?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The spinel ferrites are famous due to their unique electrical and magnetic performance. The current inspection is about the electrical and magnetic features of Holmium (Ho) substituted Co-Zn-ferrites (Co0.6Zn0.4HoxFe2−xO4; where x = 0.0 and 0.1). The Single-phase cubic configuration of prepared ferrites was perceived from X-ray diffraction data. The substituted sample has one order larger direct current (dc) resistivity as compared to the un-substituted sample. The substitution of Holmium decreased dielectric loss significantly. The dielectric study of samples was completed within the frequency range of 0.1–20 MHz. The comparison of dielectric parameters for different temperature values was measured. The magnetic characterization was done within the applied field of 2 kOe. The evaluated properties of samples like larger dc resistivity and less dielectric loss showed that the materials can be utilized in high-frequency applications.
1. Introduction
Currently, the researchers are investigating the materials that can be functioned properly in microwave absorption band and utilized as electromagnetic absorbents [Citation1,Citation2]. Such kind of materials is comprehensively used in electronic and communication devices. They have plenty of applications in scientific, industrial, military, and commercial areas, which include radar exposure techniques, wireless network systems, mobile phones, and computers. These materials are utilized as metallic surfaces in the shielding of extremely reflective surfaces by decreasing the radar cross-section of the targets and removing the issue of electromagnetic compatibility (EMC) and electromagnetic interference (EMI) [Citation3–5]. When the incoming microwave, which is actually a pairing of vibrating electric field and magnetic field, strikes upon the material's surface, the particles of material interact with either one field or both fields in order to derive the matter/light interactions under the GHz frequency section of EM spectrum [Citation6]. The interactions with both or any of the fields follow Maxwell's relations. According to Maxwell's relations, the interactions of material particles with any field will produce the response of others. Consequently, the whole EM wave dissipated [Citation7]. Moreover, the energy engrossed by the material is the energy of the EM wave, which is then converted into thermal energy because of the magnetic and dielectric response of the material. Nowadays, ferrite nanoparticles are supposed to be talented candidates for EM wave absorbers under higher frequency ranges due to their larger values of saturation magnetization and high Snoek's limit and, consequently, greater permeability figures at the gigahertz range [Citation8]. Ferrites are considered magnetic substances with EM radiation-absorbing capabilities via hysteresis loss mechanism, domain wall resonance, and eddy current effect [Citation9]. The particular type of ferrites is named spinel ferrite having the molecular formula MeFe2O4. Here, “Me” is divalent metallic ion such as Mg2+, Mn2+, Co2+, Ni2+, Cu2+, Zn2+, Cd2+etc. Among spinel ferrites, the cobalt-zinc ferrites have several useful features. Lcobalt-zinc ferrites are important components of microwave devices such as isolators, circulars, gyrators, phase shifters, and memory cores due to their high Curie temperature, high saturation magnetization, and hysteresis loop properties, which offer a performance advantage over other spinel structures [Citation10]. The well-known Co-Zn category of ferrites is soft magnetic materials with numerous high-frequency instruments like electromagnetic interference filters and multilayer chip inductors. They have many valuable features such as chemical stability, fewer loss values at higher frequency areas, permeability, resistivity, magnetization, Curie temperature [Citation11]. The substituent ions can occupy different lattice sites, they can alter the structural, electromagnetic, and other physical properties of host matter based on their site preferences [Citation12]. As a result, magnetic fields can control electrical properties. This has rekindled the desire to discover new methods of improving the properties and expectations of these materials in practical applications. The spin connection of 3d electrons causes ferroelectric and ferromagnetic properties during Fe-Fe interactions. Rare earth ions (which belong to the 4f elements) partially substitute for the Fe3+ ions in spinel ferrites, resulting in 3d-4f coupling. As a result, electrons with ferroelectric and ferromagnetic properties [Citation13] are used in spinel ferrites. The addition of rare earth metal ions, such as holmium, alters the structural and textured properties of the material. Rare Earth oxides, which are excellent electric isolators, have a resistivity of about 108 Ω-cm. These ions occupy B sites, which obstruct the movement of Fe2+ during the ferrite conduction process, increasing the compound's electrical resistivity. Holmium is an excellent dopant for all other lanthanides due to its excellent thermal and ferroelectric properties. By reducing the particle size of the host compound, incorporation of holmium ions into the spinal cord can increase electric resistance and decrease saturation magnetization [Citation14,Citation15].
In the present study, the Sol–gel method gave a homogeneous mixture of materials. This method can be adopted due to its low cost. The preparation of each sample by the sol–gel method takes lesser time as compared to other methods. So, we used the sol–gel technique to prepare this ferrite system. In the sol–gel technique, the samples can be synthesized at a relatively lesser temperature [Citation16]. The Co-Zn-ferrites have advantageous features like high resistivity and low dielectric losses. In current inspection, manufactured systems’ electrical and dielectric properties are evaluated under a wide temperature range as a function of frequency.
2. Experimental details
For preparing the samples, all metal nitrates were taken, zinc nitrate [Zn(NO3)2 (Daujung 99.90%)], cobalt nitrate [Co(NO3)2.6H2O (unit-chem 99.90%)], Holmium nitrate [Ho(NO3)3] and iron nitrate [Fe(NO3)3.9H2O (sigma Aldrich 99.90%)]. The citric acid monohydrate (Merck 99.9%) C6H6O7.H2O acted like a chelating agent. They were further taken with no impurity. The solution molarity value is chosen at 0.01mole. Along with that molarity the appropriate values were chosen for the nitrates. The liquefaction of every nitrate was done distinctly in hundred Milli litter of de-ionized water. A distinct solution of the citric acid is synthesized in the 100 Milli-litter of H2O. The amount of the citric acid has taken according with the valences of metal ions. For all-nitrates the solutions were prepared individually, also stirred well enough in a hot plate about ten minutes. Then the mixing and stirring continuously had done on a hot plate. The stirring process was continued at 80 degree centigrade. The pH of solution was kept at 7 by the addition of ammonia during stirring. After 5–6 h the solution become like a viscous gel. Then whole prepared material was placed in an oven for six hours at temperature 523 K. This gel then turned into the blackish brittle flake. Agate Mortar and pestle were used to achieve the wet blending of chemicals from the mixture of raw chemicals with deionized water. The product is made homogeneous by drying procedure and calcinated at 800 oC for three hours. The product is cooled under normal temperature conditions. A small quantity of polyvinyl alcohol is mixed as the binder. The product is ready for grinding. After completing the grinding process, the uniaxial pellets are prepared using stainless steel die under the pressure of 3–8 ton/in2. The sintering process of pellets is completed under 1000°C temperature and again cooled at room temperature slowly. The prepared spinel ferrites have no required phase at low temperatures (600°C). By studying different research papers, all the samples were sintered at high temperatures (1000°C) and got the required results [Citation17,Citation18].
The structural analysis was done using an X-ray diffractometer having CuKα as a radiation source (λ = 1.54 Å). Before the electrical and dielectric analysis, the surface of pellets is sprayed with silver paste, making the contacts better. The dielectric study was completed through an LCR metre made up of Agilent Technologies. The measurements are analysed in the 0.075–20 MHz frequency range. The Keithley instrument was used to study electrical behaviour under the temperature range of 293–473 K.
3. Results and discussion
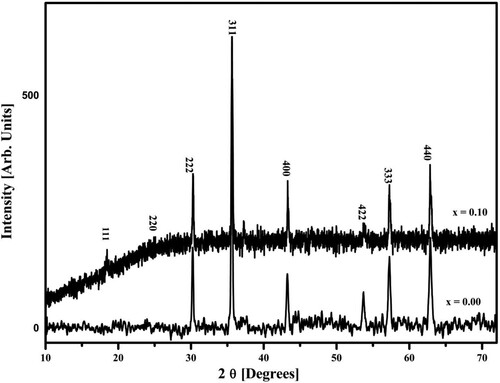
The cubic spinel structure of prepared samples was estimated from the X-ray diffraction (XRD) details presented in Figure . Sharp peaks were seen in XRD data. The XRD peaks for samples having composition x = 0.1 are wider with smaller intensity values. The diffraction planes (111), (220), (222), (311), (400), (422), (333), and (440) showed the cubic spinel structure of prepared samples. All these peaks were well-matched with standard JCPDS card (88-1942). All the samples possessed a simple cubic structure and were categorized in the Fd-3 m space group. No secondary phases were seen in the XRD pattern. The formula for the measurements of lattice constant is given as [Citation18];
(1)
(1) In Equation (1), the h k l are miller indices and d is inner planer spacing. Various numerical values for lattice constant, ionic radii, jump lengths as well as bond lengths of tetrahedral and octahedral sites were displayed in Table . The greater value of lattice constant for x = 0.1 sample is due to bigger size of Ho3+ as compared to Fe3+. These results are well matched with previous presented paper results [Citation19,Citation20].
Table 1. Different structural parameters of spinel ferrites.
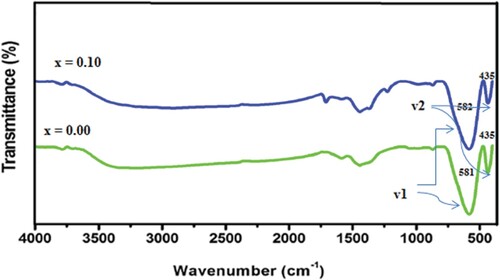
The inner atomic vibrations were estimated from Fourier-transform infrared spectroscopy (FTIR) spectra. The structural behaviour of samples is dependent upon the lattice vibrations. These vibrations vary with bonding force and mass of cations. The IR spectrum for both samples was shown in Figure . The absorption peaks of manufactured system under room temperature were positioned at 435 and 581. The existence of basic two absorption bands confirmed the successful formation of ferrites [Citation21]. Two major bands parallel to stretching vibrations of A and B sites positioned between 600 and 400 cm−1 [Citation22]. The specific band at 581 and 582 cm−1 allotted to tetrahedral category complexes whereas the absorption bands at 435 accredited to tetrahedral category complexes [Citation23].
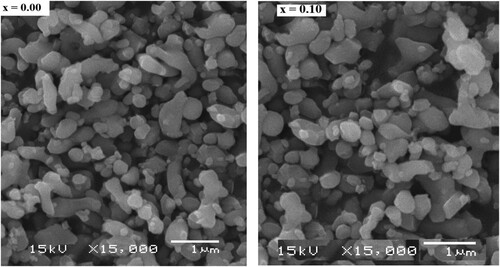
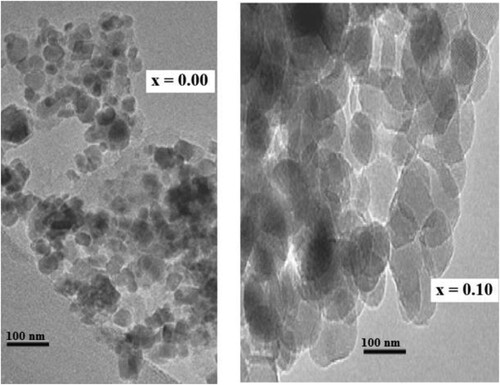
The scanning electron microscopy (SEM) images for two samples were given in Figure . The nanoparticles are agglomerated and have spherical shape as seem in SEM micrographs. The inhomogeneous mixture can be seemed clearly from images. Similar facts were also reported in previous researches by some other authors [Citation24,Citation25]. Two samples are selected for transmission electron microscopy (TEM) analysis. The TEM images were presented in Figure . TEM images showed that the particles have spherical shape and they formed clumps. The TEM data have good accordance with XRD data.
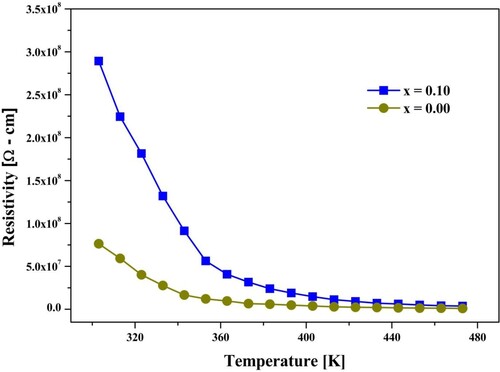
Table shows the DC electrical resistivity at room temperature of Co0.6Zn0.4HoxFe2-xO4 (x = 0.0 and x = 0.1) spinel ferrites. Co-Zni-Ferrite is extremely resistant in spinel ferrites and has high activation energy. The methods for synthesizing doped cations at sites A and B, particulate size or morphology, as well as sintering conditions affect all of spinel ferrite's electro-power properties [Citation26]. The DC electro-resistivity, as shown in Table , increases with the Ho3+ concentration. When Ho3+ (x = 0.1) is replaced, the electrical DC resistivity increases considerably. Due to electron hopping between Fe2+ and Fe3+, the Verwey mechanism could easily explain the electrical conduction of spinel ferrites. The replacement of Ho3+ with octahedral ions (B location) decreases the number of Fe3+ ions on B sites, along with the Fe3+↔Fe2+ trend, reduces the conductivity and increases the resistivity.
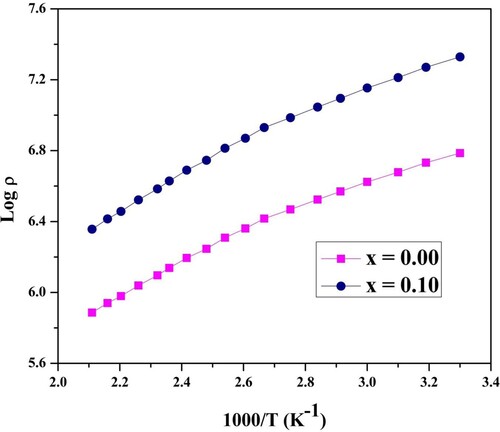
The graph of DC resistivity for temperature was given in Figure . From this figure, it is cleared that the resistivity was reduced with the surge of temperature. The reason for the high resistivity value is the presence of Ho3+ion in the Co-Zn system. The quantity of Fe-ions reduced at the octahedral site due to Ho3+ substitution, which plays a central role in the conduction mechanism [Citation26]. The DC resistivity value of pure Co-Zn-ferrites declined with the increase of temperature, obeying the eminent Arrhenius equation [Citation27]. Thus, the Co-Zn system has semiconducting nature. More conduction electrons were originated with the upsurge of temperature, which in turn reduced the resistivity. The reduction in the resistivity with the activation of drift electrons followed the hopping conduction mechanism. The decrease of resistivity with the increase of temperature does not link the charge carrier's production. The conduction mechanism is mainly completed by the electrons hopping between ferric and ferrous ions, i.e. Fe2+ ↔ Fe3+ + e−1.
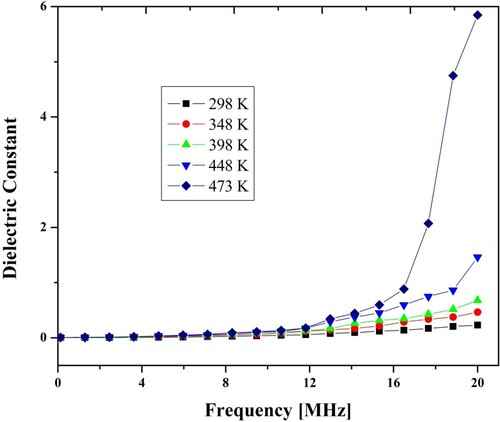
The slope of the graph presented in Figure gives the value of activation energy. Usually, the activation energy of nano-ferrites depends upon the charge carrier's mobility. It is independent of the concentration of charge carriers. Charge carriers merely stay at vacant positions. The hopping process completes the conduction procedure. The hopping procedure is determined by activation energy associated with the electrical barrier experienced by the electrons during the procedure [Citation24]. Figure contains different dielectric constant values for frequency ranges from 0.1 MHz to 20 MHz under fixed temperatures. Up to 1 MHz frequency, the dielectric constant value slowly decreases and then becomes constant at 6 MHz. Beyond this limit, its value increases. The reduction of dielectric constant below a particular frequency can be demonstrated on dipole relaxation occurrence [Citation25,Citation26]. The increase of dielectric constant beyond the specific limit can be explained on behalf of the resonance phenomenon at high frequency. The resonance happened when the applied field frequency matched with the charge exchanging frequency Fe3+↔ Fe2+. Wagner polarization model as well Maxwell theory, was used to clarify such type variations [Citation27,Citation28].
Figure 7. Variation of dielectric constant of spinel ferrite with frequency at different temperatures (x = 0.10).
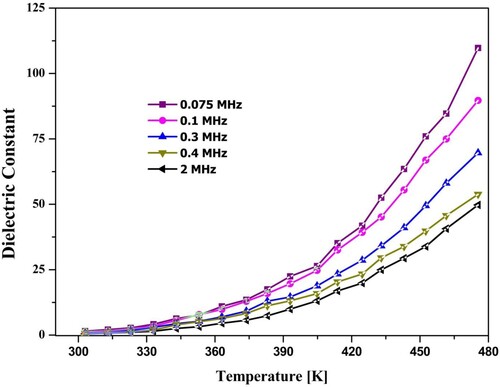
The variation of dielectric constant for different temperature values under fixed frequency range was given in Figure . For each value of frequency, the rise of temperature leads to an increase in the dielectric constant. The declined in dielectric constant was observed by substitution of the Holmium concentration. In decrease was observed through the ions substituted of the Holmium content also be clarified by the similar trend by the conduction technique of the electronic conduction. Here, the inter-conversion of electrons among Fe3+ and Fe2+ ions developed the displacement among charges increases, which supports finding out polarization of the charges through such ferrites. Therefore, the plenty of these Fe2+ ions at the octahedral site shows a competent role in the polarization of dielectric. Because of the greater ionic radius, the ions of Ho3+ take an octahedral site. Substitution of the Holmium ions for the ions of iron (site-B) blocks the mechanism of conduction because of its valence stability. It’s purposed that the transfer of electrons could not take between the Ho3+ and Fe2+ ions. Thus, a decrease in dielectric polarization is observed. However, the increase in temperature is faster in lesser frequency areas and slowed down in higher frequency areas. Usually, the dielectric constant is related directly to dielectric polarization. There are four main kinds of polarizations: electronic polarization, ionic polarization, dipolar polarization, and space charge polarization. The dipolar and space charge polarization plays a central role in the lower frequency section, and they are temperature-dependent [Citation29].
Figure 8. Variation of dielectric constant spinel ferrite with temperature at different frequencies (x = 0.1).
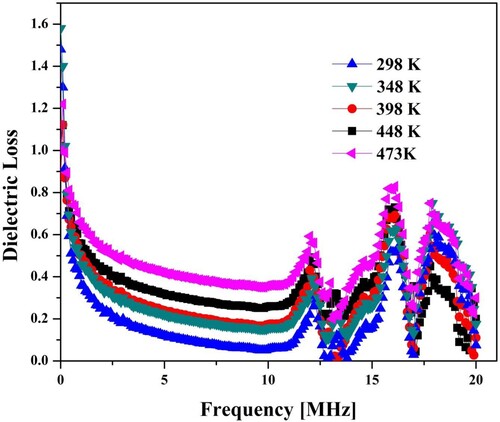
The dielectric loss versus temperature values under the specified range of frequency was displayed in Figure . The dielectric loss reduced with the rise of frequency. This variation trend is due to resonance and follows Koop's Model [Citation30,Citation31]. The phenomenon of resonance occurred when the hopping frequency matched with the applied field frequency. The data relating to the conduction process presented by Hudson explains the dielectric losses in ferrites. The substances with more excellent conductivity would have high loss values. The dielectric losses versus several frequency values under specified temperature were shown in Figure . The trend of variation of dielectric loss is similar to the trend of dielectric constant and explained similarly. The results showed that the involvement of Ho3+ ions in Co-Zn-ferrites increased DC resistivity and decreased dielectric constant and dielectric loss. These changes are demonstrated by using the concept of the hopping mechanism (Fe2+ ↔ Fe3+ + e−1). The substituted ion (Ho3+) does not take part in the conduction mechanism. These ions only bound the hopping by stopping the Fe2+ ↔ Fe3+ + e−1 exchange on the octahedral site [Citation32]. The quantity of Fe-ion lessened with the substitution of Ho3+ion [Citation33].
Figure 9. Variation of dielectric loss of spinel ferrite with frequency at different temperature (x = 0.1).
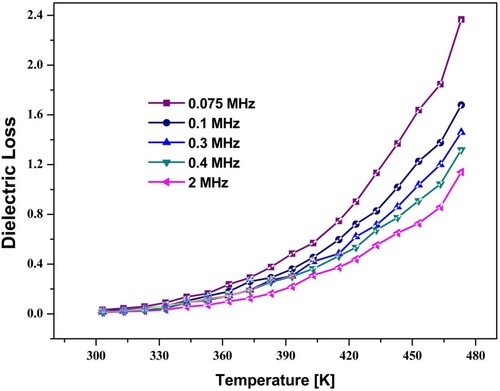
Figure 10. Variation of dielectric loss of spinel ferrite with temperature at different frequencies (x = 0.1).
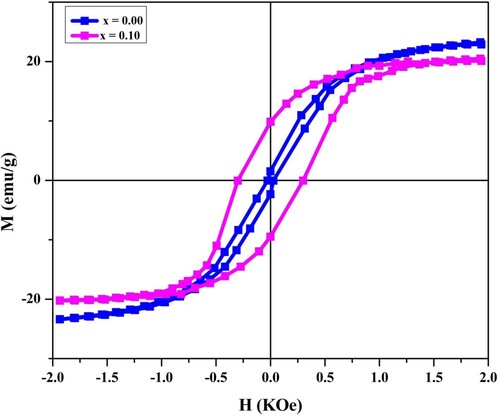
The measurements of magnetic factors were completed under the applied field of 2 kOe. Fundamentally that was ferromagnetic phenomenal. In the given magnetic field, we observed the explicit behaviour of hysterics. Narrow loops indicate the soft type of such spinel ferrites. Moreover, we used the hysteresis loop to count the various magnetic parameters such as remanence (Mr), magnetization (Ms), and coercivity (Hc). The calculation of saturation magnetization is done with magnetization curve on the Hmax also observes a falling trend by raising the Holmium contents. We observed the behaviour of soft magnetic through thin M-H loop because of their fewer coercivity values. The ferromagnetic nature was confirmed from the characterization results as given in Figure . The coercivity value enlarged while the saturation magnetization dropped. The replacement of Ho3+ ions with Fe3+ ions reduced the quantity of Fe3+ ions at the octahedral site. The magnetic moment value of RE ion is significantly less than Fe-ion [Citation32,Citation33]. Consequently, the magnetization values dropped.
The loss in flagging off the AB-exchange interfaces was the only cause of the loss in saturation magnetization and the remanence. The three types of negative exchange interactions occurred among both ions of those electrons that were unpaired on sites B and A. Among these three interactions, the interaction A-B predominates upon the remaining two, for example, interaction B-B and A-A. For the spinel ferrites given, we considered Ho3+ ions such as minimum magnetic substance value on the room temperature also attain to the sites-B(an octahedral site). In contrast, the ions of Fe3+ are occupied with both sites B as well as A. Like in the spinel ferrite, the total magnetic moments relay at the no. of the magnetic ions attaining to the octahedral (B) and tetrahedral (A) sites; create the saturation magnetization decreasing value. The Ho ions substitution (1.00 Å ionic radius) on the ions of Fe3+ (0.64 Å ionic radius) occurred on the octahedral sites also its existence on the sites of tetrahedral is founded very rare. Hence, the reduction of the octahedral site in the number of magnetic moments is expected. Thus we observed a reduction in the magnetic moment on lattice-B that consequently causes to decreases the magnetization, and eventually, in net magnetization, we observed a decrease. In the current study, by substituting Holmium, the reduction in magnetization is the best deal by the previously described work of many more researchers.
4. Conclusion
The sol–gel method was adopted to achieve Ho substituted Co-Zn-Ferrites.
All the samples possessed a simple cubic structure.
The DC resistivity value was enhanced up to 2.63 ×108 Ω-cm with Ho3+ ion substitution. The compounds with high DC resistivity are appropriate for higher frequency applications.
The decrease of resistivity with higher temperature showed the semiconducting nature of samples.
At room temperature and 20 MHz frequency, the dielectric was found to be 1.4.
Lesser values of dielectric loss and larger value of resistivity suggested that the sample (x = 0.10) may be suitable for high frequency applications.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Farid HMT, Ahmad I, Ali I, et al. Study of spinel ferrites with addition of small amount of metallic elements. J Electroceram. 2019;42:57–66.
- Kanwal M, Ahmad I, Meydan T, et al. Structural, magnetic and microwave properties of gadolinium-substituted Ca-Ba M-type hexagonal ferrites. J Electron Mater. 2018;47(9):5370–5377.
- Wu F, Sun H, AJia IA, et al. Significant internal quantum efficiency enhancement of GaN/AlGaN multiple quantum wells emitting at∼ 350 nm via step quantum well structure design. J Phys D: Appl Phys. 2017;50(24):245101.
- Munir S, Ahmad I, Laref A, et al. Effect of Nd-substitution on hexagonal ferrites for memory devices. J Appl Phys A: Mater Sci Process. 2020;126:722.
- Ashiq MN, Naz F, Malana MA, et al. Role of Co–Cr substitution on the structural, electrical and magnetic properties of nickel nano-ferrites synthesized by the chemical co-precipitation method. Mater Res Bull. 2012;47:683–686.
- Aravindh SA, Schwingenschloegl U, Roqan IS. Ferromagnetism in Gd doped ZnO nanowires: a first principles study. J Appl Phys. 2014;116(23):233906.
- Ajia IA, Edwards PR, Pak Y, et al. Generated carrier dynamics in V-pit-enhanced InGaN/GaN light-emitting diode. ACS Photonics. 2018;5(3):820–826.
- Alfaraj N, Mitra S, Wu F, et al. Photoinduced entropy of InGaN/GaN pin double-heterostructure nanowires. Appl Phys Lett. 2017;110(16):161110.
- Muhammed MM, Alwadai N, Lopatin S, et al. High-efficiency InGaN/GaN quantum well-based vertical light-emitting diodes fabricated on β-Ga2O3Substrate. ACS Appl Mater Interfaces. 2017;9(39):34057–34063.
- Flemban TH, Sequeira MC, Zhang Z, et al. Identifying the influence of the intrinsic defects in Gd-doped ZnO thin-films. J Appl Phys. 2016;119(6):065301.
- Zhang Z, Schwingenschlögl U, Roqan IS. Possible mechanism for d0 ferromagnetism mediated by intrinsic defects. RSC Adv. 2014;4(92):50759–50764.
- Kumar N, Singh RK, Satyapal HK. Structural, optical, and magnetic properties of non-stoichiometric lithium substituted magnesium ferrite nanoparticles for multifunctional applications. J Mater Sci: Mater Electron. 2020;31:9231–9241.
- Aravindh SA, Schwingenschloegl U, Roqan IS. Defect induced d0 ferromagnetism in a ZnO grain boundary. J Chem Phys. 2015;143(22):224703.
- Farid HMT, Ahmad I, Ali I, et al. Dielectric and impedance study of praseodymium substituted Mg-based spinel ferrites. J Magn Magn Mater. 2017;434:143–150.
- Limin D, Zhidong H, Yaoming Z, et al. Preparation and sinterability of Mn-Zn ferrite powders by Sol-Gel method. J Rare Earths. 2006;24:54–56.
- Ramay SM, Mahmood A, Alzayed NS, et al. Structural and magnetic properties of praseodymium substituted strontium-based spinel ferrites. J Mater Sci: Mater Electron. 2017;28:18656–18665.
- Wang J, Li Y, Wu R, et al. X-band shielding properties of Mg-9Li matrix composite containing Ni0.4Zn0.4Co0.2Fe2O4 fabricated by multi-layer composite rolling. J Alloys Compd. 2020;843(202):156053.
- Manzoor A, Khan MA, Khan MY, et al. Tuning magnetic and high frequency dielectric behavior in Li-Zn ferrites by Ho doping. Ceram Int. 2018;44(6):6321–6329.
- Bibi K, Ali I, Farid MT, et al. Electric and dielectric properties of Holmium substituted spinel ferrites. J Mater Sci: Mater Electron. 2018;29:3744–3750.
- Rezlescu N, Rezlescu E, Popa PD, et al. Comparative study between catalyst properties of simple spinel ferrite powders prepared by self-combustion route. Rom Rep Phys. 2013;65(4):1348–1356.
- Almessiere MA, Slimani Y, Rehman S, et al. Synthesis of Dy-Y co-substituted manganese-zinc spinel nanoferrites induced anti-bacterial and anti-cancer activities: comparison between sonochemical and sol-gel auto-combustion methods. Mater Sci Eng C. 2020;116:111186.
- George M, Ajeesha TL, Manikandan A, et al. Evaluation of Cu–MgFe2O4 spinel nanoparticles for photocatalytic and antimicrobial activates. J Phys Chem Solids. 2021;153:110010.
- Asiri S, Güner S, Korkmaz AD, et al. Magneto-optical properties of BaCryFe12−yO19 (0.0 ≤ y ≤ 1.0) hexaferrites. J Magn Magn Mater. 2018;451:463–472.
- Trukhanov SV, Trukhanov AV, Turchenko VA, et al. Structure and magnetic properties of BaFe11.9In0.1O19 hexaferrite in a wide temperature range. J Alloys Compd. 2016;689:383–393.
- Rhein F, Karmazin R, Krispin M, et al. Enhancement of coercivity and saturation magnetization of Al3þ substituted M-type Sr-hexaferrites. J Alloys Compd. 2017;690:979–985.
- Venturini J, Zampiva RYS, Piva DH, et al. Conductivity dynamics of metallic-to-insulator transition near room temperature in normal spinel CoFe2O4 nanoparticles. J Mater Chem C. 2018;6:4720–4726.
- Trukhanov SV, Trukhanov AV, Kostishyn VG, et al. Investigation of structure features and microwave absorption by doped barium hexaferrites. Dalton Trans. 2017;46:9010–9021.
- Zhang T, Peng X, Li J, et al. Structural, magnetic and electromagnetic properties of SrFe12O19 ferrite with particles aligned in a magnetic field. J Alloys Compd. 2017;690:936–941.
- Tabatabaie F, Fathi MH, Saatchi A, et al. Microwave absorption properties of Mn- and Ti-doped strontium hexaferrite. J Alloys Compd. 2009;470:332–335.
- Yang Y, Wang F, Shao J, et al. Influence of Nd-NbZn co-substitution on structural, spectral and magnetic properties of M-type calcium-strontium hexaferrites Ca0.4Sr0.6-xNdxFe12.0-x(Nb0.5Zn0.5)xO19. J Alloys Compd. 2018;765:616–623.
- Almessiere MA, Slimani Y, Baykal A. Impact of Nd-Zn co-substitution on microstructure and magnetic properties of SrFe12O19 nanohexaferrite. Ceram Int. 2019;45:963–969.
- Yousaf S, Ahmad I, Kanwal M, et al. Structural and electrical properties of Ba-substituted spinel ferrites. Mater Sci Semicond Process. 2021;122:105488.
- Shooshtary S, Yousefi VM, Amini MM, et al. Magnetic and microwave absorption properties of Cu/Zr doped M-type Ba/Sr hexaferrites prepared via sol-gel auto-combustion method. J Alloys Compd. 2019;773:1187–1194.