 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The present work aims to present two new concepts concerned with chiral separation. First, chiral separation of some enantiomeric drugs, using micelle-based liquid chromatography without any chiral selector, whether in the stationary phase or the mobile phase, was performed. The surfactants used were anionic; sodium dodecyl sulphate, and cationic; cetrimide. The studied analytes include phenyl-ephrine hydrochloride (PHR), cetirizine dihydrochloride (CTZ), and mebeverine hydrochloride (MBV). Successful separation of the enantiomers was attained, giving promising results that may cause a revolution in chiral separation, predicting the possibility of simply prepared aqueous micellar mobile phases of carrying chiral selector ability. The second motive of this work is studying the compatibility of hybrid micelle liquid mobile phases with chiral columns separating CTZ, using cellulose 1 chiral column as a model example. This study may be an environmentally safe substitute for the normal phase mode of elution that is usually applied for chiral separation consuming large amounts of hazardous organic solvents.
1. Introduction
Enantiomers of racemic drugs carry divergent pharmacological activities, accompanied with different pharmacokinetic and pharmacodynamic parameters [Citation1–3]. The pharmacologically inactive enantiomer may even cause undesirable side effects, which motivated analysts to perform chiral separation of several pharmaceuticals [Citation4–7] aiming to obtain maximum purity of the active enantiomer.
Carrying the isomeric nature, resolution of the racemates, comprising the enantiomeric mixture, becomes a real challenge for analysts, where isomers –in an environment lacking chirality – have identical physical and chemical properties [Citation8]. Hence, upon utilizing chromatography as a separation tool, two approaches are adopted; either converting the isomers to diastereoisomers (which are characterized by discrete physical criteria and different chemical behaviour) through combination with a chiral reagent, where separation in this case could be achieved in an achiral environment [Citation8]. Alternatively, direct separation of racemates by introducing a chiral additive in the stationary or the mobile phase makes separation of enantiomers possible [Citation8].
Chiral separations are usually performed in the normal phase mode, with the consumption of significant amounts of organic solvents (mostly hexane) in the mobile phase. Researchers are always concerned with developing green analytical methods for drug analyses by reducing the percentage of organic solvents and hence protecting the environment from many hazards.
Micellar liquid chromatography (MLC), which is mainly composed of aqueous solutions containing micelles over their critical micelle concentrations, has attracted the attention of analysts being environmentally benign and relatively economic [Citation9]. To improve separation efficiency and reduce analysis time, small ratios of organic modifiers to are introduced to MLC to yield the well-known hybrid micellar liquid chromatography HMLC mobile phases, which have been extensively applied for the analysis of pharmaceuticals, food staff, and environmental samples [Citation9].
The novelty of this work could be well established by referring to the two main topics investigated in this manuscript. The first topic is an interesting finding which arises during the application of MLC to analyse the enantiomeric mixtures of some pharmaceutical compounds, where separation of their racemates was possible by using a simply prepared MLC mobile phase on a C18 column without the addition of chiral selectors either in the mobile phase or in the stationary phase. The studied analytes include phenyl ephrine hydrochloride (PHR); 3-[(RS)-1-hydroxy-2(methylamino) ethyl] phenol hydrochloride [Citation10] (Table ), cetirizine dihydrochloride (CTZ); (±)-[2-[4-[(4-Chlorophenyl) phenylmethyl]-1-piperazinyl]ethoxy] acetic acid dihydrochloride [Citation10] (Table ) and mebeverine hydrochloride (MBV); (RS) 4-{ethyl[1-(4-methoxyphenyl)propan-2-yl]amino}butyl 3,4-dimethoxybenzoate [Citation10] (Table ). Selection of the studied drugs was based on targeting pharmaceuticals belonging to various chemical classes and having a wide variation in polarities.
Table 1. Physico-chemical properties of the studied drugs.
In all studied cases, separation was governed by charges, carried by the concerned analyte – which is dependent on the mobile phase pH – and on the charge carried by the surfactant. Hence, to generalize the separation concept, an anionic surfactant; sodium dodecyl sulphate (SDS) and a cationic surfactant; cetrimide (CTM) were investigated. A postulated mechanism for the obtained chiral separation was also introduced.
Meanwhile, the second topic of the manuscript is studying the compatibility of chiral columns with HMLC mobile phases, which was investigated taking CTZ as a model example. The obtained results proved this compatibility, which can evolve a radical change in chiral separations becoming an eco-friendly alternative analysis method.
2. Experimental
2.1. Materials and reagents
Phenyl ephrine hydrochloride (PHR), Sigma Aldrich, USA. Cetirizine dihydrochloride (CTZ), Pharco Pharmaceuticals, Egypt. Mebeverine hydrochloride (MBV), Rameda pharmaceutical company, Egypt.
Sodium dodecyl sulphate (SDS), tri-ethyl amine (TEA), and 1-propanol (HPLC grade), Sigma Aldrich, USA.
Acetonitrile and methanol (HPLC grade), TEDIA, Fairfield, USA.
o-phosphoric acid (min. assay 85%) and cetrimide (CTM) were purchased from El Nasr Pharmaceutical Chemical Company, Egypt.
2.2. Method A
2.2.1. Instrumentation
Separations were carried out on a Shimadzu SPD-20A, with a loop of 20 micro-litre capacity, and a UV/VIS. detector (model SPD-20A) was used to carry out separation. The instrument is provided with a column oven (CTO-20A) and degasser unit (DGU-207). Serial #: L 20135330505 AE, 220–230/240 V, 50–60 Hz, 160 VA, Kyoto, Japan. pH meter (Jenway), UK.
2.2.2. Chromatographic separation conditions
Separations were performed on a C18 column of 5 µm particle size and dimensions (250 × 4.6 mm); Kromaplus, Leonberg, Germany. The analytical column was thermostatically kept at 40 °C, applying a flow rate of 1.5 mL/min.
Chiral separations (in achiral environment) of enantiomers for racemic mixtures of PHR, CTZ, and MBV were conducted using the MLC mobile phase, containing an anionic surfactant and composed of 0.18 M SDS, containing 0.3% TEA and adjusted to pH 6 for PHR and CTZ, or pH 7 for MBV. Detection was adopted at 220 nm for PHR and 254 nm for both CTZ and MBV.
Moreover, chiral separation (in achiral environment) for racemic mixture for CTZ was performed using a cationic surfactant-based MLC mobile phase, which consisted of 0.18 M cetrimide containing 0.3% TEA and adjusted to pH 4 accompanied with UV detection at 254 nm.
2.3. Method B
2.3.1. Instrumentation
Separation of CTZ using the HMLC mobile phase was conducted on Azura® analytical HPLC supplied by P6.1 L pump, and equipped with a detector of model UVD 2.1 L, and a degasser DG 2.1 S, Knauer, Germany.
2.3.2. Chromatographic separation conditions
CTZ racemic mixture was separated on cellulose 1 chiral column (Lux 5µ cellulose 1 column 250 × 4.6 mm), operated at 40 °C at a flow rate of 1 mL/min., adopting 254 nm, as a detection wavelength. The HMLC mobile phase was composed of 0.15 M SDS, 10% 1-propanol, containing 0.3% TEA and adjusted to pH 7.
3. Results and discussion
All performed separations (in both methods) were carried out on columns operating at 40 °C. The applied temperature setting enhanced the repeatability of the obtained retention times tr and peak areas of the studied pharmaceutical compounds. The selected flow rates aimed to enhance peak symmetry and attain shorter chromatographic runs.
3.1. Method A
3.1.1. Optimization of separation conditions
An attempt to explore the chiral separation power of MLC mobile phases was performed using C18 column without adopting any chiral selector. 0.18M SDS was used all over the optimization procedure as lower concentrations did not have enough elution strength to separate the enantiomers of the studied drugs. It is reported that the elution strength is enhanced by increasing the surfactant concentration only if there is an interaction between the analyte and the micelles [Citation9], which is the case in this study. The main factor governing the chiral separation of the studied drugs was the pH.
Regarding PHR, (Table ), its two enantiomers could be separated over the pH range of (2.5–7). To achieve peak symmetry, pH 6 was chosen (Figure (A)), adopting UV detection at 220 nm. Similarly, MBV enantiomers could be separated all over the studied pH range. From peak symmetry perspective, pH 7 was selected for this separation (Figure (B)), where detection wavelength was set at 254 nm. Chiral separation of CTZ, on the other hand, could be attained at pH ≥ 5, if pH < 5, enantiomer # 1 overlapped with the solvent front; therefore, separation was conducted at pH 6 adopting UV detector at 254 nm (Figure (C)).
Figure 1. Chiral separation in a chiral medium (Method A) using the described MLC for the separation of racemic mixture of: (a is enantiomer #1, b is enantiomer # 2).
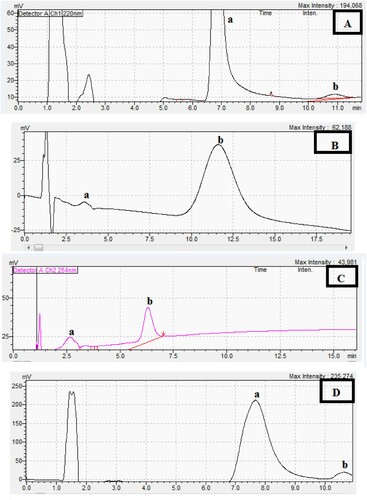
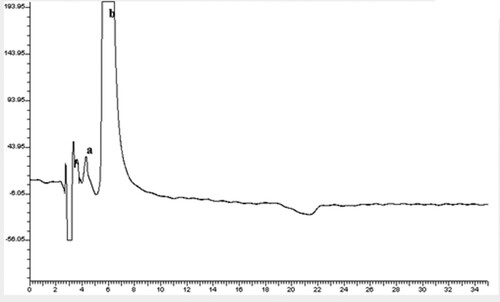
Figure 2. Chiral separation in chiral medium (Method B) using the described HMLC for the separation of racemic mixture of 200.0 µg/mL CTZ on cellulose 1 column: (a is enantiomer #1, b is enantiomer # 2).
To extent this application, a cationic surfactant; 0.18 M cetrimide was utilized using the same column operating under the same conditions. Chiral separation of CTZ was the subject of study using this surfactant. CTZ enantiomers could be separated with cetrimide when pH ≤ 5, to achieve peak symmetry, within short chromatographic run, pH 4 was adopted (Figure (D)). It is to be noted the pH values exceeding 5 failed to separate the racemates. UV detector was set at 254 nm.
3.1.2. Mechanism of chiral separation in achiral medium using SDS-based MLC
Utility of anionic surfactants like SDS enables separation of cationic drug enantiomers, bearing in mind that positive charges on racemic pharmaceuticals are induced by the knowledge of their Pka values [Citation11] and the mobile phase pH. It is proposed that cationic nitrogen atoms of the enantiomers are attracted to the negatively charged sulphate anions forming the polar heads of the micelles in aqueous solution, while the hydrophobic part of the enantiomers react with the nonpolar core of the surfactant. Since surfactants are reported to have a folded conformation in aqueous solution [Citation12], the cationic analytes are expected to bind with SDS in a pocket like structure.
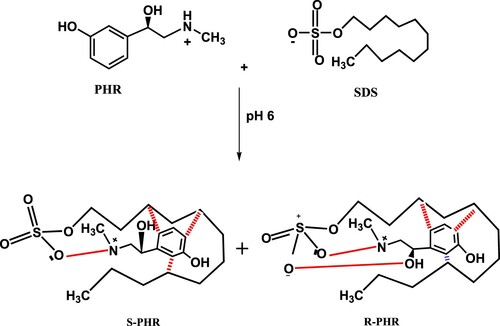
Taking PHR as an example, the pharmacologically active form (R-PHR) is expected to bind with SDS via three interaction sites, while the other racemate (S-PHR) will only have two binding centres, as previously documented by Scriba [Citation12], studying the chiral recognition mechanism in separation techniques. The two common sites of interactions in both isomers are postulated to be the cationic nitrogen atom (ion dipole bond with negatively charged oxygen of sulphate group of SDS), and the aromatic ring (Van Der Waals forces with hydrophobic part of SDS). Meanwhile, R-PHR has a third interaction site, where its benzylic hydroxyl group is suggested to form a hydrogen bond with the partially ionized oxygen atom of the sulphate group of SDS (Scheme 1), whereas the S enantiomer lacks the ability to align with this binding site. The formed inter-molecular hydrogen bond imparts lipophilicity to the R-enantiomer [Citation11], which indicates its elution after the S isomer. This postulation is established under the guidance of the fact that the vasopressor effect of R-PHR is 12 to 15 times more potent than the S isomer, where the R racemate binds to the receptor site via three interactive locations [Citation11].
Scheme 1. Postulation of chiral separation mechanism of PHR in achiral medium using the SDS-based mobile phase at pH 6.
Separation of enantiomers is initiated mainly by the difference in kinetics in their reaction with the polar head of micelles since enantiomers are reported to have different Gibb's free energy values [Citation12]. These facts suggest that micellar SDS aqueous solutions behave like chiral selectors in the mobile phase, where they react with the racemates in enantiomeric mixtures, transforming them to diastereoisomers, which have different physical and chemical properties.
Variation of physical properties imparts different sensitivities of the diastereoisomers towards the adopted wavelength of detection; hence, they show divergent intensities (Figure (A–D)). On the other hand, discrepancy in diastereoisomers’ chemical characteristics results in their elution at different retention times with an acceptable resolution (Figures (A–D)). Chiral separation of enantiomers, showing disparate sensitivities and retention times of the racemates, has been previously reported [Citation13–19].
Based on this explanation, interpretation of the obtained experimental results could be manifested. Both PHR and MBV have a basic nature as reflected from their PKa values (9 and 10.31, respectively) [Citation11]; therefore, they became completely ionized bearing positive charges all over the applicable chromatographic pH range (2.5–7), and hence separation of their enantiomeric mixtures is attainable over the entire range. Meanwhile, separation of CTZ enantiomers is quietly different, where the concerned drug carries both the acidic nature (PKa = 2.7 and 3.57) and the basic nature (PKa = 7.56) [Citation11]. Experimental studies revealed that CTZ enantiomers could be separated using SDS-based MLC mobile phases, only when pH ≥ 5, while at lower pH values of the mobile phase, enantiomer # 1 eluted with the solvent front. At pH values lower than 5, CTZ will be ionized carrying both negative and positive charges; however, it is postulated that the positive charges will be carried by both nitrogen atoms, resulting in stronger interactions between SDS and CTZ, eluting enantiomer # 1 with a solvent front. Through the elevation of pH, gradual decrease in cationic ionization of CTZ takes place, where only one nitrogen atom is expected to carry a positive charge, lessening the interaction of the analyte with SDS, and allowing separation of enantiomer # 1 from the solvent front (Figure (C)).
3.1.3. Mechanism of chiral separation in achiral medium using CTM-based MLC
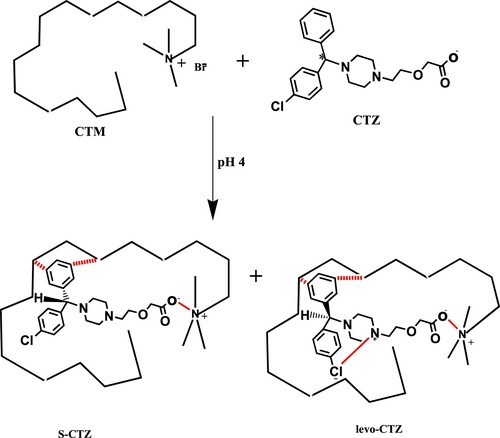
The same concept of separation could be applied upon using CTM as a surfactant. Both racemates of CTZ bind to the folded CTM through ion dipole bond (between the negatively charged oxygen atoms in carboxylate anion of CTZ and the positively charged heads of CTM micelles), in addition to Van Der Waals forces (Scheme 2). Meanwhile, the pharmacologically active levo-cetirizine is expected to have an additional binding force, where intra-molecular hydrogen bond is postulated to be formed between the protonated nitrogen of the piperazine ring and the unshared electrons of the chlorine atom in the isomer (Scheme 2), forming a more stable aryl amine-like structure. The formed intra-molecular hydrogen bond will then increase the lipophilic nature of levo-cetrizine [Citation11], where the S isomer is postulated to elute first. This interpretation is undertaken by simulation to the antidepressant drug clomipramine which is 50 times more potent than imipramine [Citation11] as it is subjected to the same interaction suggested for levo-cetrizine.
Scheme 2. Postulation of chiral separation mechanism of CTZ in achiral medium using the CTM-based mobile phase at pH 4.
Based on this postulation, the obtained separation conditions could be interpreted. The racemates of CTZ could be efficiently separated when the pH was lower than 5, where the carboxylic group of the analyte is expected to be completely ionized since the applied pH is about two units more than CTZ acidic PKa [Citation11]. Meanwhile, enantiomeric separation was hindered when the pH of the mobile phase was elevated higher than 5. Although complete ionization of the anionic group is still attained, another factor should be concerned which is the decrease in the cationic behaviour of the drug owing to its basic PKa value [Citation11]. It is expected that inhibition in the formation of the intra-molecular hydrogen bond in levo-cetrizine will take place, resulting in the failure of separation of the two racemates as both of them will have two interaction sites with CTM.
Eventually, the obtained results may constitute an evolution in chiral separation which may be possible without the utility of chiral selectors in the stationary phase, using a simply prepared and economic mobile phase, and applying a completely sustainable analytical method. Researchers are encouraged to carry out more detailed investigation concerning this point to prove the chiral nature of micellar aqueous solutions with evidence using more advanced instrumentation that clarifies chiral recognition mechanisms [Citation12], which was not available for the authors being based in a developing country. The instrumentation includes circular dichroism and vibrational circular dichroism; NMR techniques include nuclear Over Hauser effect and rotating-frame Over Hauser enhancement, Fourier transform and attenuated total reflectance IR spectroscopy [Citation12].
3.2. Method B
3.2.1. Optimization of separation conditions
This application aims to support the concept of the ability of HMLC to achieve chiral separation and prove its compatibility with chiral columns. Cellulose 1 column [Cellulose tris (3,5-dimethylphenylcarbamate)] was chosen as a model example of chiral columns owing to its extensive use in enantiomeric separation of various racemic mixtures [Citation12]. On the other hand, CTZ was selected as a typical example of racemic mixture drugs, where its enantiomeric separation was a subject of concern in the literature before [Citation20–22]. Enantiomeric separation of CTZ could be achieved over the concentration range of 0.05–0.18 M SDS; however, 0.15 M SDS was the concentration of choice as it provided better peak symmetry than other concentrations.
When the pH of the mobile phase was lower than 6, CTZ eluted with the solvent front, while its enantiomers could be separated at pH values ranging from 6 to 7. To attain peak symmetry of enantiomer # 1, pH 7 was selected.
Both 1-propanol and acetonitrile succeeded to achieve chiral separation; however, 1-propanol was used to prevent the appearance of distorted peaks. The ratio of 1-propanol was also studied, and it was found that ratios lower than 10% resulted in broad peaks of enantiomer # 1, while ratios ranging from 10 to 15%, overcame this challenge and gave almost the same separation efficiency parameters. For ecological reason, the minimum percentage of the optimum ratio range was chosen: 10%.
As a conclusion, chiral separation of CTZ on cellulose 1 column was achieved (Figure ), where the column was kept at 40 °C, adopting 254 nm as a detection wavelength, and applying a flow rate of 1 mL/min. The mobile phase consisted of 0.15 M SDS, 10% 1-propanol, containing 0.3% TEA and adjusted to pH 7.
3.2.2. Mechanism of chiral separation of CTZ on cellulose 1 column using HMLC
Several studies have been previously performed to explore the chiral recognition mechanism of cellulose columns [Citation23–26]. It has been concluded that the monosaccharide units are arranged along a helical structural, where the carbamate groups are inserted within the helix, creating the groves responsible for chirality, while the aromatic rings are located outside the helical structure [Citation12,Citation23–26]. Based on this information, it is suggested that CTZ isomers bind to SDS micelles electrostatically as described previously, then the racemates are transported by mass transfer from the micelles to the surfactant monomers adsorbed on the surface of the cellulose 1 column, keeping in mind that mass transfer takes place only in pharmaceuticals having high log P values [Citation9], which is the case in this study (log p of CTZ = 2.98 [Citation11]). Monomers of SDS are expected to attach to the chiral column via their lipophilic tails binding to the outside aromatic rings of the stationary phase. On the other hand, the polar heads are oriented towards the HMLC mobile phase, where they receive CTZ from the micelles (Scheme 3). Eventually, the ionized racemates are postulated to pass within the grooves of the chiral column, where they undergo resolution.
Scheme 3. Postulation of chiral separation mechanism of CTZ in a chiral medium using the HMLC mobile phase and cellulose 1 chiral column.
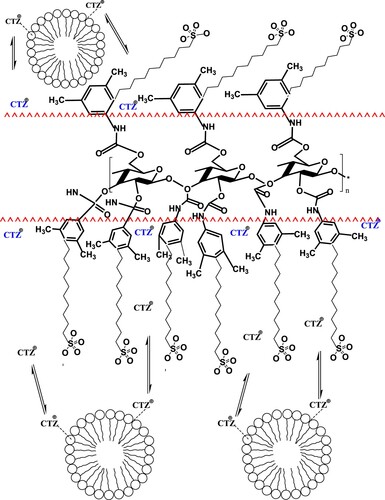
The obtained results are promising to researchers who are concerned about environmental safety, as compatibility of HMLC with chiral columns and its ability to carry out enantiomeric separation may be a substitute for the traditional analytical methods, which are usually operated in the normal mode of separation, consuming large amounts of organic solvents, and causing serious health hazards to the analysts, which is overcome in this application.
4. Conclusion
The proposed method proved two novel concepts concerning chiral separation. First, it studied the chiral selector ability of simply prepared aqueous MLC-based mobile phases, where C18 column was used as a stationary phase. Both anionic surfactants (SDS) and cationic surfactants (CTM) were investigated to perform chiral separation of some racemic mixture drugs, namely PHR, MBV, and CTZ. Promising results were obtained, where racemates of the studied enantiomers could be efficiently separated. Postulation of the separation mechanism was suggested based on the previously reported articles, which suggest the possibility of micelle solutions to have a chiral selector probability. Secondly, the compatibility of HMLC mobile phases with chiral columns was studied, where chiral separation of CTZ, using cellulose 1 column, was taken as a model example. The proposed method proved this compatibility, which may replace normal phase chromatography as a popular mode in chiral separation, hence reducing the consumption of organic solvents and accomplishing application of green chemistry in chiral separation.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Brunton LL. Goodman and Gilman's: the pharmacological basis of therapeutics. 12th ed.. USA: McGrow Hill; 2011.
- Rang HP, Ritter JM, Flower RJ, et al. Rang and dale's pharmacology. 8th ed. Livingstone: Elsevier; 2014.
- Sweetman SC. Martindale: the complete drug reference. 26th ed. London: Pharmaceutical Press; 2009.
- Rochat B, Amey M, Van Gelderen H, et al. Determination of the enantiomers of citalopram, its demethylated and propionic acid metabolites in human plasma by chiral HPLC. Chirality. 1995;7(6):389–395.
- Ameyibor E, Stewart JT. Enantiomeric HPLC separation of selected chiral drugs using native and derivatized β-cyclodextrins as chiral mobile phase additives. J Liq Chromatogr Rel Technol. 1997;20(6):855–869.
- Penmetsa KV, Reddick CD, Fink SW, et al. Development of reversed phase chiral HPLC methods using mass spectrometry compatible mobile phases. J Liq Chromatogr Relat Technol. 2000;23(6):831–839.
- Lobenhoffer JM, Reiche I, Tröger U, et al. Enantioselective quantification of omeprazole and its main metabolites in human serum by chiral HPLC – atmospheric pressure photoionization tandem mass spectrometry. J Chromatogr B. 2007;857(2):301–307.
- Davankov VA. Analytical chiral separation methods (IUPAC recommendations 1997). Pure Appl Chem. 1997;69(7):1469–1474.
- Ruiz-Ángel MJ, Carda-Broch S, Torres-Lapasió JR, et al. Retention mechanisms in micellar liquid chromatography. J Chromatogr A. 2009;1216(10):1798–1814.
- United States pharmacopeial convention: United States pharmacopoeia 30; national formulary 25. US pharmacopoeia convention.
- Beale JM, Block JH. Organic medicinal and pharmaceutical chemistry. 12th ed. London: Wolters Kluwer, Lippincott Williams & Wilkins; 2010.
- Scriba GKE. Chiral recognition mechanisms in analytical separation sciences. Chromatographia. 2012;75(15–16):815–838.
- Hyun MH. Development and application of crown ether-based HPLC chiral stationary phases. Bull Korean Chem Soc. 2005;26(8):1153–1163.
- Ali I, Gaitonde VD, Aboul-Enein HY, et al. Chiral separation of β adrenergic blockers on CelluCoat column by HPLC. Talanta. 2009;78(2):458–463.
- Schmid MG, Schreiner K, Reisinger D, et al. Fast chiral separation by ligand-exchange HPLC using a dynamically coated monolithic column. J Sep Sci. 2006;29(10):1470–1475.
- Mohr S, Taschwer M, Schmid MG. Chiral separation of cathinone derivatives used as recreational drugs by HPLC-UV using a CHIRALPAKW AS-H column as stationary phase. Chirality. 2012;24(6):486–492.
- Tang Y, Zielinski WL, Bigott HM. Separation of nicotine and nornicotin enantiomers via normal phase HPLC on derivatized cellulose chiral stationary phases. Chirality. 1998;10(4):364–369.
- Ali I, Naim L, Ghanem A, et al. Chiral separations of piperidine-2,6-dione analogues on Chiralpak IA and Chiralpak IB columns by using HPLC. Talanta. 2006;69(4):1013–1017.
- Ôi N, Kitahara H. Enantiomer separation by HPLC with some urea derivatives of L-valine as novel chiral stationary phases. J Liq Chromatogr. 1986;9(2–3):511–517.
- Chmielewska A, Konieczna L, Bączek T. A novel two-step liquid-liquid extraction procedure combined with stationary phase immobilized human serum albumin for the chiral separation of cetirizine enantiomers along with M and P parabens. Molecules. 2016;21(12):1654–1667.
- Eom HY, Kanga M, Kang SW, et al. Rapid chiral separation of racemic cetirizine in human plasma using subcritical fluid chromatography-tandem mass spectrometry. J Pharm Biomed Anal. 2016;117:380–389.
- Zhou J, Luo P, Chen S, et al. Enantioseparation of six antihistamines with immobilized cellulose chiral stationary phase by HPLC. J Chromatogr Sci. 2016;54(4):531–535.
- Ma S, Shen S, Lee H, et al. Mechanistic studies on the chiral recognition of polysaccharide-based chiral stationary phases using liquid chromatography and vibrational circular dichroism: reversal of elution order of N-substituted alpha-methyl phenylalanine esters. J Chromatogr A. 2009;1216(18):3784–3793.
- Kasat RB, Wee SY, Loh JX, et al. Effect of the solute molecular structure on its enantioresolution on cellulose tris (3,5-dimethylphenylcarbamate). J Chromatogr B. 2008;875(1):81–92.
- Kasat RB, Wang NHL, Franses EI. Experimental probing and modeling of key sorbent–solute interactions of norephedrine enantiomers with polysaccharide-based chiral stationary phases. J Chromatogr A. 2008;1190(1–2):110–119.
- Yamamoto C, Yashima E, Okamoto Y. Computational studies on chiral discrimination mechanism of phenyl carbamate derivatives of cellulose. Bull Chem Soc Jpn. 1999;72(8):1815–1825.
