ABSTRACT
One of the synthetic pyrethroid pesticides is Cypermethrin (CYP) which has been widely used in Egypt. CYP is primarily believed to be safe for ordinary application. Many studies presented its neural and reproductive toxicity in nonmammalian, mammalian and different animal species. Up till now, there is not enough information known about the reproductive toxicity mechanism induced by the CYP and there is no sufficient data for the protection against its toxicity. Our study focuses on the toxic effects induced by CYP in the testis and brain tissues by detecting some apoptosis markers (Caspase3, p53 and Bcl-2) and estimating FSH, LH and testosterone hormones in serum. Sesame oil (S. oil) is considered as a potent antioxidant and dietary supplement but its molecular protective effects are still unclear. Four groups contain healthy adults’ rats (age- and weight-matched): the normal control group, S. oil group (2 ml/kg b.wt./day), CYP group (3 ml/kg b.wt./day) and the last group treated with S. oil plus CYP. The treatment was continuous for 60 days. In CYP group biochemical analysis showed a reduction in the serum levels of testosterone, LH and FSH hormones, an increase in brain and testis Caspase3 and p53 expression besides the detectable decrease in Bcl-2 expression. Pretreatment with S. oil effectively protected against such biochemical changes, counteracted the CYP-altering the expression of Caspase3, p53 and upregulated Bcl-2 expression in testis and brain tissues. In Conclusion, S. oil has an ameliorative potency against biochemical alternations and apoptosis produced by CYP in tissues of the brain and testis of rats.
1. Introduction
The toxicity of streams and rivers with chemical pollutants has become one of the main global ecological risks. Because of the transportation of pollutants into the environment from industrial zones and agricultural areas, various aquatic ecologies are rich with different chemical xenobiotic [Citation1,Citation2]. The extensive pesticide usage in general agricultural programmes and health protection has produced serious health hazards and pollution of the environment, mainly in developing countries, involving problems of chronic and acute animal and human harming besides injury to other non-targeted organisms [Citation3]. Insecticides have been assumed chemical mutagens and certain reports have revealed that several agrochemical components have genotoxic properties, causing DNA damage or mutations [Citation4]. All over the world about 220,000 deaths and three million cases of severe pesticide toxicity have been stated annually, nearly 99% of which related to the third world countries [Citation5]. The variety of health side effects involve injury to the nervous, respiratory, endocrine, reproductive system and sometimes cancer [Citation6,Citation7].
In recent years, the pesticides pyrethroid toxicity to mammals has got wide considerations due to the reality that animals exposed to these insecticides have exhibited alterations in their pathological and physiological features [Citation8]. Organophosphates pesticides have been exchanged by pyrethroids for various agricultural and house usages due to the severe limitations on organophosphate pesticides [Citation9]. In the past two decades cypermethrin (CYP), one insecticide of synthetic pyrethroids type II, has been widely used as a dominant insecticide in Egypt for fighting insects of veterinary and agricultural pests as well as a human concern [Citation3,Citation10]. CYP is cyano-(3-phen-oxyphenyl)-2,2 dimethylcyclopropane-1-carboxylate. It is a viscous semi oiled, yellowish brown coloured and odorless chemical with molecular formula: C22 H19 Cl2 No3. CYP applies to fight pests attacking vegetable, fruit and cotton crops, such as landscape care or structural pest control. Consequently, this leads to its release into the aquatic environment and subsequently to plants, fishes and finally human [Citation9]. In various experimental systems CYP can cause a series of immunotoxic, genotoxic effects, reproductive toxicity and it is involved in many neurological disorders’ pathogenesis [Citation11–13]. The US Environmental Protection Agency has classified CYP as a carcinogen [Citation14]. The organism removes unwanted cells, without causing an inflammatory response, by a programmed cell death (apoptosis) which is an extremely controlled process [Citation10,Citation15]. In some previously published reports, environmental pollutants as pesticides were recorded to induce cell apoptosis. Although wide research work is in progress on many aspects of synthetic pyrethroid, involving pharmacological properties, metabolism and ecotoxicity, the antioxidants protective effects against pyrethroid toxicity has received little attention.
Sesame oil (S. oil) is the extract of the plant Sesanum Indicum, Pedaliaceae family. S. oil has phenolic compounds, polyunsaturated fats and lipids, non-protein amino acids, cacogenics glycosides, mucilage, phospholipids, ketones, thiazole, alkaloids, aldehyde, disulphide, vitamins as B2, B1, E and C and a trace of elements such as magnesium, calcium, iron, copper, phosphorus and zinc [Citation16,Citation17]. S. oil contains abundant lignans like sesamolin and sesamin (lipid-soluble lignans), sesaminol diglycoside and sesaminol triglucoside (water-soluble lignan glycosides and sterol) with varied antioxidant attributes which have the capability of improving fertility potency of the male reproductive system [Citation18]. Sesaminol and Sesamin are the main phenolic ingredients of S. oil with profound pharmacological influences, including antihypertensive, antimutagenic, anti-oxidant, antithrombotic and anti-inflammatory effect [Citation19]. By inhibiting the production of free radical reactive oxygen species, S. oil decreased lipid peroxidation [Citation20] and abolishes many organ injuries that are caused by endotoxin in rats. However, the molecular protective effects of S. oil are still unclear. The widespread usage of CYP gives motivation to study its probable sub-acute toxicity and harmful impacts on the brain and testis, which are the principal target organs for this study. The protection against the CYP toxicity has not enough data. Thus, our research was designed to: (i) well understand the ability of CYP to induce toxicity and apoptosis in the brain and testis tissues of male albino rats by investigating the variations of the transcriptional genes on the apoptosis pathway, such as p53, B-cell lymphoma/leukaemia-2 gene (Bcl-2), and Caspase3 (Cas3) using qRT-PCR, besides biochemical changes of sex hormones (testosterone (T), luteinizing hormone (LH) and follicle-stimulating hormone (FSH)), and (ii) the probability of S. oil to reduce the toxic effects by CYP.
2. Materials & methods
2.1. Kits and chemicals
CYP, ethidium bromide and agarose were purchased from Sigma. Aldrich (St. Louis, MO, USA). The Sesame oil was purchased from The CAP. PHARM company, Egypt. Nucleic acid (DNA) ladder was purchased from MBI, Ferments, Thermo Fisher Scientific, USA. Oligo DT primer and Qiazol for RNA extraction were purchased from QIAGEN (Valenica, CA, USA). The kit used in the present study, such as FSH and LH were purchased from THE PADTAN ELM CO. (Tehran, IRAN).
2.2. Experimental design and animals
The albino rats were purchased from the International Institute for research (St. Nadi Elsid, El Dokki, Egypt). Forty male albino rats aging 9 weeks, weighing 120–170 g, were collected randomly and given free access to food and water. Rats were assigned for experimental design after two weeks of acclimatization. Rats were divided into four subgroups, each 10 as follows:
The normal control group: The rats received a free tendency to water and food and don’t receive any treatment to measure the normal level of basic parameters.
The Sesame oil group (S. oil rats): They were given S. oil by a gastric tube, at a dose of 2 ml/kg b.wt./day for 60 days [Citation21] (the dose was equivalent to 4 mL/kg daily for 30 days).
CYP group: They received CYP orally at a dose of 3 ml/kg b.wt./day for 60 days. The dose of CYP was estimated based on sublethal dose (1/62 LD50) of CYP since a lethal dose of it is 187–326 mg/kg for male according to Environmental Protection Agency (EPA) [Citation22].
CYP + S. oil group: As a pretreatment manner, this group received sesame oil (2 ml/kg b.wt./day) through oral administration 30–60 min after CYP (3 ml/kg b.wt./day) administration for 60 days.
2.3. Serum extraction and biochemical indexes measurements
After blood collection, at room temperature the blood was left to clot and for 15 min samples were put in the refrigerator at 4°C then they were centrifuged at 3000 r/min for 10 min. The sera were kept frozen (−20°C) until they were used. Testosterone analysis was performed by an automatic analyzer Tossoh System (Hitachi Bothering Mannheim, 912 Automatic Analyzer) using specific kits. All the detections were performed according to the kit instructions.
2.4. Quantitative real-time PCR
Total RNA was isolated from brain and testis tissues using the Qiagen tissue extraction Kit (Qiagen, USA) according to instructions of the manufacturer. By using spectrophotometry (dual wavelength Beckman, spectrophotometer, USA), the purity (A260/A280 ratio) and the concentration of RNA were obtained. Total RNA (0.5–2 µg) was used for cDNA conversion using high capacity cDNA reverse transcription Kit (Fermentas, USA) according to the manufacturer’s protocols. Real-time qPCR analysis and amplification were done using an Applied Biosystem with software version 3.1 (StepOne™, USA). The qPCR assay with the primer sets was optimized at the annealing temperature. The relative quantification was calculated according to Applied Biosystem software according to the ΔΔCt method. The RQ is the fold change compared to the calibrator (untreated sample). The sequence of primer was shown on Table .
Table 1. Primer pairs used for q-PCR.
2.5. Statistical analysis
The data was expressed as means ± standard error (SE). The statistical significance of the differences between groups was processed using statistical package for the social sciences statistical software (SPSS) version 20 for Windows (SPSS, IBM, Chicago, IL, USA). Values at P < 0.05 were significant, P < 0.01 were highly significant and P < 0.001 were very highly significant.
3. Results
3.1. Effect of cypermethrin and sesame oil treatment on brain Caspase3, p53, and Bcl-2 gene expression
Gene expression analysis showed significant (P < 0.001) up regulation of p53 and Cas3 expression in the brain of the CYP-treated group when compared with both the normal control and S. oil groups. By comparison to CYP group we found that CYP-supplemented rats with the S. oil have reduced the elevation of expression level of the p53 and Cas3 significantly (p < 0.001) (Figure a,b, respectively). So, S. oil reversed the pro apoptotic behaviour of CYP.
Figure 1. PCR analysis of brain p53 (A) Caspase 3 (B), and Bcl-2 (C) expression. CONTROL: normal control, OIL: Sesame oil, Cyper: Cypermethrin and Cyper + Oil: Cypermethrin rats treated with Sesame oil. Data are Mean ± SE (n = 10). ***P < 0.001 versus Control, and ###P < 0.001 versus CYP.
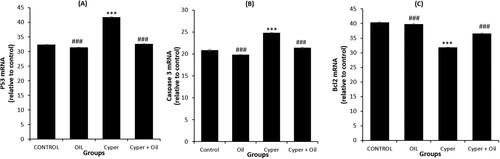
On the contrary, CYP group showed significantly (P < 0.001) down regulated brain Bcl-2 expression when compared with both the normal control and S. oil rats. The supplementation of S. oil produced a marked (P < 0.001) up regulation of brain Bcl-2 when compared with the CYP group (Figure c).
Concerning one-way ANOVA, it was found that the effect between groups on brain Casp3, p53, and Bcl-2 gene expression was significant (P < 0.001) throughout the experiment.
3.2. Effect of cypermethrin and sesame oil treatment on testis Caspase3, p53 and Bcl-2 gene expression
Data represented in Figure illustrate Cas3, p53, and Bcl-2 expression in testis of the normal control, S. oil, CYP and CYP + S. oil -administered rats. PCR analysis revealed a significant (P < 0.001) upregulation of Cas3 and p53 expression in the testis of the CYP treated rats when compared to the normal control and S. oil rats. The S. oil significantly decline the expression level of Cas3 and p53 (P < 0.001) in the CYP + S. oil group compared to the CYP group (Figure a,b, respectively).
Figure 2. PCR analysis of testis Caspase3 (A), p53 (B), and Bcl-2 (C) expression of CONTROL: normal control, OIL: Sesame oil, Cyper: Cypermethrin and Cyper + Oil: Cypermethrin rats treated with Sesame oil. Data are Mean ± SE (n = 10). ***P < 0.001 versus Control, and ###P < 0.001 versus CYP.
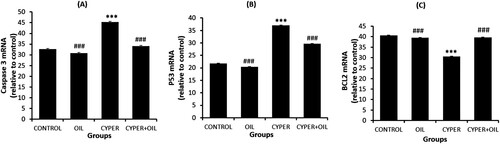
The findings revealed that Bcl-2 was significantly (P < 0.001) downregulated in the testis tissue after CYP administration compared to both the normal control and S. oil groups. Pretreatment with the S. oil (CYP + S. oil group) produced significant (P < 0.001) upregulation in the expression of Bcl-2 compared to the CYP rats (Figure c).
One-way ANOVA showed that the general effect between groups was significant (P < 0.001) throughout the experiment.
3.3. Effect of cypermethrin and sesame oil treatment on LH, FSH and testosterone hormones
The analysis results of T, LH and FSH levels are shown in Figure . The data showed that CYP administration declined the level of the serum FSH significantly (P < 0.001) when compared with both the normal and S. oil control groups (Figure a). More or less similar, the results revealed a significant decline in both T and LH levels (P < 0.001) of CYP administrated groups compared to both the normal and S. oil control ones (Figure b,c), whilst the pretreatment with the S. oil along with the CYP led to a significant (P < 0.001) increase in FSH, LH and T when compared to the CYP group. One-way ANOVA revealed that the general effect between groups on FSH, LH, and T were significant (P < 0.001) thought the experiment.
Figure 3. Sesame oil has reversed the effect of CYP on follicle-stimulating hormone (FSH) (A), luteinizing hormone (LH) (B) and testosterone (C) hormones’ level. CONTROL: normal control, OIL: Sesame oil, Cyper: Cypermethrin and Cyper + Oil: Cypermethrin rats treated with Sesame oil. Data are Mean ± SE (n = 10). ***P < 0.001 versus Control, and ###P < 0.001 versus CYP.

4. Discussion
Synthetic pyrethroid insecticides as CYP has controlled insect pests for over 20 years in several crops [Citation23]. Because of its widespread usage in several fields, CYP might cause side effects on non-target species (human and health environment) and the worry about its safety has grown [Citation24]. Previous studied showed that the exposure to CYP causes minor tremors, hyper-excitability, sunken eyes, thick eye discharge and a decrease in the spontaneous locomotor activity [Citation25–27]. These signs might be because of the disturbance of normal functioning of the nervous system and deposition of CYP as well as its metabolites in brain of adult mammals, inducing the shift in the quantity of growth hormones and mental functions besides neurotoxicity [Citation28,Citation29]. These effects are resulted by modifying sodium channels in mammals [Citation30,Citation31]. Up till now, not enough is known about the reproductive toxicity mechanism of CYP. Moreover, testes are one of the most sensitive organs against the unwanted stress, environmental and heat pollutants [Citation32]. In a number of prior reports as tests for different molecular models in both in vitro and in vivo, treatment with environmental chemicals as pesticides can induce cell apoptosis (mitochondrial pathway) in other organisms [Citation33–35] and this is true for CYP [Citation36–40].
As a response to various neurotoxins, some transcription factors which regulates neuronal cell death are p53 and Cas3 [Citation41,Citation42]. Bcl-2 is one member of the Bcl-2 anti-apoptosis protein family. If the anti-apoptotic protein is lower than the pro-apoptotic protein then apoptosis will occur [Citation43].
Therefore, our investigation was designed to (i) study the probable p53, Cas3 and Bcl-2 role in CYP-mediated stimulation of apoptosis in brain and testis of adult albino male rats’ cells besides the biochemical alterations of reproductive hormones by long-term exposure to CYP and (ii) the ameliorative effect of S. oil administration. In our results, treatment with S. oil without exposure to CYP revealed no significant variations in all investigated parameters compared to the normal control group showing its safe usage.
Processes of apoptotic signalling are associated with variations of apoptotic molecules, including cytochrome c and p53 [Citation44]. When the cell has been in an apoptotic state, p53 activation is an effective indicator and/ as well as the release of the cytochrome c into the cytosol from the mitochondria [Citation45]. Besides, the reduction in the Bcl-2 is considered a significant correlation in cell death or apoptosis [Citation46]. Apoptotic protease activating factor-1 (Apaf1) interacts with cytochrome c in the cytosol to activate caspase 9 [Citation10]. Then, this initiator caspase activates one of the important regulators of apoptosis, Cas3 [Citation47].
In the present study, the results obtained showed that CYP could be an apoptosis inducer and its effects are via activation of some apoptotic regulated genes in the brain and testis tissues, including p53 and Cas3 which were significantly up-regulated. In contrast, levels of anti-apoptotic genes as the Bcl-2 expression were down-regulated. The over-expression of pro-apoptotic gene of p53 which was observed in CYP group might be because of cell death as a result of interruption of energy utilization pathway induced by the CYP effect on ATP, and as a result, increase the up-regulation of Cas3 as abovementioned. Cas3 is a protein that has an essential role in both the death receptor and the mitochondrial pathway (extrinsic and intrinsic apoptosis, respectively) [Citation48]. Studies have revealed that the greater expression of cleaved-Cas3 is directly connected with apoptosis in different organisms [Citation49]. Several prior reports [Citation29,Citation50–52] presented parallel results from our study, where CYP induced apoptosis and up-regulated p53 and Cas3 gene expression besides the down-regulation of Bcl2 expression in neuronal cell and testis tissue. Also, studies of Molavi et al. [Citation53] and Indrayanti et al. [Citation43], which revealed that CYP induced RNA damage and over-expression of Cas3 and p53 in granulosa and theca cells besides decreased expression of Bcl-2 in the ovaries of CYP exposed group, are in accordance with our results.
Based on literature data and our results there is no doubt that CYP induces testicular toxicity and a neurotoxic impact which is demonstrated by induced apoptosis through up-regulation of p53 and Cas3 besides the down-regulation of Bcl2 expression in the brain and testis tissue. These effects are because of CYP induced oxidative stress which can cause lipid peroxidation [Citation54], cell structure damage and eventually induce DNA damage and apoptosis [Citation43]. These are demonstrated by several studies [Citation23,Citation55].
Recently, Sesamin was revealed to have neuroprotective roles in many cells and animal designs of neurological disease [Citation56–59]. Our biochemical investigation revealed that treatment with S. oil alleviated the brain and testis injury by reducing the levels of up-regulation of p53 and Cas3 expression and reversed the Bcl-2 levels in brain and testis tissues, as reported also by Ying et al. [Citation60]. Several evidences recommended that the S. oil protective effects against oxidative injury could be because of its anti-oxidative properties [Citation61] where oxidative stress can induce apoptosis [Citation62,Citation63]. Sesamol, sesamin and sesaminol as phenolic lignans-type compounds give S. oil its antioxidant activity [Citation60]. Also, S. oil contains vitamin E, which has a protective role against toxicity as reported by Abd El- Hameed et al. [Citation64]. Thus, S. oil could protect against the CYP toxicity through modulating the oxidative status as an antioxidant agent. Consequently, S. oil protect against lipid peroxidation, cell structure and DNA damage. This suggests that S. oil may contribute as an anti-apoptotic agent.
Some pyrethroids or their metabolites are regarded as effective endocrine disruptors in animals and for the reproductive system, which was considered as a sensitive target to these disruptors. CYP toxicity showed its activity by changes in hormones or enzymes and other molecules in serum of CYP-intoxicated rats [Citation65,Citation66].
In this study, we observed a reduction in serum T, LH and FSH level in the CYP exposed rats, but S. oil treatment enhanced their level. The diminution in serum T level is possible because of the direct effect of CYP on testicular tissue. On pesticide exposure, the reduction in pituitary gonadotrophins (LH and FSH) and T secretions have been previously reported [Citation67–70]. Reduction in T, LH and FSH suggest that away from testicular tissue, CYP may similarly be affected hypothalamus-pituitary axis. Leydig cells were stimulated by LH to produce the T, hence decrease in LH may additionally be a share factor for T low level. The low level of T, LH and FSH inhibit effective spermatogenesis, resulting in low numbers of functional sperms and low fertility [Citation69]. These suggest the reproductive toxicity of the CYP.
Also, CYP accumulation in testicular tissue enhanced oxidative stress [Citation71], causes extreme formation of free radical and membrane degeneration. Therefore, treatment with S. oil minimizes the damage caused by CYP accumulation by its free radical scavenging activity [Citation62]. S. oil enhanced T level, which may be because of the increase in cell viability. The increase in FSH and LH level may be because of the direct effect of S. oil on hypothalamus-pituitary axis [Citation72]. Thus, the alleviated effect of S. oil against CYP toxicity may be associated with its ability to inhibit the lipid peroxidation and its antioxidant effect.
5. Conclusion
We suggested that the levels of mRNA-expression of some main genes associated with cell apoptosis were significantly altered in CYP treated rats. Consistently, the expression of p53 and Cas3 were up-regulated in brain and testis tissue after CYP exposure besides the down-regulation of Bcl-2. These results revealed the ability of CYP to induce cell apoptosis and alteration in reproductive hormones. This study showed that S. oil ameliorated CYP induced testicular injury and neurotoxicity by acting as an anti-apoptotic agent and by enhancing the sex hormones level. Taken together, these results show that S. oil may be a possible therapeutic agent for the improvement of the injury of the brain and testis after exposure to CYP. Further researches are required to understand and comprehend the mechanism of actions purified molecules of S. oil on the reproductive system.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Brack W, Schirmer K, Kind T, et al. Effect-directed fractionation and identification of cytochrome P4501A-inducing halogenated aromatic hydrocarbons in a contaminated sediment. Environ Toxicol Chem. 2002;21(12):2654–2662.
- Díez S, Ábalos M, Bayona JM. Organotin contamination in sediments from the Western Mediterranean enclosures following 10 years of TBT regulation. Water Res. 2002;36(4):905–918.
- Assayed ME, Khalaf AA, Salem HA. Protective effects of garlic extract and vitamin C against in vivo cypermethrin-induced teratogenic effects in rat bone-marrow. Mutat Res. 2010;48(11):3153–3158.
- Bolognesi C. Genotoxicity of pesticides: a review of human biomonitoring studies. Mutat Res – Rev Mutat Res. 2003;543(3):251–272.
- Ahmad L, Khan A, Khan MZ, et al. Cypermethrin induced anaemia in male rabbits. Pak Vet J. 2009;29(4):191–195.
- Sangha GK, Kaur K, Khera KS, et al. Toxicological effects of cypermethrin on female albino rats. Toxicol Int. 2011;18(1):5–8.
- Naz S, Rana SA, Javed M, et al. Effect of two different rodenticides on serum biochemistry of house rats (Rattus rattus). Pak Vet J. 2010;30(4):239–241.
- Glass R. Chronic and long-term effects of pesticides use in agriculture: current knowledge and limits. Toxicol Lett. 2008;180:S21–S23.
- David M, Shivakumar HB, Shivakumar R, et al. Toxicity evaluation of cypermethrin and its effect on oxygen consumption of the freshwater fish, Tilapia mossambica. Indian J Environ Toxicol. 2003;13:99–102.
- Pallardy M, Biola A, Lebrec H, et al. Assessment of apoptosis in xenobiotic-induced immunotoxicity. Methods A Companion to Methods Enzymol. 1999;19(1):36–47.
- Sood R. Medical laboratory technology method and interpretations. 5th ed. London: Jaypee Brothers Medical Publishers (P) Ltd; 2006.
- Yousef MI, El-Demerdash FM, Kamel KI, et al. Changes in some hematological and biochemical indices of rabbits induced by isoflavones and cypermethrin. Toxicology. 2003;189(3):223–234.
- Steel RGD, Torrie JH. Principle and procedure of statistics. A biometrical approach. New York: Mc Gvaus-Hill Book Company; 1989.
- Li Y, Wei L, Cao J, et al. Oxidative stress, DNA damage and antioxidant enzyme activities in the pacific white shrimp when exposed to acute pH stress. Chemosphere. 2009;144:234–240.
- Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science (80-). 1998;281(5381):1322–1326.
- Shittu Lukeman AJ, Shittu Remilekun K, Olufemi O, et al. Hypoglycaemia and improved testicular parameters in Sesamum radiatum treated normo-glycaemic adult male Sprague Dawley rats. African J Biotechnol. 2009;8(12):2878–2886.
- Konan AB, Datté JY, Yapo PA. Nitric oxide pathway-mediated relaxant effect of aqueous sesame leaves extract (Sesamum radiatum Schum. & Thonn.) in the guinea-pig isolated aorta smooth muscle. BMC Complement Altern Med. 2008;8:1–8.
- Shittu L, Bankole MA, Oguntola JA, et al. Sesame leaves intake improve and increase epididymal spermatocytes reserve in adult male Sprague Dawley rat. Sci Res Essays. 2007;2(8):319–324.
- Sankar D, Sambandam G, Ramakrishna Rao M, et al. Modulation of blood pressure, lipid profiles and redox status in hypertensive patients taking different edible oils. Clin Chim Acta. 2005;355(1–2):97–104.
- Hsu DZ, Chen KT, Chien SP, et al. Sesame oil attenuates acute iron-induced lipid peroxidation-associated hepatic damage in mice. Shock. 2006;26(6):625–630.
- Shuaib MDDM. Effect of energy drinks on rat hippocampus and the possible neuroprotective role of sesame oil: histological, immunohistochemical and molecular study. Med J Cairo Univ. 2019;87(9):3871–3881.
- U.S. Environmental Protection Agency. Jan. 3, 1989. Pesticide Fact Sheet Number 199: Cypermethrin. USEPA, Office of Pesticide Programs, Registration Div., Washington DC.
- Maund SJ, Travis KZ, Hendley P, et al. Probabilistic risk assessment of cotton pyrethroids: V. Combining landscape-level exposures and ecotoxicological effects data to characterize risks. Environ Toxicol Chem. 2001;20(3):687–692.
- Bradberry SM, Cage SA, Proudfoot AT, et al. Poisoning due to pyrethroids. Toxicol Rev. 2005;24(2):93–106.
- Whiteman M, Chu SH, Siau JL, et al. The pro-inflammatory oxidant hypochlorous acid induces Bax-dependent mitochondrial permeabilisation and cell death through AIF-/EndoG-dependent pathways. Cell Signal. 2007;19(4):705–714.
- Kaur J, Sandhu HS. Subacute oral toxicity of cypermethrin and deltamethrin in buffalo calves. Indian J Anim Sci. 2001;71(12):1150–1152.
- Singh A, Mudawal A, Shukla RK, et al. Effect of gestational exposure of cypermethrin on postnatal development of brain cytochrome P450 2D1 and 3 A1 and neurotransmitter receptors. Mol Neurobiol. 2014;52(1):741–756.
- Singh A, Mudawal A, Shukla RK, et al. Effect of gestational exposure of cypermethrin on postnatal development of brain cytochrome P450 2D1 and 3A1 and neurotransmitter receptors. Mol Neurobiol. 2015;52(1):741–756.
- Pandey A, Jauhari A, Singh T, et al. Transactivation of P53 by cypermethrin induced miR-200 and apoptosis in neuronal cells. Toxicol Res (Camb. 2015;4(6):1578–1586.
- Tan J, Soderlund DM. Actions of tefluthrin on rat Nav1.7 voltage-gated sodium channels expressed in Xenopus oocytes. Pestic Biochem Physiol. 2011;101(1):21–26.
- Oliveira EE, Du Y, Nomura Y, et al. A residue in the transmembrane segment 6 of domain I in insect and mammalian sodium channels regulate differential sensitivities to pyrethroid insecticides. Neurotoxicology. 2013;38:42–50.
- Aitken RJ, Roman SD. Antioxidant systems and oxidative stress in the testes. Oxid Med Cell Longev. 2008;1(1):15–24.
- Xu WN, Bin LW, Liu ZP. Trichlorfon-induced apoptosis in hepatocyte primary cultures of Carassius auratus gibelio. Chemosphere. 2009;77(7):895–901.
- Aluigi MG, Guida C, Falugi C. Apoptosis as a specific biomarker of diazinon toxicity in NTera2-D1 cells. Chem Biol Interact. 2010;187(1–3):299–303.
- Vaithinathan S, Saradha B, Mathur PP. Methoxychlor induces apoptosis via mitochondria- and FasL-mediated pathways in adult rat testis. Chem Biol Interact. 2010;185(2):110–118.
- Madsen C, Claesson MH, Röpke C. Immunotoxicity of the pyrethroid insecticides deltametrin and α-cypermetrin. Toxicology. 1996;107(3):219–227.
- Patel S, Pandey AK, Bajpayee M, et al. Cypermethrin-induced DNA damage in organs and tissues of the mouse: evidence from the comet assay. Mutat Res – Genet Toxicol Environ Mutagen. 2006;607(2):176–183.
- Shelley LK, Balfry SK, Ross PS, et al. Immunotoxicological effects of a sub-chronic exposure to selected current-use pesticides in rainbow trout (Oncorhynchus mykiss). Aquat Toxicol. 2009;92(2):95–103.
- Jurkiewicz M, Averill-Bates DA, Marion M, et al. Involvement of mitochondrial and death receptor pathways in tributyltin-induced apoptosis in rat hepatocytes. Biochim Biophys Acta – Mol Cell Res. 2004;1693(1):15–27.
- Chiappini F, Alvarez L, Lux-Lantos V, et al. Hexachlorobenzene triggers apoptosis in rat thyroid follicular cells. Toxicol Sci. 2009;108(2):301–310.
- Johnson MD, Yu L-R, Conrads TP, et al. Proteome analysis of DNA damage-induced neuronal death using high through put mass spectrometry. Cell Death Differ. 2000;7:868–879.
- D’Amelio M, Cavallucci V, Cecconi F. Neuronal caspase-3 signaling: not only cell death. Cell Death Differ. 2010;17(7):1104–1114.
- Indrayanti I, Rahardjo B, Sujuti H. Effects of per oral cypermethrin exposure on Bcl-2 expression in granulose cells and antral follicle count of Rattus norvegicus ovaries. Maj Obstet Ginekol. 2019;26(3):123.
- Zhao M, Zhang Y, Wang C, et al. Induction of macrophage apoptosis by an organochlorine insecticide acetofenate. Chem Res Toxicol. 2009;22(3):504–510.
- Wang T, Chen F, Chen Z, et al. Honokiol induces apoptosis through p53-independent pathway in human colorectal cell line RKO. World J Gastroenterol. 2004;10(15):2205–2208.
- Lessenger JE. Five office workers inadvertently exposed to cypermethrin. J Toxicol Environ Health. 1992;35(4):261–267.
- Lindenboim L, Yuan J, Stein R. Bcl-x(S) and Bax induce different apoptotic pathways in PC12 cells. Oncogene. 2000;19(14):1783–1793.
- Lavrik IN. Systems biology of apoptosis signaling networks. Syst Biol Apoptosis. 2010:1–203.
- Abu-Qare AW, Abou-Donia MB. Biomarkers of apoptosis: release of cytochrome c, activation of caspase-3, induction of 8-hydroxy-2′-deoxyguanosine, increased 3-nitrotyrosine, and alteration of p53 gene. J Toxicol Environ Heal – Part B Crit Rev. 2001;4(3):313–332.
- Raszewski G, Lemieszek MK, Łukawski K, et al. Chlorpyrifos and cypermethrin induce apoptosis in human neuroblastoma cell line SH-SY5Y. Basic Clin Pharmacol Toxicol. 2015;116(2):158–167.
- Chauhan LKS, Varshney M, Pandey V, et al. ROS-dependent genotoxicity, cell cycle perturbations and apoptosis in mouse bone marrow cells exposed to formulated mixture of cypermethrin and chlorpyrifos. Mutagenesis. 2016;31(6):635–642.
- Jin Y, Zheng S, Fu Z. Embryonic exposure to cypermethrin induces apoptosis and immunotoxicity in zebrafish (Danio rerio). Fish Shellfish Immunol. 2011;30(4–5):1049–1054.
- Molavi M, Razi M, Malekinejad H, et al. Vitamin E improved cypermethrin-induced damages in the ovary of rats; evidence for angiogenesis and p53 involvement. Pestic Biochem Physiol. 2014;110(1):27–35.
- Giray B, Gürbay A, Hincal F. Cypermethrin-induced oxidative stress in rat brain and liver is prevented by Vitamin E or allopurinol. Toxicol Lett. 2001;118(3):139–146.
- Abd EL-Reheem ES, Mahmoud AM, Abd El-Hameed AM, et al. Effect of cinnamaldehyde on the toxicity of cyclophosphamide in the testes of rats: influence of pre- and post-treatment schedule. Nat Sci. 2016;14:44–51.
- Hou RCW, Huang HM, Tzen JTC, et al. Protective effects of sesamin and sesamolin on hypoxic neuronal and PC12 cells. J Neurosci Res. 2003;74(1):123–133.
- Hou RCW, Wu CC, Yang CH, et al. Protective effects of sesamin and sesamolin on murine BV-2 microglia cell line under hypoxia. Neurosci Lett. 2004;367(1):10–13.
- Park HJ, Zhao TT, Lee KS, et al. Effects of (-)-sesamin on 6-hydroxydopamine-induced neurotoxicity in PC12 cells and dopaminergic neuronal cells of Parkinson’s disease rat models. Neurochem Int. 2015;83–84:19–27.
- Fujikawa T, Miguchi S, Kanada N, et al. Acanthopanax senticosus harms as a prophylactic for MPTP-induced Parkinson’s disease in rats. J Ethnopharmacol. 2005;97(2):375–381.
- Liu YL, Xu ZM, Yang GY, et al. Sesamin alleviates blood-brain barrier disruption in mice with experimental traumatic brain injury. Acta Pharmacol Sin. 2017;38(11):1445–1455.
- Hsu DZ, Liu MY. Effects of sesame oil on oxidative stress after the onset of sepsis in rats. Shock. 2004;22(6):582–585.
- Suman G, Naravaneni R, Jamil K. In vitro cytogenetic studies of cypermethrin on human lymphocytes. Indian J Exp Biol. 2006;44(3):233–239.
- Yin Y, Terauchi Y, Solomon GG, et al. Involvement of p85 in p53-dependent apoptotic response to oxidative stress. Nature. 1998;391(6668):707–710.
- Abd El- Hameed AM, Soliman HA, Abd El- Reheem ES. Protective role of wheat germ oil in clozapine-induced oxidative stress and biochemical alterations in liver of male albino rats. J Am Sci. 2013;9(1):1782–1789.
- Stamati PN, Hans L, Howard CV. Pesticides as endocrine disrupters: identification of hazards for female reproductive function. Reprod Heal Environ. 2007;2:227–248.
- Zalata A, Atwa A, El-Naser Badawy A, et al. Tumor necrosis factor-α gene polymorphism relationship to seminal variables in infertile men. Urology. 2013;81(5):962–966.
- Biswas NM, Ghosh P. Effect of lead on male gonadal activity in albino rats. Kathmandu Univ Med J. 2004;2((1):43–46.
- Pareek TK, Joshi AR, Sanyal A, et al. Insights into male germ cell apoptosis due to depletion of gonadotropins caused by GnRH antagonists. Apoptosis. 2007;12(6):1085–1100.
- Monet Kuntz C, Hochereau de Reviers MT, Terqui M. Variations in testicular androgen receptors and histology of the lamb testis from birth to puberty. J Reprod Fertil. 1984;70(1):203–210.
- Joshi SC, Bansal B, Jasuja ND. Evaluation of reproductive and developmental toxicity of cypermethrin in male albino rats. Toxicol Environ Chem. 2011;93(3):593–602.
- Sharma P, Singh R. Protective role of curcumin on lindane induced reproductive toxicity in male wistar rats. Bull Environ Contam Toxicol. 2010;84(4):378–384.
- El-Sheshtawy SM, El-Gobary GIA, Omar NA, et al. Ameliorating the toxic effects of cypermethrin by sesame oil in male rabbits. Slov Vet Res. 2019;56:51–59.
