairments, cell membrane impairment, liver hepatitis or cirrhosis.
Interestingly, the serum aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP) and albumin concentrations were not significantly altered by the treatment with alkaloid, flavonoid and phenol extracts of S. acuta. This is an indication that the administration of phytochemicals to the rats has not compromised the integrity of the liver, i.e. the functional integrity of the liver has been preserved. However, the alterations in total protein concentrations in rats treated with 300 mg/kg bw of flavonoid extract could be attributed to protein turnover during the metabolism of the flavonoid, such an increase in total proteins could lead to dehydration which is detrimental to cellular homeostasis, thus effecting the health of the animals [Citation57].
Serum electrolytes, urea and creatinine, on the other hand, indicate the function integrity of the kidney [Citation55]. In this study, urea and creatinine concentrations were not affected by the administration of the phytochemicals, thus suggesting that the functional capacity of the kidney has not been compromised [Citation58] However, the significant alterations (p > 0.05) in the concentrations of sodium and chloride in the albino rats dosed with the phytochemical extracts are an indication that the functional integrity of the kidney as regards to this metabolite has been compromised. Nevertheless, the mild alterations recorded in the electrolyte may not be of clinical relevance without histopathological backup [Citation59]. Fortunately, it was observed that the alkaloid and phenol extracts of Sida acuta do not alter the normal cellular architectures of the liver and kidney. In fact, the integrity of the hepatocytes and renal corpuscles was improved at higher doses of the alkaloid and phenolic extracts better than the control group. It is, therefore, reasonable to conclude that the mild alteration observed in some of the biochemical parameters could be attributed to the initial metabolic adaptations of the animals [Citation60] to the phytochemicals and are not of clinical significance as regards to the integrity of the liver and kidney.
5. Conclusion
The study suggests that alkaloid, flavonoid and phenolic extracts of Sida acuta leaf possess antiplasmodial properties with phenolic extract (600 mg/kg bw) possessing the highest plasmodial suppression (64.64%) among other extracts and close to the positive control (chloroquine) which caused plasmodial suppression of 76.79%. The phytochemicals were also found to be safe as revealed by biochemical, haematological and histopathological findings of sub-chronic toxicity studies.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Ounjaijean S, Kotepui M, Somsak V. Antimalarial activity of Tinospora baenzigeri against Plasmodium berghei-infected mice. J Trop Med. 2019: 1–6. doi:10.1155/2019/5464519.
- WHO. World Malaria Report, World Health Organization, Geneva, Switzerland, 2017, http://wwwwhoint/malaria/world_malaria_report_2017/en/indexhtml.
- Mzena T, Swai H, Chacha M. Antimalarial activity of Cucumis metuliferus and Lippia kituiensis against Plasmodium berghei infection in mice. Res Rep Trop Med. 2018;9:81–88.
- Ajayi EIO, Adeleke MA, Adewumia TY, et al. Antiplasmodial activities of ethanol extracts of Euphorbia hirta whole plant and Vernonia amygdalina leaves in Plasmodium berghei-infected mice. J Taibah Univ Sci. 2017;1:831–835.
- Lawal B, Shittu OK, Abubakar A, et al. Human genetic markers and structural prediction of plasmodium falciparum multi-drug resistance gene (Pfmdr1) for ligand binding in pregnant women attending General Hospital Minna. J Environ pub Health. 2018;2018:1–13.
- Rout S, Mahapatra RK. Plasmodium falciparum: multi-drug resistance. Chem Biol Drug Des. 2019;93:737–759.
- Pan WH, Xu XY, Xu N, et al. Antimalarial activity of plant metabolites. Intern J Mol Sci. 2018;19(5):1382–1389.
- Lawal B, Shittu OK, Kabiru AY, et al. Potential antimalarials from African natural products: A review. J. Intercult Ethnopharmacol. 2015;4(4):318–343.
- Benjumea DM, Gómez-Betancur IC, Vásquez J, et al. Neuropharmacological effects of the ethanolic extract of Sida acuta. Revista Brasileira de Farmacognosia. 2016;26(2):209–215.
- Mohideen S, Sasikala E, Gopal V. Pharmacognostic studies on Sida acuta Burm.f. Ancient sci of Life. 2002;22(1):57.
- Khare M, Srivastava SK, Singh AK. Chemistry and pharmacology of genus Sida (Malvaceae); a review. J. Med. Aromatic Plant Sci. 2002;24:430–440.
- Olivier TT, Armel JS, Francis NT. Ethnomedicinal uses, phytochemical and pharmacological profiles, and toxicity of Sida acuta Burm. F.: a review article. The Pharma Innovat J. 2017;6(6):1–6.
- Aarthi A, Murugan K, Madhiyazhagan P, et al. Studies on the effect of sida acuta and Vetiveri azizanioides against the malarial vector, anopheles stephensi and malarial parasite, plasmodium berghei. Inter J Pure Appl Zool. 2014;2(1):51–60.
- Konate K, Souza A, Coulibaly AY, et al. In vitro antioxidant, lipoxygenase and xanthine oxidase inhibitory activities of fractions from Cienfuegosia digitata Cav, Sida alba L. and Sida acutaBurn f. (Malvaceae). Pak J Biol Sci. 2010;13:1092–1098.
- Malairajan P, Gopalakrishnan G, Narasimhan S, et al. Antiulcer activity of Sida acuta Burm. Nat Prod Sci. 2006;12:150–152.
- Akilandeswari S, Senthamarai R, Valarmathi R, et al. Wound healing activity of Sida acuta in rats. Int J Pharm Technol. 2010;2:585–587.
- Ramachandran AV. Cardioprotective effect of Sida rhomboidea. Roxb extract against isoproterenol induced myocardial necrosis in rats. Exp Toxicol Pathol. 2011;63:351–356.
- Arciniegas A, Pérez-Castorena A, Nieto-Camacho A. Anti-hyperglycemic, antioxidant, and anti-inflammatory activities of extracts and metabolites from Sida acuta and Sida rhombifolia. Química. 2017;40:176–181.
- Karou SD, Savadogo A, Canini A, et al. Antibacterial activity of alkaloids from Sida acuta. Afr J Biotechnol. 2005;4:1452–1457.
- Jindal A, Kumar PA. Antibacterial activity of Sida acuta Burm. f. against human pathogens. Asian J Pharm Clin. Res. 2012;5:33–35.
- Karou D, Mamoudou H, Sanon S, et al. Antimalarial activity of Sida acuta Burm. f. (Malvaceae) and Pterocarpus erinaceus Poir. (Fabaceae). J Ethnopharmacol. 2003;89:291–294.
- Banzouzi JT, Prado R, Menan H, et al. Studies on medicinal plants of Ivory Coast: investigation of Sida acuta for in vitro antiplasmodial activities and identification of an active constituent. Phytomedicine. 2004;11:338–341.
- Jigam AA, Mahmood F, Lawal B. Protective effects of crude and alkaloidal extracts of Tamarindus indica against acute inflammation andnociception in rats. J Acute Dis. 2017;6(2):78–81.
- Umar SI, Lawal B, Mohammed BA, et al. Antioxidant and antimicrobial activities of naturally occurring flavonoids from M. heterophylla and the safety evaluation in Wistar rats. Iran J Toxicol. 2019;13(4):39–44.
- Prakash A, Varma RK, Ghosal S. Alkaloid constituents of Sida acuta, Sida humilis, Sida. rhombifolia and Sida spinosa. Planta Med. 1981;43:384–388.
- Gonzales MVM, Tolentino AG. Extraction and isolation of the alkaloids from the Samanea Saman (Acacia) Bark: its antiseptic potential. Inter J Sci Technol. 2014;3:1–6.
- Yahaya MM. Isolation and purification of flavonoids from the leaves of locally produced Carica Papaya. Intern J Sci Technol Res. 2015;4(12):282–284.
- Atansuyi K, Ibukun EO, Ogunmoyole T. Antioxidant properties of free and bound phenolic extract of the leaves of Jatropha tanjorensis In vitro. J Med Plants Res. 2012;6(31):4667–4674.
- OECD. (2008). OECD guideline for testing of chemicals. Test No. 425: acute oral toxicity: up-and-down procedure. doi:10.1787/9789264071049-en.
- Ryley JF, Peters W. The antimalarial activity of some Quinolone Esters. Annals of Trop Med Parasitol. 1970;64(2):209–222.
- Organization for Economic Cooperation and Development. (1981). Repeated dose dermal toxicity: 21/28-day oral toxicity study in rodents. Guidelines for the Testing of Chemicals/Draft Updated Test Guideline 410.
- Yusuf AA, Lawal B, Yusuf MA, et al. Free radical scavenging, antimicrobial activities and effect of sub-acute exposure to Nigerian Xylopia Aethiopica seed extract on liver and kidney functional indices of albino rat. Iran J Toxicol. 2018;12(3):51–58.
- Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28:56–63.
- Weatherburn MW. Phenol-hypochlorite reaction for determination of ammonia. Analyt Chem. 1967;39:971–974.
- Bartels H, Bohmer M, Heierli C. Serum creatinine determination without protein precipitation. Clin Chem Acta. 1972;37:193–197.
- Weichselbaum TE. An accurate and rapid method for the determination of proteins in small amounts of blood serum and plasma. Amer J Clin Pathol. 1946;10:40–49.
- Doumas BT, Watson WA, Biggs HG. Albumin standards and the measurement of serum albumin with Bromcresol Green. Clin Chem Acta. 1971;31(1):87–96.
- Maruna RFL. Quantitative estimation of sodium (Na), potassium (K) in human serum by colorimetric methods. Clin Chem Acta. 1958;2:581–585.
- Tietz NW. Clinical guide to laboratory tests. 3rd ed. Philadelphia (PA): WB Saunders Company; 1996; pp. 286–288.
- Dacie JV, Lewis SM. Practical Haematology. 11th Edn. London: Elsevier; 2002; pp: 380-382.
- Igwebuike UM, Eze UU. Morphology of the caeca of the african pied crow (corvus albus). Anim Res Inter. 2010;7(1):1121–1124.
- Sultana B, Anwar F, Ashraf M. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plants extracts. Molecules. 2009;14(6):2167–2180.
- Konaté K, Bassolé I, Hilou A, et al. Toxicity assessment and analgesic activity investigation of aqueous acetone extracts of Sida acuta Burn f. and Sida cordifolia L. (Malvaceae), medicinal plantsof Burkina Faso. BMC complement. Altern. Med. 2012;12:120, doi:10.1186/1472-6882-12-120.
- Carvalho LH, Brandao MGL, Santos-Filho D, et al. Antimalarial activity of crude extracts from Brazilian plants. Studied in vivo in Plasmodium berghei-infected mice and in vitro against Plasmodium falciparum in culture. Braz J Med Biol Res. 1991;24:1113–1123.
- Noedl H, Wongsrichanalai C, Wernsdorfer WH. Malaria drug sensitivity testing: new assays, new perspectives. Trends in Parasitol. 2003;19:175–181.
- Adebayo JO, Yakubu MT, Egwim EC, et al. Effect of ethanolic extract of Khaya senegalensis stem bark on some biochemical parameters on rat kidney. J Ethnopharmacol. 2003;88:69–72.
- Builders M, Alemika T, Aguiyi J. Antimalarial activity and isolation of phenolic compound from Parkia biglobosa. J Pharm Biol Sci. 2014;9(3):78–85.
- Awoke N, Arota A. Profiles of hematological parameters in Plasmodium falciparum and Plasmodium vivax malaria patients attending Tercha General Hospital, Dawuro Zone, South Ethiopia. Infect Drug Resist. 2019;12:521–527.
- Chinchilla M, Guerrero OM, Abarca G, et al. An in vivo model to study the anti-malariac capacity of plant extracts. Rev Biol Trop. 1998;46(1):1–7.
- Berinyuy EB, Lawal B, Olalekan AA, et al. Hematological status and organs/body-weight parameters in Wister rats during chronic administration of Cassia occidentalis. Inter Blood Res Rev. 2015;4(3):1–7.
- Ozkan C, Kaya A, Akgul Y. Normal values of haematological and some biochemical parameters in serum and urine of New Zealand white rabbits. World Rab Sci. 2012;20:253–259.
- Lawal B, Shittu OK, Rotimi AA, et al. Effect of methanol extract of Telfairia occcidentalis on haematological parameters in Wister rats. J Med Sci. 2015;15(5):246–250.
- Mishra N, Tandon VL. Haematological effects of aqueous extract of ornamental plants in male Swiss albino mice. Vet World. 2012;5:19–23.
- Umar SI, Ndako M, Jigam AA, et al. Anti-plasmodial, Anti-inflammatory, antinociceptive and safety profile of Maytenus senegalensis root bark extract on hepato-renal integrity in experimental animals. Comp Clin Pathol. 2019;28(8):1–8.
- Shittu OK, Lawal B, Blessing Uchenna AB, et al. Alteration in biochemical indices following chronic administration of methanolic extract of Nigeria bee propolis in Wister rats. Asian Pac J Trop Dis. 2015;5(8):654–657.
- Yusuf AA, Lawal B, Abubakar AN, et al. In-vitro antioxidants, antimicrobial and toxicological evaluation of Nigerian Zingiber officinale. Clin Phytosci. 2018;4(12):1–8.
- Akanji MA, Salau AK, Yakubu MT. Safety evaluation of aqueous extract of Cratevaadansonii leaves on selected tissues of rats. Fount Nat Appl Sci. 2013;2(1):17–28.
- Bashir L, Shittu OK, Busari MB, et al. Safety evaluation of giant African land snails (Archachatina marginata) Haemolymph on hematological and biochemical parameters of albino rats. J Adv Med Pharm Sci. 2015;3(3):122–130.
- Ogunlana OO, Ogunlana OE, Ntube CA, et al. Phytochemical screening and in vivo antioxidant activity of ethanolic extract of Caesalpinia bonduc (L.) Roxb. Global Res J Pharm. 2012;1(1):1–4.
- da Silva G, Tanica M, Rocha J. In vivo anti-inflammatory effect and toxicological screening of Maytenus heterophylla and Maytenus senegalensis extracts. Human and Exp Toxicol. 2010;30(7):693–700.
