ABSTRACT
This study evaluated the anti-diabetic and hepatic protective effect of the aqueous extract of the Aloe perryi (AP) in streptozotocin-induced diabetic rats. Rat were divided into control, diabetic, diabetic + glimepiride, diabetic + AP (150 or 300 mg/Kg) groups. AP (300 mg/Kg) alone lowered fasting serum glucose levels but increased insulin levels and HOMA-β. At both doses, AP significantly decreased hepatic levels of CHOL and LDL-C and reduced serum levels of TGs, CHOL, and LDL-C, without altering levels of HDL-C in the diabetic rats. Also, they improved liver architectures and reduced serum levels of ALT and AST. Concomitantly, they suppressed hepatic levels of MDA, and increase hepatic levels of SOD, GSH, CAT, and GPx. Except for lipids, the effect of the extract on all other parameters was more profound with the higher dose of the extract. In conclusion, AP extract exerts hypoglycaemic, insulin-releasing, and hepatic antioxidant potentials in diabetic rats.
Introduction
Diabetes mellitus is a complex disorder involving carbohydrates, fat, and protein metabolism [Citation1]. It results from either insulin deficiency, low insulin secretion, or reduced tissue sensitivity to insulin [Citation2]. Recent statistics show that over 425 million individuals worldwide have diabetes; which is estimated to increase to 642 million by 2045 [Citation3,Citation4]. Hypertension, dyslipidemia, obesity, and cardiovascular diseases are often associated with diabetes [Citation5,Citation6].
Some drugs are commonly used in diabetes management and dyslipidemia or atherosclerosis; however, these have disadvantages including low drug efficiency, toxicity, and side effects. For instance, after 5 years of treatment with sulfonylureas, 34% of diabetics reported a loss of treatment effectiveness [Citation7]. Another common side effect is hypoglycemia due to the over-stimulation of insulin release into the blood [Citation8]. Some glucose-lowering drugs such as thiazolidinediones may be associated with weight gain and increased low-density lipoprotein (LDL-C) levels [Citation9]. Such significant adverse reactions associated with conventional treatments mean it is essential to search for new, natural compounds to overcome the problems of hyperlipidemia and diabetes.
During the last decades, researchers have given much attention to the use of herbal medicines as a treatment of chronic disorders including diabetes and cardiovascular disease as they have fewer side effects and are cheaper [Citation10–13]. Besides, the world health organization (WHO) is encouraging the introduction and development of herbal medicine in primary health care [Citation14]. Within this view, more than 800 plants have been listed as conventional remedies for lowering hyperglycemia and hyperlipidemia [Citation15,Citation16].
The Aloe plant is a non-food shrub that has long fleshy leaves with serrated edges and a jelly-like content, yellow-orange tubular flowers, and tubular fruits [Citation17]. The plant is a member of the Liliaceae or the lily family [Citation18]. Currently, more than 500 different species were reported to belong to the Aloe family [Citation19], with the species A. vera to be the most studied [Citation17,Citation20]. The chemical analysis of the A. vera revealed the presence of vitamins (i.e. A, E, and C), enzymes (i.e. peroxidases, cellulase, lipase, alkaline phosphatase, carboxypeptidase; catalase, amylase, bradykinesia), and, hormones (i.e. Auxins and gibberellins), Mineral (i.e. Ca, Fe, Mg, Mn, Zn, etc), sugars and glycoproteins, phenolic compounds (i.e. Anthraquinones), fatty acids (i.e. β-sitosterol, campesterol, and lupeol), and essential amino acids (i.e. salicylic acid), lignins, and saponins [Citation17,Citation21]. Accordingly, the presence of such antioxidant, anti-inflammatory, antimicrobial, laxatives, anti-septic, wound-healing compounds allowed the Aloe species to be used in skincare, beauty materials production, food preservatives, and to treat various disorders such as diabetes mellitus (DM), cancer, bacterial and viral infections, inflammatory systemic and skin disorders such as dermatitis, psoriasis Vulgaris, and colitis [Citation17].
Aloe perryi (A. perryi) is grown in the most common Aloe species in the Arabian Peninsula. The extracts from the A. perryi have extensively studied in traditional medicine and used to treat eye infection and constipation with recent confirmed scientific evidence with promising anti-tumorigenesis, anti-bacterial, anti-parasite, and gastrointestinal regulatory effects [Citation22–25].
Recently, Aldayel et al. [Citation26] have characterized the chemical composition of A. perryi and A. vera methanolic extract and have shown that both extract are rich, anthrones, chromones, anthraquinones, flavonoids, where the levels of all these components are higher in A. perryi. Besides, the same authors have shown that the A. perryi and A. vera methanolic extract has a potent free radical scavenging and antioxidant abilities and can suppress the α-glucosidase enzyme, thus implicating postprandial hypoglycemic and anti-diabetic effects [Citation26,Citation20].
Up-to-date, no in vivo evidence supports the hypoglycemic and hypolipidemic effect of A. perryi in humans and animals, which was worthy to be considered. Therefore, in this study, we aimed to evaluate the hypoglycaemic, hypolipidemic, and hepatic protective effect of A. perryi Baker aqueous extract in streptozotocin-induced DM in rats.
Materials and methods
Plant material
Aloe perryi (A. perryi ) Baker (Saber Socotri) as a resin dried parts were obtained from the local market in Hadibu, Socotra Island, Yemen in 2017. The plant identified at the Pharmacognosy Department, Faculty of Pharmacy, Sana'a University, Yemen, (voucher specimen: Mo-Sq9) [Citation27].
Preparation of A. perryi Baker aqueous extract
Briefly, the A. perryi Baker resin dried powder (10 g) was added to 100 mL distilled water, sonicated for 5 min, and centrifuged at room temperature at a speed of 5000 rpm for 10 min to collect the supernatants. The remaining A. perryi Baker resin residues were re-extracted using a similar procedure and all supernatants were added together. The A. perryi Baker resin was dried under a rotatory evaporator and resin dried extract (2 g/10 g dried weight) was reconstituted in 20 mL distilled water to give a final concentration of 1 g/mL.
Estimation of the total phenolic content
Different concentrations of A. perryi Baker resin extracts (10, 20, 30, 40, and 50 mg/mL) were analyzed for total polyphenol content using Folin–Ciocalteu method [Citation28]. Aliquots of 0.5mL of standard solutions of gallic acid (0.2, 0.4, 0.6, 0.8, and 1mg/mL) or the A. perryi extract were mixed with 2 mL 2% sodium carbonate, 2.4 mL deionized water, and 0.1 mL Folin–Ciocalteu reagent. All samples were then incubated for 60 min at room temperature and the absorbance was measured at 750 nm against the blank (deionized water) using a spectrophotometer (Shimadzu, model 1650PC). The results were expressed as Gallic acid equivalents (GAE). All experiments were performed in 3-independent experiments.
Estimation of the total flavonoid content
Different concentrations of A. perryi Baker resin dried powder extracts (10, 20, 30, 40, and 50 mg/mL) were assessed for total flavonoid content using the formation of a flavonoid-aluminum complex as the basis of a colorimetric method [Citation29]. In brief, 0.5 mL of each standard catechin solution (0.2, 0.4, 0.6, 0.8, and 1mg/mL) and the A. perryi extracts were combined with 2.2 mL distilled water and 0.15 mL 5% NaNO2 solution and incubated at room temperature for 6 min. Then, 0.15 mL 10% AlCl3 solution was added to each sample and incubated at room temperature for another 6 min. After that, 2 mL 4% NaOH solution was added to each sample, vortexed well, and then incubated at room temperature for 15 min. The results were expressed as Catechin equivalents (CE). The absorbance was recorded at 510 nm. All experiments were performed in 3-independent experiments.
Animals
Male Albino Wistar rats (280–320g) were provided from the Experimental animal care center, College of Pharmacy, King Saud University, Saudi Arabia. Rats were kept in controlled environmental conditions in stainless steel cages with a 12h dark/light cycle, 22 ± 1°C, and 50-55% humidity, individually. The experimental protocol was approved by the institutional review board (IRB Number: 18-0050) of Princess Nourah Bint Abdulrahman University, Riyadh, KSA which allowed us to use up to 60 rats for this experiment.
Induction of experimental diabetes
This has been done in accordance with the method described by [Citation30]. Briefly, Streptozotocin (STZ) (CAS No. 18883-66-4; ThermoFisher, Germany) was freshly dissolved in warmed 0.1 M, sodium citrate buffer (pH=4.5) to the desired concentration. Each rat was intraperitoneal (i.p) injected with this STZ solution at a final concentration of 60 mg/Kg. During the first 12 h, all rats were provided with a 5% glucose solution to prevent sudden hypoglycemia. Three days later, rats with blood glucose levels of more than 250 mg/dL were considered diabetic and were included in this study.
Experimental procedure
After the induction of DM, control and diabetic rats were segregated randomly into 5 groups (n=10/each). (1) A control group: were control healthy non-diabetic rats: received an equivalent volume of distilled water as a vehicle; (2) DM1: were rats with pre-established type 1 DM and received an equivalent volume of distilled water, as a vehicle; (3) DM1 + GLB (appositive control group): were diabetic rats and were administered glimepiride (UFC Biotechnology, NY, United States) (10mg/Kg/day, i.p.) sulfonylureas that lower blood glucose levels and stimulate the release of insulin from the pancreatic β-cells [Citation31]; (4) DM1+AP150: were diabetic rats and administered A. perryi Baker aqueous extract (150 mg/Kg); and (5) DM1+AP300: were diabetic rats and administered A. perryi Baker aqueous extract (300 mg/Kg). The administration of the vehicle and the extract were given by gavage and the experiments were conducted for 6 weeks on a daily basis. Body weeks were measured every week.
Blood and tissue collection and processing
All rats fasted for 12 h after the last day of week 6 and then anesthetized (xylazine hydrochloride/ketamine hydrochloride (10 and 90 mg/Kg, i.p)) [Citation32]. Once anesthesia was confirmed, blood samples were withdrawn directly into EDTA and plain tubes, centrifuged 3000 rpm to collect sera, which were kept at at-20°C until the time of the analysis. After that, all rats were ethically killed, and their livers were removed, placed on ice-containing dishes, and cut into small pieces. Small parts were immersed in liquid nitrogen (N2) and stored at −80 for preparing homogenates and other biochemical analyses. Other pieces were fixed in 10-buffer formalin and transferred to the histology laboratory for evaluation. To prepare tissue homogenates for the biochemical analysis, parts of the livers were homogenized in 9 volumes of ice-cold PBS in ice, centrifuged (14,000×g/4°C/15 min), and collected supernatants were frozen −80°C for further biochemical analysis.
Hepatic lipid extraction
This has been done to measure various lipids in frozen liver using the chloroform/methanol protocol as previously described [Citation33,Citation34]. Briefly, 1 g of frozen livers was homogenized with chloroform and methanol solution (19 mL, 2:1, v/v) for 3 times each of 3 min in ice. Then, the samples were cooled in the fridges for 60 min and then centrifuged (1400 xg/4°C/10 min). For each sample, the lower organic layer containing the lipid fractions was removed and the solvent was evaporated in a rotatory evaporator. The resultant dried material was reconstituted in 200 µl isopropanol. All samples were stored at −20°C and used later for the analysis of various lipids using the provided kits.
Protein extraction
This has been performed as described by others [Citation35]. Briefly, equal tissue weights (0.25 g) from each frozen liver sample was homogenized in 10 mL 0.1 M NaOH/3.5% NaCl solution. All tubes were incubated at 60 °C for 1.5 h and then centrifuged (4°C/30 min/4000 ×g) to collect the supernatants which were frozen at −20 °C and used later to calculated protein concentrations using the modified Lawry method [Citation36].
Biochemical analysis in the serum
Fasting serum glucose and insulin levels were measured using the respective colorimetric and ELISA assay kits (Cat. No. 10009582 & Cat. No. 589501, Cayman Chemical, Ann Arbor, MI, USA, respectively). All analyses were conducted per the manufacturer's instruction. In both analyses, an M-2 Spectramax microplate reader was used for reading the absorbance of all wells. The homeostasis model assessment of β-cell function (HOMA-β) was calculated using the following formula (20 × fasting insulin/fasting glucose − 3.5). The homeostasis model assessment of insulin resistance (HOMA-IR) index was calculated according to the following formula [fasting insulin (ng/mL) × fasting blood glucose (mg/dL)/405].
Determination of serum levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST)
Serum levels of ALT were determined using a colorimetric assay kit (Cat. No. MBS2540581, MyBioSource, CA, USA). The method of analysis depends on measuring the levels of pyruvic acid phenylhydrazone that form a series of reactions that involve (1) conversion of alanine and α-ketoglutarate to pyruvic acid and (2) the interaction of this acid with phenylhydrazine. The levels of AST in all sera samples were measured using an assay kit (Cat. No. MBS2540582, MyBioSource, CA, USA). The principle of the test depends on the ability of AST to generate oxaloacetic acid from the transfer of keto and amino groups between aspartic acid and α- ketoglutaric acid. A next decarboxylation step of the oxaloacetic acid produces pyroracemic acid which can react with 2,4- dinitrophenylhydrazine (DNPH) to yield a stable molecule named 2,4-dinitrophenylhydrazone. In both tests, all measurements were done at 50 nm using an M-2 Spectramax microplate reader.
Determination of serum and hepatic lipids
The measurement of both CHOL and HDL-C in both the sera samples and liver extracts was using a commercial fluorometric assay kit (Cat. No. STA-384, Cellbiolabs, CA, USA). The principle of the assay is to measure H2O2 levels (at 570 nm) produced from the hydrolysis of CHOL esters CHOL (by CHOL esterase) to CHOL, which is then oxidized by certain reagents to CHOL 4-en-3-one and hydrogen peroxide (H2O2). The total concentrations of HDL-C or CHOL in every sample was calculated using a specific standard curve. The levels of TGs in the sera samples and liver extracts samples were calculated using a commercially available colorimetric assay kit (Cat. NO. STA-396, Cellbiolabs, CA, USA). In the test, the lipase converts total TGs to produce free glycerol. A next oxidation reaction of the free glycerol produces H2O2, which can be detected at 570 nm. The levels of LDL-C were calculated in the sera samples and liver extracts using a commercially available assay kit (Cat. No. 80069, Crystal Chem, CA, USA). The principle of the test is to prevent the oxidation of various lipids (i.e. chylomicron, LDL-C, VLDL-C) from oxidation by with CHOL oxidase and CHOL esterase by precipitating them after interaction with and polyethylene-glycol methyl ether (PEGME) and polyvinyl sulfonic acid (PVS) whereas the unconjugated HDL is oxidized by these enzymes. Then LDL-C is released from this complex and undergoes certain reactions to produce H2O2, which was measured at an absorbance of 600 nm (M-2 Spectramax microplate reader). All analyses were performed according to the manufacturer's protocols.
Biochemical determination in the liver homogenates
Levels of Malondialdehyde (MDA), superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx), in all liver homogenates, were measured using a specific rat’s ELISA kit (Cat. No. MBS738685, Cat. No. MBS036924; Cat. No. MBS9712526, and Cat. No. MBS774703 MyBioSource, CA, USA, respectively). Total homogenate levels of reduced glutathione (GSH) were measured using an assay kit (Cat# 7511-100-K, R&D systems, MN, USA). All analyses were performed per the manufacturer’s established methods. The absorbance of each well plate for each analysis was performed on the M-2 Spectramax microplate reader.
Histological evaluation
This has been done by staining the tissues with hematoxylin and eosin (H and E). In brief, freshly collected tissues were fixed in 10% buffered formalin solution for 20 h. After that, dehydration was performed in ascending alcohol concentration. The following clearance step was done in xylene. After this step, the tissues parasitized, cut at 3-5 µM, and then stained with H&E. Photographs were captured using Nikon Eclipse light microscope (model ME600).
Statistical analysis
Statistical analysis was done on Graphpad Prism software (version 8). The differences between body weights over the different periods of types between all groups of rats were analyzed using 2-way ANOVA followed by Tukey’s t-test. Differences between all other measured parameters were done using 1-way ANOVA and Newman–Keuls Multiple Comparison Test. All data were given as mean ± standard deviation (M ± SD). The degree of significance was considered at p < 0.05.
Results
Total polyphenol and flavonoid contents
We have determined the phenolic and flavonoid contents in increasing concentrations of the A. perryi aqueous extract. We have found increased concentrations of both phenolic and flavonoid contents with increasing the extract concentration (Table ). However, at any measured concentration, the phenol contents were higher than the flavonoid contents (Table ). Total phenols varied from 27-76mg GAE whereas flavonoid content ranged from 24-53mg CE (Table ).
Table 1. Total flavonoids and phenolic content in the aqueous extract of the Aloe perryi Baker.
Effect of A. perryi extracts on body weight
The weekly alterations in rats’ body weights through the experimental period are depicted in Table . A significant weight loss was observed in diabetic rats (DM1) starting from week 1 until the end of week 6 (Table ). All other tested groups including DM1 + GLB, DM1 + AP150, and DM1 + AP300 showed a significant decrease all through the 6 weeks as compared to control rats (Table ). However, when compared to DM1-induce rats, body weights were significantly increased in the DM1 + GLB and DM1 + AP300 during the 4th, 5th, and 6th week. However, the administration of AP150 significantly but slightly increased the final body weights of treated rats as compared to diabetic rats weeks only by the end of week 6. However, the ANOVA analysis revealed that the increase in final body weights in DM1 + GLB and DM1 + AP300 were not significantly varied during the 5th and 6th weeks of the experiments as compared with each other but were significantly higher than those seen in DM1 + AP150 (Table ).
Table 2. The weekly change in body weight (g) of all experimental groups of rats.
Effect of A. perryi extracts on glucose and insulin levels
Fasting serum levels of glucose were significantly increased whereas fasting insulin levels were significantly decreased in DM1-induced rats as compared to control non-diabetic rats (Figure (A,B)). Besides, calculated HOMA-IR and HOMA-β were significantly decreased in DM1-induced rats as compared to control rats (Figure (C,D)). However, the levels of glucose were significantly decreased and the levels of fasting insulin, HOMA-IR and HOMA-β were significantly increased in DM1 + GLB and DM1 + AP300-treated rats as compared to DM1-induced rats with no significant differences among them when compared to each other (Figure (A-D)). However, although DM1 + AP150 showed a significant increase in fasting glucose levels, they showed no significant levels of fasting insulin, HOMA-IR and HOMA-β as compared to DM1-induced rats or other treated groups (Figure (A-D)).
Figure 1. Serum levels of fasting glucose (A) and fasting insulin (B) levels, as well as calculated homeostasis model assessment of insulin release, β cell function (HOMA-β) and insulin resistance (HOMA-IR) in all experimental groups of rats. Data are expressed as mean ± SD (n=10). Values were considered significantly different at p < 0.05. a: significantly different as compared to control non-diabetic rats. b: significantly different as compared to streptozotocin-induced diabetic rats (DM1). c: significantly different as compared to DM1 + glimepiride (GLB)-treated rats. d: significantly different as compared to DM1 + Aloe perryi (AP) (150 mg/Kg)-treated rats. AP300: Aloe perryi (AP) (300 mg/Kg). HOMA-IR = [fasting insulin (ng/mL) × fasting blood glucose (mg/dL)/405], and HOMA-β = 20 × fasting insulin/fasting glucose − 3.5.
![Figure 1. Serum levels of fasting glucose (A) and fasting insulin (B) levels, as well as calculated homeostasis model assessment of insulin release, β cell function (HOMA-β) and insulin resistance (HOMA-IR) in all experimental groups of rats. Data are expressed as mean ± SD (n=10). Values were considered significantly different at p < 0.05. a: significantly different as compared to control non-diabetic rats. b: significantly different as compared to streptozotocin-induced diabetic rats (DM1). c: significantly different as compared to DM1 + glimepiride (GLB)-treated rats. d: significantly different as compared to DM1 + Aloe perryi (AP) (150 mg/Kg)-treated rats. AP300: Aloe perryi (AP) (300 mg/Kg). HOMA-IR = [fasting insulin (ng/mL) × fasting blood glucose (mg/dL)/405], and HOMA-β = 20 × fasting insulin/fasting glucose − 3.5.](/cms/asset/881096cd-ea47-4802-ab04-ca3db8852ff0/tusc_a_1855859_f0001_ob.jpg)
Effect of A. perryi extracts on lipid profile
Serum and hepatic levels of CHOL, TGs, LDL-C were significantly increased whereas serum and hepatic levels of HDL-C were significantly decreased in DM1-induced rats as compared to control non-diabetic rats (Figure (A-D) and Figure (A-D)). However, DM1 + GLB showed a significant reduction in serum and hepatic levels of CHOL, TGs, and LDL-C as compared to DM1-induced rats (Figure (A-D) and Figure (A-D)). On the other hand, hepatic levels of CHOL and LDL-C, as well as serum levels of CHOL, TGs, and LDL-C were significantly decreased in both DM1 + AP150 and DM1 + AP300-treated rats and were not significantly different as compared to their levels in DM1 + GLB-treated rats (Figure (A-D) and Figure (A-D)). However, all treatments didn’t modify levels of HDL as compared to DM1-induced rats (Figure (A-D) and Figure (A-D)).
Figure 2. Serum levels of high-density lipoprotein cholesterol (HDL-C) (A), total triglycerides (TGs) (B), total cholesterol (CHOL) (C), and low-density lipoprotein cholesterol (LDL-C) (D) in all experimental groups of rats. Data are expressed as mean ± SD (n=10). Values were considered significantly different at p < 0.05. a: significantly different as compared to control non-diabetic rats. b: significantly different as compared to streptozotocin-induced diabetic rats (DM1). GLB: Glimepiride. AP150: Aloe perryi (150 mg/Kg). AP300: Aloe perryi (AP) (300 mg/Kg).
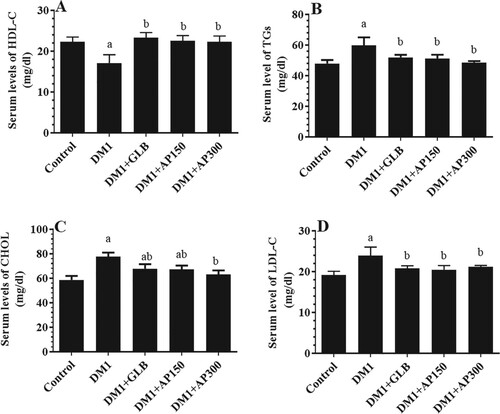
Figure 3. Hepatic levels of high-density lipoprotein cholesterol (HDL-C) (A), total triglycerides (TGs) (B), total cholesterol (CHOL) (C), and low-density lipoprotein cholesterol (LDL-C) (D) in all experimental groups of rats. Data are expressed as mean ± SD (n=10). Values were considered significantly different at p < 0.05. a: significantly different as compared to control non-diabetic rats. b: significantly different as compared to streptozotocin-induced diabetic rats (DM1). c: significantly different as compared to DM1 + glimepiride (GLB)-treated rats. AP150: Aloe perryi (150 mg/Kg). AP300: Aloe perryi (AP) (300 mg/Kg).
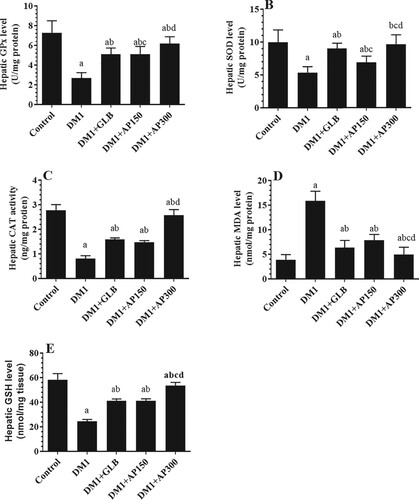
Effect of A. perryi extracts on liver function, architecture, and oxidative stress markers
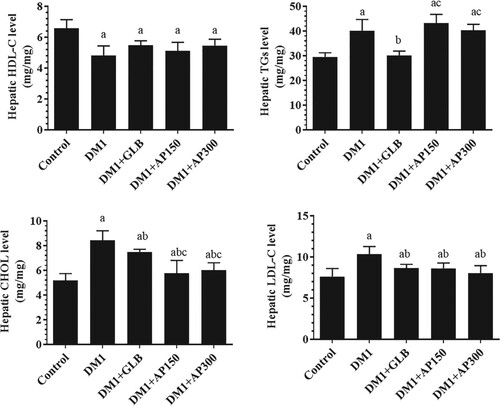
Circulatory levels of ALT and AST were significantly increased in the sera of DM1-induced rats as compared to control rats (Figure (A&B)). Besides, the liver section of these rats showed damaged and swelled hepatocytes, constricted central vein, increased accumulation of lipid vacuoles, and inflammatory cell infiltration (Figure (B-C)). Also, levels of SOD, GPx, CAT, and GSH were significantly decreased whereas hepatic levels of MDA were significantly increased in the liver homogenates of DM1-induced rats as compared to control rats (Figure (A-E)). Serum levels of both ALT and AST, as well as hepatic levels of MDA, were significantly decreased but hepatic levels of GSH, SOD, GPx, and CAT were significantly increased in all treated groups including DM1 + GLB, DM1 + AP150, and DM1 + AP300-treated rats as compared to DM1-induced rats (Figure (A&B) and Figure (A-E)). Moreover, the liver sections of all these treated groups showed big improvement with a significant reduction in hepatocyte damage, lipid accumulation, central vein diameter, and inflammatory cell infiltration (Figure (D-F)). However, the improvements in all these markers, as well as in the structures of the liver tissues were more profound in the diabetic rats, which were treated with GLB and AP300 (Figure (A&B), Figure (A-F), and Figure (A-E)).
Figure 4. Serum levels of alanine aminotransferase (ALT) (A) and aspartate aminotransferase (AST) (B) in all experimental groups of rats. Data are expressed as mean ± SD (n=10). Values were considered significantly different at p < 0.05. a: significantly different as compared to control non-diabetic rats. b: significantly different as compared to streptozotocin-induced diabetic rats (DM1). c: significantly different as compared to DM1 + glimepiride (GLB)-treated rats. d: significantly different as compared to DM1 + Aloe perryi (AP) (150 mg/Kg)-treated rats. AP300: Aloe perryi (AP) (300 mg/Kg).
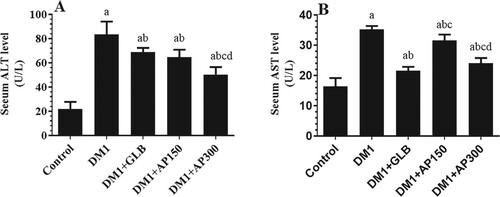
Figure 5. Histological features in the livers of all groups of rats as stained with hematoxylin and eosin (H&E) (200x). A: was taken form a control rat and showed normal liver architecture with normally sized hepatocytes (long arrow) radiating from a central vein with intact normal-sized sinusoids (short arrow). B and C: were taken from streptozotocin-induced diabetic rats (DM1) and showed small central vein, swelled (long arrow), and damaged (short arrow) hepatocytes. Lipid accumulation was evident in most of the hepatocytes with the appearance of lipid vacuoles (Red arrow) and inflammatory cells (Curved arrow). D, E, and F: were taken from DM1 + glimepiride (GLB), DM1 + Aloe perryi (AP) (150 mg/Kg), and DM1 + Aloe perryi (AP) (300 mg/Kg)-treated rats and showed much improvement in the structure of the hepatocytes. However, few fat vacuoles (short arrow) and less damaged hepatocytes (long arrow) are still observed in DM1 + glimepiride (GLB) and DM1 + Aloe perryi (AP) (150 mg/Kg)-treated groups. Almost normal liver structure was observed in DM1 + AP300-treated rats.
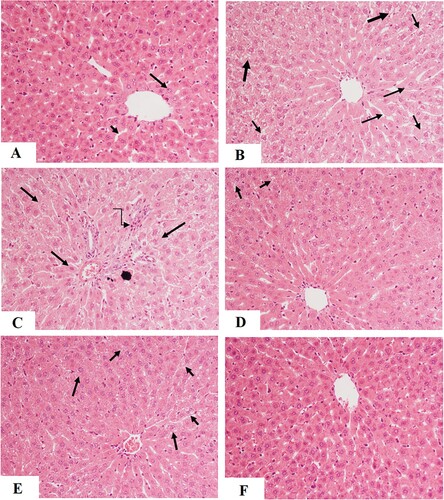
Figure 6. Hepatic levels of glutathione peroxidase (GPx) (A) superoxide dismutase (SOD) (B), catalase (CAT) (C), malondialdehyde (MDA) (D) and glutathione (GSH) (E) in all experimental groups of rats. Data are expressed as mean ± SD (n=10). Values were considered significantly different at p < 0.05. a: significantly different as compared to control non-diabetic rats. b: significantly different as compared to streptozotocin-induced diabetic rats (DM1). c: significantly different as compared to DM1 + glimepiride (GLB)-treated rats. d: significantly different as compared to DM1 + Aloe perryi (AP) (150 mg/Kg)-treated rats. AP300: Aloe perryi (AP) (300 mg/Kg).
Discussion
This study is the first one to examine the potential protective roles of A. perryi Baker against STZ-induced hyperglycemia, insulin deficiency, and liver damage. The main findings of this study showed that the aqueous water extract of the A. perryi, as tested at 150 and 300 mg/Kg, not only reduced fasting serum glucose levels and increased fasting serum insulin levels, but also suppressed hepatic CHOL and LDL-C synthesis and reduced serum levels of CHOL, LDL-C, and TGs in STZ-induced rats. Besides, both doses of the extract-improved architectures of the livers reduced serum injury marker enzymes and suppressed hepatic oxidative stress by attenuating lipid peroxidation and increasing the levels of SOD, CAT, GPx, and GSH. However, a more profound effect in all measured parameters was observed with the higher dose, an effect that was comparable to that afforded by Glimepiride.
Intraperitoneal administration of STZ (55-60 mg/Kg) is the most common protocol that induces T1DM in rats and results in similar manifestations to those reported in humans [Citation37]. In general, STZ is rapidly taken by the GLUT2 glucose transporter in the pancreatic β cells and induces their degeneration by acting through different mechanisms including the generation of carbonium radicals and highly reactive ROS, increasing the production of nitric oxide (NO) through activating nitric oxide synthases (NOS), inducing inflammatory cytokines production and DNA fragmentation and methylation [Citation37–41]. These events lead to the destruction of 60-80% of β-cells, thus reducing insulin production and inducing sustained hyperglycemia [Citation37]. Besides, STZ-induced T1DM is associated with severe loss of body weights due to increased muscle wasting, degradation of muscle proteins, and inducing adipose tissue and muscle glycolysis and lipolysis as a result of insulin deficiency [Citation42–46]. Of note, the test, HOMA-β is used usually to quantify β-cell function in T1DM patients and animals and is positively correlated with the cell mass [Citation47,Citation48]. On the other hand, HOMA-IR is the major index of insulin resistance and is always common with obesity, metabolic syndrome, and T2DM [Citation48].
Sulfonylureas such as glimepiride (Amaryl) and glibenclamide (glyburide) are anti-diabetic drugs to treat hyperglycemia in T1DM and T2DM as they stimulate the release of insulin production and secretion from the survival β cells through membrane depolarization-induced Ca+2 influx mediated by suppressing the membranous ATP-sensitive K+ channels [Citation49–51]. However, although, their use is restricted in humans due to poor bioavailability, limited action, the prevalence of hypoglycemia, and cardiovascular risk [Citation51], several studies have used sulfonylureas as a standard diabetic drug that can be compared to other anti-diabetic compounds [Citation50]. In this study, we have selected glimepiride as our reference drug at a dose (10 mg/Kg/day) that is very close and comparable to the one used in humans (8 mg/Kg) [Citation51] and compared its effects with those exhibited by A. perryi Baker extract.
Similar to these studies, we have also found higher fasting glucose levels, fasting reduced insulin levels, and low values of HOMA-β and HOMA-IR in STZ-induced diabetic rats, thus confirming a state of T1DM due to reduced insulin secretion. However, the reduction in HOMA-IR is expected in this animal model due to a lack of insulin rather than impairing its peripheral signaling. Also, diabetic rats showed a significant reduction in their final body weights which could be expiated by the associated hypoinsulinemia. On the other hand, the administration of glimepiride and A. perryi Baker extract (only at 300 mg/Kg) significantly increased blood insulin levels, reduced fasting hyperglycemia, and significantly improved rat’s final body weights to a level that is comparable to the one observed in glimepiride-treated rats. In this study, we have administered A. perryi extracts to diabetic rats at two doses 150 and 300 mg/Kg on daily basis [Citation51–53]. Since the studies on the anti-diabetic effect of this species are rare, we have adopted these doses from similar studies using the sister species A.vera which have been demonstrated to exhibit hypoglycaemic and insulin-releasing effect in rats when used at closer doses (200 and 300 mg/Kg) [Citation51–53]. Herein, the administration of A. perryi extracts to diabetic rats at a dose of 150 mg/Kg reduced fasting glucose levels with no alternation in the serum levels of insulin nor the levels of HOMA-β but reduced the values of HOMA-IR, thus indicating a possible role in improving peripheral insulin signaling at this low dose. On the contrary, the significant increase in fasting insulin levels and HOMA-IR with the concomitant reduction in the values of HOMA-β in the diabetic rats which received the higher dose of the extract suggest an insulin-releasing potential of the extract. However, the increase in HOMA-IR in this group of rats could be explained by the increase in remaining higher levels of glucose. Therefore, the increase in insulin levels/action could, at least, explain the significant progressive improvement in rats’ body weights. However, since food intake was not reordered in this study, it could be also possible that the extract may also participate in the increment of the body weights of rats by increasing their appetite. Unfortunately, food intake was not monitored in this study and could influence these result. Overall, these findings indicate that the hypoglycemic effect of A. perryi involves both increasing insulin secretion and insulin-independent hypoglycemic effect, which may involve improving insulin sensitivity. However, the hypoglycemic and insulin-releasing effect of glimepiride could be explained by the mechanism explained above.
However, our data still not sufficient to explain the precise mechanisms that explain the hypoglycemic effect of A. perryi. This could be mediated by stimulating the generation (proliferation) and/or increasing insulin secretion from the remaining β-cells, reducing ROS generation in β-cells, stimulating glucose peripheral disposal, and suppressing hepatic gluconeogenesis. At this stage, further studies are required to confirm these effects. Although these data are the first to be shown for A. perryi, several lines of evidence support the hypoglycaemic effects of other Aloe species at similar relevant doses. Within this view, administration of A.vera (A. barbadensis) gel extract or ethanolic leaves extract (200 and 300 mg/Kg) to control, glucose loaded, and or to STZ-diabetic rats significantly attenuated blood glucose levels and reduced the levels of glycosylated hemoglobin (Hb1AC) through suppressing the activity of gluconeogenic enzymes and possibly by increasing the survival of β-cells and/or stimulating insulin release from the β-cells [Citation51–53]. Similar hypoglycaemic effects of A. vera aqueous leaf extracts were also reported in STZ and alloxan-induced diabetic rats as well as in human subjects with T2DM at doses various ranges between 100-300 mg/Kg [Citation54–56].
Another important finding in this study is the anti-hyperlipidemia effect of the extract of A. perryi. In general, hepatic lipid metabolism is a very delicate balance that requires high expression of the Sterol regulatory element-binding proteins (SREBPs). In the liver, SREBP1 induces FAs and TGs synthesis whereas SREBP2 mainly regulates CHOL synthesis [Citation57]. Under normal conditions, insulin stimulates hepatic lipid synthesis by increasing the expression of SREBP1/2 [Citation58]. At the same time, insulin is required to activate the endothelium-bound glycoprotein enzyme, lipoprotein lipase (LPL) that hydrolyzes circulatory TGs and generates HDL-C [Citation59]. In the absence of insulin as in T1DM, the activity of LPL is diminished and the lipolysis in peripheral tissues is activated [Citation59]. These events lead to hypertriglyceridemia and an increased influx of free fatty acids (FFAs) to the liver, events that stimulate the synthesis of VLDL-C and LDL-C, and reduce the production of HDL-C, leading to dyslipidemia. Besides, although insulin is required for the activation of SREBP2, hyperglycemia alone, and independent of insulin, stimulates hepatic expression and activity of both SREBP1 and SREBP2, thus increasing hepatic cholesterol and TGs synthesis, leading to hepatic steatosis and dyslipidemia [Citation60,Citation61].
Indeed, STZ-induced DM is associated with hypercholesterolemia and hypertriglyceridemia that is characterized by high hepatic and circulatory levels of TGs, TC, LDL-C, and VLDL-C with a coincided reduction in HDL-C [Citation62]. These were also observed in the livers of STZ-induced T1DM in the rats of the current study, this confirming a lipid disorder. However, glimepiride significantly reduced hepatic and serum levels of TGs, CHOL, and LDL-C and significantly but slightly attenuated HDL-C levels. These effects could be explained basically by the concurrent hypoglycemic and insulin-releasing effects of this drug as previously demonstrated in patients with T1DM [Citation63]. Of note, the administration of both doses of A. perryi significantly reduced hepatic levels of CHOL and LDL-C but didn’t affect the hepatic levels of TGs. However, it significantly reduced serum levels of CHOL, LDL-C, TGs but didn’t modify levels of hepatic nor serum levels of HDL. The significant reduction in hepatic levels of TC and LDL-C indicates that the extract of A. perryi interferes with the de novo synthesis of CHOL possibly through suppressing intestinal cholesterol synthesis, inhibiting of SREBP2, or inactivating the hydroxymethyl glutaryl CoA (HMGCoA) reductase, the key enzymes in hepatic CHOL synthesis [Citation64]. However, since hepatic TGs content did not change in the livers of T1DM-induced rats after receiving the A. perryi extracts, we could expect that the extract has nothing to do with the expression/activation of SREB1 nor the process of fatty acids (FAs) synthesis or oxidation. Besides, the reduction in serum levels of TGs could be explained by the improvement of TGs levels due to the enhancement of LPL due to increased insulin levels, as discussed above. Nevertheless, it is well established that the levels of TGs synthesis are inversely proportional to the circulatory levels of HDL-C [Citation65]. Given the unchanged levels of TGs in the livers of diabetic rats, which administered both doses of A. perryi extract, it is reasonable to see low levels of circulatory HDL-C. However, these mechanisms need further clarification in more detailed experiments.
The hypolipidemic effect of Aloe plants was mostly demonstrated using the species A. vera. Indeed, administration of A. vera gel powder extract (150 mg/kg) to alloxan-induced diabetic rats significantly lowered serum levels of VLDL-C, LDL-C, TC, and TGs but increased serum levels of HDL-C [Citation64,Citation66]. In the same way, A. vera polysaccharides prevented the development of alcoholic fatty liver in rats by suppressing TGs synthesis and hepatic lipid accumulation through increasing the FAs oxidation mediated by increasing the expression and activity of AMPK/PPARα axis without affecting the expression of SREBP1 or FAs synthesis genes [Citation67]. Also, the administration of A. vera extracts (25, 50, or 100 mg/kg) to T2DM rats reduced hepatic TGs synthesis and prevented hepatic steatosis [Citation68]. Therefore, the variation in the effect of different Aloe species on TGs synthesis needs further investigation to accurately describe their hypolipidemic effects.
Nonetheless, DM is an oxidative stress disorder where an overproduction of ROS and depletion of antioxidants in various tissue are the major hallmarks of the disease and are responsible for several diabetic complications including retinopathy, nephropathy, cardiotoxicity, hepatic damage, and endothelial dysfunction [Citation69–72]. Hyperglycemia, and independent of any other factor is believed to be the sole major trigger that produces ROS and initiates oxidative stress in most tissues through different mechanisms including autoxidation, increasing the production of diacylglycerol (DAG) and advanced glycation products (AGE), and activation of numerous pathways such as protein kinase C (PKCs), hexosamine, and polyol pathways [Citation73]. In the liver, like any other tissue, ROS not only induces tissue damage by increasing lipid peroxidation but also overwhelms antioxidant enzymes and thiols, induces inflammation, inactivate proteins and other enzymes, and impairs insulin signaling, thus creating a positive feedback loop of sustained hyperglycemia [Citation69,Citation73,Citation74]. Of note, although ROS in DM is triggered by hyperglycemia during the early stages, their production during the later stages is not related to hyperglycemia [Citation69,Citation75]. Therefore, the use of drugs that control glucose levels was not shown to protect against DM-induced hepatic damage where concomitant antioxidant therapy was shown to be an effective strategy [Citation69,Citation75].
Hepatic damage due to hyperglycemia-induced oxidative stress is well documented in experimental animal models of STZ-induced DM where various plant extracts or the use of antioxidants afforded production [Citation75–78]. Similar to these studies, higher levels of hepatic injury markers (i.e. ALT and AST) with degenerative changes in the liver structure we observed in DM-induced rats. These effects were associated with increased hepatic MDA levels and concurrent decrease in the activities/levels of CAT, SOD, GPx, and GSH. However, the administration of glimepiride or A. perryi extract at both doses preserved liver structure and revised the alterations in the levels of serum liver markers and hepatic levels of MDA and antioxidant enzymes. These effects could be attributed to their observed hypoglycaemic effect. However, sulfonylureas such as Glibenclamide was shown to protect the livers and kidneys in STZ-treated rats by increasing SOD and CAT, independent of its hypoglycaemic effects, thus suggesting direct antioxidant effects [Citation79,Citation80]. In the same line, Gliclazide improved antioxidants in T2DM patients as well in experimental studies, in vivo, and in vitro [Citation81–83]. On the other hand, the antioxidant potential of A. perryi was demonstrated in vitro [Citation27]. Although the hepatoprotective effect of A. perryi was poorly investigated, many other studies support the hepatoprotective, ROS scavenging, and antioxidant stimulatory effects of A. vera, at concentration between 100-300 mg/kg, in several animal models of liver injury including STZ, carbon tetrachloride (CCL4), paracetamol, aflatoxins B1, alcohol, petroleum products, benzene hexachloride-induced hepatotoxicity [Citation50,Citation67,Citation84,Citation85,Citation86]. Besides, the antioxidant potential of A. vera was shown to be more effective than those afforded by α-tocopherol, in vitro [Citation87].
However, the medicinal beneficial effects of A. vera is attributed to its chemical composition that is rich vitamins, enzymes, hormones, minerals, sugars, flavonoids, glycoproteins, phenolic compounds, fatty acids, and essential amino acids, lignins, and saponins [Citation17,Citation21]. However, in this study, we have also shown that the extract A. perryi is very rich also in flavonoids and phenolic compounds, which are known for their antioxidant, hypolipidemic, and antioxidant effects [Citation88,Citation89]. A recent study investigates the full phytochemical analysis of A. perryi, HPLC-UV, and LC-MS showed that anthrones, and chromones as major phenolic contents [Citation26]. The presence of such ingredients may explain the observed biological effect of the extracts of A. perryi Baker in this study.
Despite this, this study still has some limitations. Besides, the effect of A. perryi on molecular pathways regulating intestinal lipid absorption, hepatic lipid synthesis, glucose metabolism, and insulin sensitivity and signaling was not investigated in this study to explain our descriptive results are highly encouraged to draw a possible mechanism of action. Besides, further studies are needed to determine the pharmacokinetics of A. perryi extract in the blood and tissues to determine their availability and effects. Furthermore, further studies are needed to extrapolate the doses used in the animals to humans by using more advanced mathematical models.
Conclusion
The findings of the present are introductory for future studies on the possible anti-diabetic effect of A. perryi Baker. Our data showed the ability of A. perryi Baker aqueous to reduce fasting blood glucose, increase insulin action, and attenuate abnormal lipid metabolism in STZ-diabetic rats. However, many tests and examinations are needed to understand the underlying mechanisms and identify the active ingredients.
Acknowledgements
The authors are grateful to the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia, (Grant No 39/S/241) for funding this work.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes
CE = catechin equivalent. GAE: gallic acid equivalent. Data were analyzed as mean ± SD of three experiments each performed in triplicates.
Data are expressed as mean ± SD (n=10/group) at p < 0.05. Means were compared using two-way ANOVA followed by Tukey’s t-test. *: significantly different as compared to initial body weight. a: significantly different as compared to control rats. b: significantly different as compared to STZ-induced diabetes mellitus rats (DM1), c: significantly different as compared to DM1 + glimepiride (GLB)-treated rats. c: significantly different as compared to DM1 + Aloe perryi (150 mg/kg) (AP150)-treated rats. d: significantly different as compared to DM1 + Aloe perryi (300 mg/kg) (AP300)-treated rats.
References
- Chukwuma CI, Matsabisa MG, Ibrahim MA, et al. Medicinal plants with concomitant anti-diabetic and anti-hypertensive effects as potential sources of dual acting therapies against diabetes and hypertension: A review. J Ethnopharmacol. 2019;235:329–360. doi:10.1016/j.jep.2019.02.024.
- Nazarian-Samani Z, Sewell RDE, Lorigooini Z, et al. Medicinal plants with multiple effects on diabetes mellitus and Its complications: a systematic review. Curr Diab Rep. 2018;18(10):72, Published 2018 Aug 13. doi:10.1007/s11892-018-1042-0.
- International Diabetes Federation (IDF). Key points for IDF Diabetes Atlas 2017. Diabetes research and clinical practice. Volume 64 - Issue 4 - December 2017.
- Herman WH. The global burden of diabetes: an overview. In: Dagogo-Jack S, editor. Diabetes mellitus in developing countries and underserved communities. Chem: Springer; 2017. p. 1–5. ISBN 978-3-319-41557-4.
- Parikh NI, Aurora MS, Dash R, et al. Assessment of obesity and cardiovascular risk in South Asians. Curr Cardiovasc Risk Rep. 2015;9:425, doi:10.1007/s12170-014-0425-2.
- Klop B, Elte JW, Cabezas MC. Dyslipidemia in obesity: mechanisms and potential targets. Nutrients. 2013;5(4):1218–1240. Published 2013 Apr 12. doi:10.3390/nu5041218.
- Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy [published correction appears in N Engl J Med. 2007 Mar 29;356(13):1387-8]. N Engl J Med. 2006;355(23):2427–2443. doi:10.1056/NEJMoa066224.
- Upadhyay J, Polyzos SA, Perakakis N, et al. Pharmacotherapy of type 2 diabetes: An update. Metab Clin Exp. 2018;78:13–42. doi:10.1016/j.metabol.2017.08.010.
- Dey L, Attele AS, Yuan CS. Alternative therapies for type 2 diabetes. Altern Med Rev. 2002;7(1):45–58.
- Gupta PD, De A. Diabetes mellitus and its herbal treatment. Int J Res Pharm Biomed Sci. 2012;3(2):706–721.
- Tabassum N, Ahmad F. Role of natural herbs in the treatment of hypertension. Pharmacogn Rev. 2011;5(9):30–40. doi:10.4103/0973-7847.79097.
- DiNardo MM, Gibson JM, Siminerio L, et al. Complementary and alternative medicine in diabetes care. Curr Diab Rep. 2012;12(6):749–761. doi:10.1007/s11892-012-0315-2.
- Hamza N, Berke B, Umar A, et al. A review of Algerian medicinal plants used in the treatment of diabetes. J Ethnopharmacol. 2019;238:1–28. doi:10.1016/j.jep.2019.111841.
- de Boer HJ, Cotingting C. Medicinal plants for women's healthcare in Southeast Asia: a meta-analysis of their traditional use, chemical constituents, and pharmacology. J Ethnopharmacol. 2014;151(2):747–767. doi:10.1016/j.jep.2013.11.030.
- Kooti W, Farokhipour M, Asadzadeh Z, et al. The role of medicinal plants in the treatment of diabetes: a systematic review. Electron Physician. 2016;8(1):1832–1842. Published 2016 Jan 15. doi:10.19082/1832.
- Jacob B, Narendhirakannan RT. Role of medicinal plants in the management of diabetes mellitus: a review. 3 Biotech. 2019;9(1):4, doi:10.1007/s13205-018-1528-0.
- Surjushe A, Vasani R, Saple DG. Aloe vera: a short review. Indian J Dermatol. 2008;53(4):163–166. doi:10.4103/0019-5154.44785.
- Telefo PB, Moundipa PF, Tchouanguep FM. Oestrogenicity and effect on hepatic metabolism of the aqueous extract of the leaf mixture of Aloe buettneri, Dicliptera verticillata, Hibiscus macranthus and Justicia insularis. Fitoterapia. 2002;73(6):472–478. doi:10.1016/s0367-326x(02)00177-6.
- Cock IE. The Genus Aloe: phytochemistry and therapeutic uses including treatments for gastrointestinal conditions and chronic inflammation. Prog Drug Res. 2015;70:179–235. doi:10.1007/978-3-0348-0927-6_6.
- Kumar R, Singh AK, Gupta A, et al. Therapeutic potential of Aloe vera-A miracle gift of nature. Phytomedicine. 2019a;60:1–11. doi:10.1016/j.phymed.2019.152996.
- Radha MH, Laxmipriya NP. Evaluation of biological properties and clinical effectiveness of Aloe vera: A systematic review. J Tradit Complement Med. 2014;5(1):21–26. Published 2014 Dec 23. doi:10.1016/j.jtcme.2014.10.006.
- Ali NA, Jülich WD, Kusnick C, et al. Screening of Yemeni medicinal plants for antibacterial and cytotoxic activities. J Ethnopharmacol. 2001;74(2):173–179. doi:10.1016/s0378-8741(00)00364-0.
- Al-Fatimi M, Friedrich U, Jenett-Siems K. Cytotoxicity of plants used in traditional medicine in Yemen. Fitoterapia. 2005;76(3-4):355–358. doi:10.1016/j.fitote.2005.02.009.
- Mothana RA, Al-Musayeib NM, Matheeussen A, et al. Assessment of the in vitro antiprotozoal and cytotoxic potential of 20 selected medicinal plants from the island of Soqotra. Molecules. 2012;17(12):14349–14360. Published 2012 Dec 3. doi:10.3390/molecules171214349.
- Al-Oqail MM, El-Shaibany A, Al-Jassas E, et al. In vitro anti-proliferative activities of Aloe perryi flowers extract on human liver, colon, breast, lung, prostate and epithelial cancer cell lines. Pak J Pharm Sci. 2016;29(2 Suppl):723–729.
- Aldayel TS, Grace MH, Lila MA, et al. LC-MS characterization of bioactive metabolites from Two Yemeni Aloe spp. with antioxidant and antidiabetic properties. Arab. J. Chem. 2020;2020(13):5040–5049. doi:10.1016/j.arabjc.2020.02.003.
- Mothana RA, Lindequist U, Gruenert R, et al. Studies of the in vitro anticancer, antimicrobial and antioxidant potentials of selected Yemeni medicinal plants from the island Soqotra. BMC Complement Altern Med. 2009;9:7, Published 2009 Mar 25. doi:10.1186/1472-6882-9-7.
- Shaghaghi M, Manzoori JL, Jouyban A. Determination of total phenols in tea infusions, tomato and apple juice by terbium sensitized fluorescence method as an alternative approach to the Folin-Ciocalteu spectrophotometric method. Food Chem. 2008;108(2):695–701. doi:10.1016/j.foodchem.2007.11.008.
- Roshanak S, Rahimmalek M, Goli SA. Evaluation of seven different drying treatments in respect to total flavonoid, phenolic, vitamin C content, chlorophyll, antioxidant activity and color of green tea (Camellia sinensis or C. assamica) leaves. J Food Sci Technol. 2016;53(1):721–729. doi:10.1007/s13197-015-2030-x.
- Cheng D, Liang B, Li Y. Antihyperglycemic effect of Ginkgo biloba extract in streptozotocin-induced diabetes in rats. Biomed Res Int. 2013: 1–7. doi:10.1155/2013/162724.
- Sato J, Ohsawa I, Oshida Y, et al. Effects of glimepiride on in vivo insulin action in normal and diabetic rats. Diabetes Res Clin Pract. 1993;22(1):3–9. doi:10.1016/0168-8227(93)90126-p.
- Kim HC, Song JM, Kim CJ, et al. Combined effect of bisphosphonate and recombinant human bone morphogenetic protein 2 on bone healing of rat calvarial defects. Maxillofac Plast Reconstr Surg. 2015;37(1):16, Published 2015 Jul 2. doi:10.1186/s40902-015-0015-3.
- Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226(1):497–509.
- Al-Otaibia SN, Alshammari GM, AlMohanna FH, et al. Antihyperlipidemic and hepatic antioxidant effects of Leek leaf methanol extract in high fat diet-fed rats. Front Life Sci. 2020;13(1):373–385. doi:10.1080/26895293.2020.1792355.
- Mæhre HK, Dalheim L, Edvinsen GK, et al. Protein determination-method matters. Foods. 2018;7(1):5, Published 2018 Jan 1. doi:10.3390/foods7010005.
- Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265–275.
- Wang-Fischer Y, Garyantes T. Improving the reliability and utility of streptozotocin-induced Rat diabetic model. J Diabetes Res. 2018. 8054073. Published 2018 Sep 23. doi:10.1155/2018/8054073
- Cardinal JW, Margison GP, Mynett KJ, et al. Increased susceptibility to streptozotocin-induced beta-cell apoptosis and delayed autoimmune diabetes in alkylpurine-DNA-N-glycosylase-deficient mice. Mol Cell Biol. 2001;21(16):5605–5613. doi:10.1128/MCB.21.16.5605-5613.2001.
- Pacher P, Obrosova IG, Mabley JG, et al. Role of nitrosative stress and peroxynitrite in the pathogenesis of diabetic complications. Emerging new therapeutical strategies. Curr Med Chem. 2005;12(3):267–275. doi:10.2174/0929867053363207.
- Ghanema A II, Sadek KM. Olive leaves extract restored the antioxidant perturbations in red blood cells hemolysate in streptozotocin induced diabetic rats. World Acad Sci Eng Technol. 2012;6(4):124–130. doi:10.5281/zenodo.1083815.
- Sadek KM, Shaheen H. Biochemical efficacy of vitamin D in ameliorating endocrine and metabolic disorders in diabetic rats. Pharm Biol. 2014;52(5):591–596. doi:10.3109/13880209.2013.854812.
- Enoksson S, Caprio SK, Rife F, et al. Defective activation of skeletal muscle and adipose tissue lipolysis in type 1 diabetes mellitus during hypoglycemia. J Clin Endocrinol Metab. 2003;88(4):1503–1511. doi:10.1210/jc.2002-021013.
- Musabayane CT, Mahlalela N, Shode FO, et al. Effects of Syzygium cordatum (Hochst.) [Myrtaceae] leaf extract on plasma glucose and hepatic glycogen in streptozotocin-induced diabetic rats. J Ethnopharmacol. 2005;97(3):485–490. doi:10.1016/j.jep.2004.12.005.
- Montano ME, Molpeceres V, Mauriz JL, et al. Effect of melatonin supplementation on food and water intake in streptozotocin-diabetic and non-diabetic male Wistar rats. Nutr Hosp. 2010;25(6):931–938.
- Juárez-Rojop IE, Díaz-Zagoya JC, Ble-Castillo JL, et al. Hypoglycemic effect of Carica papaya leaves in streptozotocin-induced diabetic rats. BMC Complement Altern Med. 2012;12:236, Published 2012 Nov 28. doi:10.1186/1472-6882-12-236.
- Choudhary M, Aggarwal N, Choudhary N, et al. . effect of aqueous and alcoholic extract of Sesbania sesban (Linn.) Merr. root on glycemic control in streptozotocin-induced diabetic mice. Drug Dev Ther. 2014;5(2):115–122. DOI: 10.4103/2394-2002.139616.
- Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi:10.1007/BF00280883.
- Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27(6):1487–1495. doi:10.2337/diacare.27.6.1487.
- Proks P, Reimann F, Green N, et al. Sulfonylurea stimulation of insulin secretion. Diabetes. 2002;51(Suppl 3):S368–S376. doi:10.2337/diabetes.51.2007.s368.
- Rajasekaran S, Sivagnanam K, Subramanian S. Antioxidant effect of Aloe vera gel extract in streptozotocin-induced diabetes in rats. Pharmacol Rep. 2005;57(1):90–96.
- Kabadi UM, Kabadi M. Comparative efficacy of glimepiride and/or metformin with insulin in type 2 diabetes. Diabetes Res Clin Pract. 2006;72(3):265–270. doi:10.1016/j.diabres.2005.10.024. Epub 2006 Jan 10. PMID: 16406190.
- Rajasekaran S, Sivagnanam K, Ravi K, et al. Hypoglycemic effect of Aloe vera gel on streptozotocin-induced diabetes in experimental rats. J Med Food. 2004;7(1):61–66. doi:10.1089/109662004322984725.
- Ayesha NS, Gunasekaran A, Manickam S, et al. Antidiabetic activity of aloe vera and histology of organs in streptozotocin induced diabetic rats. Curr Sci. 2008;94(8):1070–1076. DOI:10.2307/24100806.
- Rajasekaran S, Ravi K, Sivagnanam K, et al. Beneficial effects of aloe vera leaf gel extract on lipid profile status in rats with streptozotocin diabetes. Clin Exp Pharmacol Physiol. 2006;33(3):232–237. doi:10.1111/j.1440-1681.2006.04351.x.
- Adesokan AA, Akanji MA, Aderibigbe A. Serum glucose and lipid levels in alloxan-induced diabetic rats following oral administration of aloe barbadensis miller juice extract. TJHS. 2006;13(2):11–14. DOI: 10.4314/tjhc.v13i2.36691.
- Sharma B, Siddiqui S, Ram G, et al. Hypoglycemic and hepatoprotective effects of processed Aloe vera Gel in a Mice model of alloxan induced diabetes mellitus. J Diabetes Metab. 2013;4:9, DOI: 10.4172/2155-6156.1000303.
- Moslehi A, Hamidi-Zad Z. Role of SREBPs in liver diseases: A Mini-review. J Clin Transl Hepatol. 2018;6(3):332–338. doi:10.14218/JCTH.2017.00061.
- Shimomura I, Bashmakov Y, Ikemoto S, et al. Insulin selectively increases SREBP-1c mRNA in the livers of rats with streptozotocin-induced diabetes. Proc Natl Acad Sci U S A. 1999;96(24):13656–13661. doi:10.1073/pnas.96.24.13656.
- Almeida D, Braga CP, Novelli ELB, et al. Evaluation of lipid profile and oxidative stress in STZ-induced rats treated with antioxidant vitamin. Braz Arch Biol Technol. 2012;55:527–536. doi:10.1590/S1516-89132012000400007.
- Mourrieras F, Foufelle F, Foretz M, et al. Induction of fatty acid synthase and S14 gene expression by glucose, xylitol and dihydroxyacetone in cultured rat hepatocytes is closely correlated with glucose 6-phosphate concentrations. Biochem J. 1997;326(Pt 2):345–349. doi:10.1042/bj3260345.
- Matsuzaka T, Shimano H, Yahagi N, et al. Insulin-independent induction of sterol regulatory element-binding protein-1c expression in the livers of streptozotocin-treated mice. Diabetes. 2004;53(3):560–569. doi:10.2337/diabetes.53.3.560.
- Andallu B, Vinay Kumar AV, Varadacharyulu N. Lipid abnormalities in streptozotocin-diabetes: Amelioration by Morus indica L. cv Suguna leaves. Int J Diabetes Dev Ctries. 2009;29(3):123–128. doi:10.4103/0973-3930.54289.
- Najim HD, Majeed IA, Rahmah AM. Effects of Metformin, glimepiride and their combination on glycemia and lipid profile of NIDDM patients-A study in Iraqis. Int J Adv Pharm Biol Chem. 2013;2(2):2277–4688.
- Friesen JA, Rodwell VW. The 3-hydroxy-3-methylglutaryl coenzyme-A (HMG-CoA) reductases. Genome Biol. 2004;5(11):248, doi:10.1186/gb-2004-5-11-248.
- Bitzur R, Cohen H, Kamari Y, et al. Triglycerides and HDL cholesterol: stars or second leads in diabetes? Diabetes Care. 2009;32(Suppl 2):S373–S377. doi:10.2337/dc09-S343.
- Yasin MS, Ferdosi MFH, Nasir F, et al. Effect of Aloe vera gel on lipid profile in alloxan induced diabetic mice. Mycopath. 2011;9(2):67–70.
- Cui Y, Ye Q, Wang H, et al. Hepatoprotective potential of Aloe vera polysaccharides against chronic alcohol-induced hepatotoxicity in mice. J Sci Food Agric. 2014;94(9):1764–1771. doi:10.1002/jsfa.6489.
- Kim K, Kim H, Kwon J, et al. Hypoglycemic and hypolipidemic effects of processed Aloe vera gel in a mouse model of non-insulin-dependent diabetes mellitus. Phytomedicine. 2009;16(9):856–863. doi:10.1016/j.phymed.2009.02.014.
- Maritim AC, Sanders RA. Watkins JB 3rd. diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol. 2003;17(1):24–38. doi:10.1002/jbt.10058.
- Styskal J, Van Remmen H, Richardson A, et al. Oxidative stress and diabetes: what can we learn about insulin resistance from antioxidant mutant mouse models? Free Radic Biol Med. 2012;52(1):46–58. doi:10.1016/j.freeradbiomed.2011.10.441.
- Schmatz R, Perreira LB, Stefanello N, et al. Effects of resveratrol on biomarkers of oxidative stress and on the activity of delta aminolevulinic acid dehydratase in liver and kidney of streptozotocin-induced diabetic rats. Biochimie. 2012;94(2):374–383. doi:10.1016/j.biochi.2011.08.005.
- Thakur P, Kumar A, Kumar A. Targeting oxidative stress through antioxidants in diabetes mellitus. J Drug Target. 2018;26(9):766–776. doi:10.1080/1061186X.2017.1419478.
- Ighodaro OM. Molecular pathways associated with oxidative stress in diabetes mellitus. Biomed Pharmacother. 2018;108:656–662. doi:10.1016/j.biopha.2018.09.058.
- Kakkar R, Kalra J, Mantha SV, et al. Lipid peroxidation and activity of antioxidant enzymes in diabetic rats. Mol Cell Biochem. 1995;151(2):113–119. doi:10.1007/BF01322333.
- Abolfathi AA, Mohajeri D, Rezaie A, et al. Protective effects of Green Tea extract against hepatic tissue injury in streptozotocin-induced diabetic rats. Evid Based Complement Alternat Med. 2012;740671. doi:10.1155/2012/740671
- Mehenni C, Atmani-Kilani D, Dumarçay S, et al. Hepatoprotective and antidiabetic effects of Pistacia lentiscus leaf and fruit extracts. J Food Drug Anal. 2016;24(3):653–669. doi:10.1016/j.jfda.2016.03.002.
- Taghizadeh M, Rashidi AA, Taherian AA, et al. The protective effect of hydroalcoholic extract of Rosa canina (Dog Rose) Fruit on liver function and structure in streptozotocin-induced diabetes in rats. J Diet Suppl. 2018;15(5):624–635. doi:10.1080/19390211.2017.1369205.
- Molehin OR, Oloyede OI. Attenuation of oxidative stress and hepatic damage by white butterfly (Clerodendrum volubile) leaves in streptozotocin-induced diabetes in rats. J Basic Clin Physiol Pharmacol. 2018;30(1):81–89. doi:10.1515/jbcpp-2018-0083.
- Elmalí E, Altan N, Bukan N. Effect of the sulphonylurea glibenclamide on liver and kidney antioxidant enzymes in streptozocin-induced diabetic rats. Drugs R D. 2004;5(4):203–208. doi:10.2165/00126839-200405040-00003.
- Siddique MAH, Begum A, Begum S, et al. Comparison of antioxidative effects of biguanides and sulfonylureas monotherapy on total antioxidant status in newly-diagnosed patients with type 2 diabetes mellitus. Diabetes Case Rep. 2016;1:1, DOI: 10.4172/2572-5629.1000104
- O'Brien RC, Luo M, Balazs N, et al. In vitro and in vivo antioxidant properties of gliclazide. J Diabetes Complications. 2000;14(4):201–206. doi:10.1016/s1056-8727(00)00084-2.
- Fava D, Cassone-Faldetta M, Laurenti O, et al. Gliclazide improves anti-oxidant status and nitric oxide-mediated vasodilation in type 2 diabetes. Diabet Med. 2002;19(9):752–757. doi:10.1046/j.1464-5491.2002.00762.x.
- Sliwinska A, Rogalska A, Szwed M, et al. Gliclazide may have an antiapoptotic effect related to its antioxidant properties in human normal and cancer cells. Mol Biol Rep. 2012;39(5):5253–5267. doi:10.1007/s11033-011-1323-z.
- Etim OE, Farombi EO, Usoh IF, et al. The protective effect of aloe vera juice on lindane induced hepatotoxicity and genotoxicity. Pak J Pharm Sci. 2006;19(4):337–340.
- Chandan BK, Saxena AK, Shukla S, et al. Hepatoprotective potential of Aloe barbadensis Mill. against carbon tetrachloride induced hepatotoxicity. J Ethnopharmacol. 2007;111(3):560–566. doi:10.1016/j.jep.2007.01.008.
- Gbadegesin MA, Odunola OA, Akinwumi KA, et al. Comparative hepatotoxicity and clastogenicity of sodium arsenite and three petroleum products in experimental Swiss Albino Mice: the modulatory effects of Aloe vera gel. Food Chem Toxicol. 2009;47(10):2454–2457. doi:10.1016/j.fct.2009.07.002.
- Hu Q, Hu Y, Xu J. Free radical-scavenging activity of Aloe vera (Aloe barbadensis Miller) extracts by supercritical carbon dioxide extraction. Food Chem. 2005;91(1):85–90. DOI: 10.1016/j.foodchem.2004.05.052.
- Kumar S, Yadav A, Yadav M, et al. Effect of climate change on phytochemical diversity, total phenolic content and in vitro antioxidant activity of Aloe vera (L.) Burm.f. BMC Res Notes. 2017;10(1):60, Published 2017 Jan 25. doi:10.1186/s13104-017-2385-3.
- Hossain MA, Muhammad DS. A study on the total phenols content and antioxidant activity of essential oil and different solvent extracts of endemic plant Merremia borneensis. Arab J. Chem. 2005;8(1):66–71. doi:10.1016/j.arabjc.2011.01.007.
