Abstract
Lysine acetylsalicylate (LAS) is an analgesic used for the prevention of cardiovascular disease with few adverse though its effect on the hepatorenal and hematological parameters remains unclear. 45 mature male Wistar rats were equally divided into three groups. Group I, II, and III received daily intramuscular doses of 0.25 mL saline, 40, and 80 mg/kg b. wt. of LAS respectively for 2-weeks. After 2, 4, and 8 weeks from beginning of drug administration, 5 rats/group were assessed for hepatorenal function, complete blood-count, and histopathological findings of liver and kidney. LAS induced a significant increase (p ≤ 0.05) in serum levels of aspartate aminotransferase, alanine aminotransferase, urea, and creatinine in a dose-dependent manner, but a significant decrease (p ≤ 0.05) observed in the hematological parameters. Histopathological alterations were a dose-dependent manner, and persisted for 8-weeks at the higher dose. In conclusion, LAS induced reversible deleterious effects on hepatorenal functions and various hematological parameters in a dose-dependent manner.
Introduction
In human and veterinary medicine, the non-steroidal anti-inflammatory drugs (NSAIDs) are advised for alleviating inflammation, pain, and fever through inhibition of cyclooxygenases and prostaglandin synthesis [Citation1,Citation2]. In spite of great effort for improving the efficacy, safety, and potency of NSAIDs, adverse effects such as hepatorenal toxicity [Citation3, Citation4], gastrointestinal irritations [Citation5], interference with hemostasis [Citation3, Citation4], cardiovascular [Citation6, Citation7] and reproductive problems are still common [Citation4, Citation8–10]. Acetylsalicylic acid (ASA) or aspirin, obtained from the bark of the white willow tree, remains the most prescribed analgesics and antipyretics [Citation2, Citation11, Citation12]. It is used for the prevention of heart strokes and attacks [Citation13], myocardial infarctions [Citation14, Citation15] and colorectal cancer [Citation16, Citation17]. Prevalence studies in the United States showed a marked shift in use for the prevention of cardiovascular disease (CVD), which can be attributed to ASA use [Citation18]. Among those 40 years and older, 23.4% reported taking daily doses of ASA for primary prevention of CVD, and out of these, 22.8% did so without a physician's order [Citation18]. ASA at low doses irreversibly inhibits cyclooxygenase enzyme at platelets, preventing thromboxane's synthesis, thereby avoiding cardiovascular problems [Citation19, Citation20]. However, new studies questioned its overall benefit for primary prevention, as it is shown to increase the risk of major bleeding with no significant reduction in CVD risk and all-cause mortality [Citation21, Citation22].
Lysine acetylsalicylate (LAS) is water-soluble, making it useful for parenteral (i.m. and i.v.) administrations [Citation23]. Only for a short period of time, ASA and LAS can be identified in the plasma as a result of its hydrolysis to salicylic acid (SAL) by carboxylesterases in plasma, liver, and red blood cells (RBCs) [Citation24–26]. Because of this fast deacetylation, it was expected that the anti-inflammatory effects of acetylsalicylate by-products are primarily attributed to SAL [Citation27]. LAS applies a distinct analgesic effect compared to other agents and its bioavailability after i.m. administration was similar to i.v. and significantly exceeds orally administered agents [Citation28].
The pharmacokinetic parameters of LAS wererecorded and LAS was found to be widely distributed in all tissues; all of its pharmacokinetic characteristics were influenced by body weight, height, and body surface area. Repeated administration of high doses of LAS in patients with low body weights may give rise to toxic effects [Citation29, Citation30]. Toxicity studies showed no neurotoxicity after a chronic intrathecal administration of LAS in rats [Citation31]. Furthermore, the role of LAS in preventing the toxicity of several materials such as paraquate [Citation32] and aluminum was also observed [Citation33]. LAS is used in various levels of prevention of heart diseases and stroke [Citation34], presenting potent antiplatelet complex with fewer gastrointestinal adverse effects than aspirin [Citation35]. Some revisions have compared aspirin with LAS in patients with persistent coronary artery disease and showed higher efficacy on platelet inhibition with LAS [Citation1, Citation36–38].
There is not enough available data concerning the influence of LAS on hepatorenal and blood images. The present study was designed to investigate the effect of LAS on the complete blood count, serum levels of AST, ALT, urea and creatinine, as well as its effects on the histopathological findings in the liver and kidney. The hypothesis of this study was that subacute administration of LAS is either safe or adverse to mammals, and these effects are either reversible or irreversible using male rats as a model.
Materials and methods
Drugs and chemicals
LAS was obtained from ASPEGIC@ (900 mg/vial equal to 500 mg acetylsalicylate; Produced by AMRIYA Pharmaceutical Industries, Alexandria, Egypt under license of laboratories SYNTHELABO FRANCE, LE PLESSIS ROBINSON – FRANCE). All other chemicals used in this study were of analytical grade and were provided by the local suppliers.
Animals
A total of 45 adult male Wistar albino rats weighing approximately 170 ± 10 g each (8-week age) were used. The animals were housed in a room with a constant temperature (25 ± 2.0°C) and humidity (55 ± 5%) in polypropylene cages with alternating 12-h light/dark cycles and received a normal pellet diet specially prepared for laboratory rodents and water ad libitum. Before conducting the experiment, the animals were acclimatized for two weeks.
Experimental design and sample collection
Three groups were used in this study, and each group contained an equal number of rats. Group I, the control group, received only the vehicle (0.9% saline, 0.25 mL/rat), while Group II received 40 mg/kg b. wt. of LAS once daily via i.m. administration (calculated according to Paget and Barnes) [Citation39], and Group III received 80 mg/kg b. wt. of LAS once daily. The duration of the experimental treatment was two weeks. For the sample collection directly after drug administration, 5 rats from each group were fasted for 12 h, anesthetized with light ether and sacrificed by decapitation for blood collection to assess the biochemical parameters. This was repeated after 4 and 8 weeks from the beginning of the experiment (sampling was as given in Table ).
Table 1. Duration of the experiment and sampling schedule
This design shows the sub-acute duration of LAS administration and the study of its actions in the sub-chronic period. Previous studies showed a variable duration of administration. In the present study the sampling was extended until the 8th week to investigate the possible recovery effects of LAS, which may extend to 3 months [Citation33, Citation40].
Blood analysis
Hemoglobin concentration was determined as cyano-methemoglobin using Sahl's method according to the method described by Benjamin [Citation41]. Platelets, erythrocytes, and total leukocyte counts were performed using a double improved Neubauer hemocytometer (Dacie and Lewis) [Citation42] As described briefly, total white blood cell (WBC) and red blood cell counts were determined in a Neubauer counting chamber after dilution of blood samples with specific diluents using the hemocytometer technique. Platelet count was estimated from Giemsa-stained blood smears and microscopically examined under high magnification.
Biochemical analysis
Total protein and albumin were measured as described previously using the biuret and bromocresol green methods by Lubran [Citation43] and Doumas and Biggs [Citation44], respectively. Serum protein reacts with cupric ions in an alkaline medium to produce a violet colour. The intensity of the colour, which has a maximum absorption at 540 nm, is proportional to the protein concentration. The globulin value was obtained by subtracting albumin from total protein values using the method described by Coles [Citation45]. Serum AST, ALT, urea, and creatinine levels were measured using commercial kits from Diamond Diagnostic (Egypt).
Histopathological studies
Liver and kidney sections were collected immediately after decapitation. The sections were fixed in neutral formalin 10%, dehydrated in graded ethyl alcohol (50-100%), and embedded in paraffin wax. Then, the samples were cut into 4–5 μm thick and stained with H&E for microscopic investigation [Citation46].
Statistical analysis
The obtained data were presented in the current study as means ± standard error (SE) and analyzed using ANOVA. Statistical significance was set to P ≤ 0.05. Significant differences between means were compared using Duncan multiple range tests, and calculations were carried out using the SAS system [Citation47].Values within each raw not sharing a common superscript letter (a, b, and c) are significantly different (p < 0.05).
Ethical statement
The experimental protocol used in this study was carried out in accordance to the Guide for Care and Use of Animals, which was established by the National Research Council (NRC, 1995). This study was approved by the local university ethics committee for animal research.
Results
Effect of Lysine acetylsalicylate on haematological pictures of male rats
Daily intramuscular injection of lysine acetylsalicylate at a dose of 80 mg/kg b. wt. for 14 days in adult male rats induced a significant decrease in the counts of red blood cells, white blood cells, platelets, and hemoglobin percent throughout the experimental period as compared with the other groups. The administration of 40 mg/kg bw. significantly decreased red blood cells and white blood cells after 2 and 4 weeks, but hemoglobin percent and platelet count significantly decreased only after two weeks from the beginning of its administration (Table ).
Table 2. Effect of daily i. m. injection of different doses of Lysine acetylsalicylate (LAS) for 14 days on the hematological pictures of male rats at different periods.
Effect of lysine acetylsalicylate on serum total protein, albumin, and globulin levels in male rats
Daily intramuscular injection of lysine acetylsalicylate at a dose of 40 mg/kg b. wt. for 14 days in adult male rats induced a significant decrease in total protein, albumin, and globulin levels after two weeks, while there was a non-significant effect on these parameters at 4 and 8 weeks after the injection (Table ). Daily intramuscular injection of LAS at a dose of 80 mg/kg bw. for 14 days in adult male rats induced a significant decrease in total protein, albumin, and globulin levels after 2 and 4 weeks, while there was a non-significant effect on these parameters 8 weeks after the injection (Table ).
Table 3. Effect of daily i. m. injection of different doses of Lysine acetylsalicylate for 14 days on serum total protein, albumin and globulin, ALT, AST, urea and creatinine levels of male rats, at different periods.
Effect of lysine acetylsalicylate on serum ALT, AST, urea, and creatinine levels in male rats
From Table , it can be observed that daily intramuscular injection of lysine acetylsalicylate at 40 or 80 mg/kg bw. for 14 days in adult male rats induced significant elevations in the serum levels of ALT, AST, urea, and creatinine after both 2 and 4 weeks from the beginning of treatment as compared with the control group. There were no significant effects on these parameters eight weeks after the start of treatment (Table ).
Histopathological findings in the liver
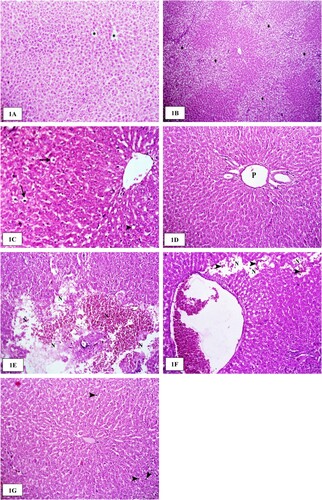
Microscopically examined livers of rats administered with the vehicle showed a normal histological structure of hepatocytes that were radially arranged around the central veins (Figure A). Two weeks post-LAS (40 mg/kg b. wt.), livers revealed multifocal to coalescent areas of vacuolar and hydropic degeneration of the hepatocyte cells, particularly in the periportal areas (Figure B). Four weeks post-LAS (40 mg/kg b. wt.), livers revealed mild hydropic and fatty degeneration of hepatocytes (Figure C). The degenerated hepatocytes exhibited a marked enlargement of the cells due to empty vacuoles that distended the cell cytoplasm. Eight weeks post-LAS (40 mg/kg b. wt.), the livers showed dilatation and congestion of the portal blood vessels with normal histological appearance of the hepatocytes (Figure D). Two weeks post-LAS (80 mg/kg b. wt.), livers revealed focal areas of coagulative necrosis characterized by the retention of hepatic cord architecture and shrunken hepatocytes with hypereosinophilic cytoplasm and pyknotic nuclei. Multifocal areas of lytic necrosis, with loss of cord architecture, were replaced with erythrocytes and eosinophilic material (edema) (Figure E). Four weeks after LAS treatment (80 mg/kg b. wt.), the livers of the examined treated rats revealed congestion of the central and portal veins with moderate mononuclear inflammatory cellular infiltration in the portal areas. Multifocal vacuolar and hydropic degeneration of the hepatocyte cells in the periportal zone of the hepatic lobules were characteristically observed. Moreover, small areas of lytic necrosis, with loss of cord architecture, were replaced by erythrocytes (Figure F). Eight weeks post-LAS (80 mg/kg b. wt.), the livers showed the activation and enlargement of Kupffer cells with a normal architecture (Figure G).
Figure 1. The histological structure of the liver after lysine acetylsalicylate (LAS) administration in rats. (A) Liver of a rat administered with the vehicle showing the normal histological structure of hepatocytes that were radially arranged around the central veins (asterisk). H&E stain ×200. (B) Liver of a rat two weeks' post-administration of LAS (40 mg/kg b. wt.) showing multifocal to coalescent areas of vacuolar and hydropic degeneration (asterisk) of hepatocyte cells in the periportal areas. H&E stain ×100. (C) Liver of a rat four weeks post-administration of LAS (40 mg/kg b. wt.) showing mild hydropic (arrows) and fatty degeneration (arrowhead) of hepatocyte cells. H&E stain ×400. (D) Liver of a rat eight weeks post-administration of LAS (40 mg/kg b. wt.) showing dilatation of the portal vein (P) with normal histological appearance of hepatocytes. H&E stain ×200. (E) Liver of a rat two weeks post-administration of LAS (80 mg/kg b. wt.) showing multifocal areas of lytic necrosis (N), with loss of cord architecture and replaced by erythrocytes and eosinophilic material (edema). H&E stain ×200. (F) Liver of a rat four weeks post-administration of LAS (80 mg/kg b. wt.) showing small areas of lytic necrosis (N), with loss of cord architecture and replaced by erythrocytes (arrowheads). H&E stain ×200. (G) Liver of a rat eight weeks post-administration of LAS (80 mg/kg b. wt.) showing activation of Kupffer cells (arrowheads) and normal histological characteristics of hepatocytes. H&E stain ×200.
Histopathological findings in the kidney
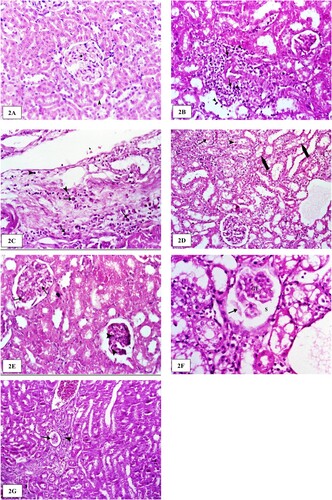
Microscopic examination of the kidneys of rats administered with the vehicle revealed normal histological appearance of the glomeruli and convoluted renal tubules (Figure A). Two weeks after LAS treatment (40 mg/kg b. wt.), the examined kidneys showed mild congestion of the renal blood vessels. In addition, the perivascular and periglomerular interstitium of the renal cortex was expanded by mononuclear inflammatory cells in moderate numbers, mostly lymphocytes and macrophages (Figure B). Four weeks post-LAS (40 mg/kg b. wt.), the examined kidneys revealed degenerative changes characterized by vacuolar and hydropic degeneration of the epithelium lining. Degenerated tubular epithelial cells were swollen with vacuolated cytoplasm and the perivascular interstitium of the renal cortex was expanded by edema admixed with mononuclear inflammatory cells in moderate numbers, mostly lymphocytes and plasma cells (Figure C). Eight weeks post-LAS (40 mg/kg b. wt.), the examined kidneys revealed degenerative changes in the lining epithelium of the proximal and distal convoluted tubules characterized by vacuolar and hydropic degeneration were commonly noticed. Two weeks post-LAS (80 mg/kg b. wt.), the kidneys showed marked pathological changes in the renal cortex. Glomerular tufts exhibited mesangial/endothelial cell necrosis with hypereosinophilic cytoplasm and pyknotic nuclei (Figure E). Four weeks post-LAS (80 mg/kg b. wt.), the examined kidneys revealed shrinkage of the glomerular tuft and ectatic uriniferous spaces containing pale, eosinophilic, homogenous material within glomeruli. The lining epithelium of the proximal and distal convoluted tubules was swollen and exhibited vacuolar and hydropic degeneration (Figure F). Eight weeks post-LAS (80 mg/kg b. wt.), the examined kidneys revealed large vesicular nuclei and pale basophilic cytoplasm in the regenerating tubules. Multifocally and predominantly around the glomeruli, the interstitium was expanded by aggregates of many lymphocytes and variable amounts of fibrosis. Occasionally, within glomeruli, uriniferous spaces were ecstatic and contained pale, eosinophilic, homogenous material (protein), as well as shrinkage of the glomerular tuft (Figure G).
Figure 2. The histological structure of the kidney after lysine acetylsalicylate (LAS) administration in rats. (A) Kidneys of rats administered with the vehicle revealed the normal histological appearance of the glomeruli and convoluted renal tubules. (B) Kidney of a rat two weeks post-administration of LAS (40 mg/kg b. wt.) showing perivascular mononuclear inflammatory cells, mainly lymphocytes (arrowhead) and macrophages (arrow). H&E stain ×400. (C) Kidney of a rat four weeks post-administration of LAS (40 mg/kg b. wt.) showing perivascular edema admixed with moderate numbers of mononuclear inflammatory cells, mainly lymphocytes (arrowheads) and plasma cells (arrows). H&E stain ×400. (D) Kidney of a rat eight weeks post-administration of LAS (40 mg/kg b. wt.) showing vacuolar and hydropic degeneration (thick arrows) of the proximal and distal convoluted tubules. Note also the homogenous eosinophilic hyaline (arrow) and cellular (arrowhead) casts in the lumen of some renal tubules. H&E stain ×200. (E) Kidney of rat two weeks post-administration of LAS (80 mg/kg b. wt.) showing glomerular tuft degeneration characterized by the vacuolization (arrow) of the lining cells. Note also the mesangial /endothelial cell necrosis with hypereosinophilic cytoplasm and pyknotic nuclei (arrowhead). H&E stain ×200. (F) Kidney of a rat four weeks post-administration of LAS (80 mg/kg b. wt.) showing shrinkage of the glomerular tuft (GT) and ectatic uriniferous spaces (S) containing pale, eosinophilic, homogenous material (arrow) within glomeruli. Vacuolar and hydropic degeneration of renal epithelium lining the convoluted tubules was noted. H&E stain ×400. (G) Kidney of a rat eight weeks post-administration of LAS (80 mg/kg b. wt.) showing atrophy (arrow) of the glomerular tuft accompanied by periglomerular inflammatory cellular infiltration (arrowhead). H&E stain ×200.
Discussion
LAS (a water-soluble form of aspirin administered via injection) and aspirin induce similar anti-inflammatory activity in vivo. The study hypostasis was that subacute administration of LAS is either safe or adverse to mammals, and these effects are either reversible or irreversible using male rats as a model, and the outcome of the study seems to support and contribute to our hypothesis. To the best of our knowledge, this is the first study to show the influence of a sub-acute administration of LAS on complete blood count, liver, and kidney function.
LAS (80 mg/kg, b. wt. i.m. for two weeks) induced a significant decrease in the counts of red blood cells, white blood cells, platelets, and hemoglobin percent throughout the experimental period. The addition of 40 mg/kg b. wt. significantly decreased red blood cells and white blood cells after 2 and 4 weeks, while the hemoglobin and platelet counts were significantly reduced after 2 weeks from the beginning of its administration. It is well known that blood is a composite of 2-phase fluid: formed components and plasma. The formed components contain red blood cells, white blood cells, and reticulocytes (platelets). The main task of red blood cells is to carry and supply oxygen to the outlying tissues within the body. However, the complex fluid dynamics occurring in microvessels are mainly manipulated by the mechanical and flow properties of red blood cells [Citation48]. Hence, modifications within the biomechanical properties of red blood cells probably cause a decrease in oxygen and supplement supply to marginal tissues [Citation49]. White blood cells, besides their essential role in the immune system and inflammation, play a role in modulating the blood stream [Citation50].
Aspirin at low doses was reported to have no significant effects on the number of red blood cells [Citation51]. Meanwhile, a decrease in the white blood cell number associated with a remarkable decrease in the count of monocytes possibly indicates that the phagocytic function of the body has been affected by aspirin [Citation52]. Aspirin can cause anemia and reduction in the blood oxygen-carrying capacity, and hence the amount of oxygen delivered to marginal tissues [Citation53] through a significant decrease in the values of PCV and Hb [Citation54]. Additionally, our results are in accordance with Meischer;[Citation55] Rainsford;[Citation56] Navratil et al.[Citation57], who stated that low doses of aspirin significantly decrease red blood cells and white blood cell counts, and attributed this reduction to the inhibitory action on bone-marrow hematopoiesis caused by aspirin. Many authors have reported the mechanisms of these effects, and Meischer [Citation55] and Rainsford [Citation58] stated that aspirin induced potential acetylation of bone marrow macromolecules, causing blood disorders. Sarkar et al., [Citation59] Tomoda et al., [Citation60] Modi, and Merchant [Citation61] reported that aspirin modifies the membrane permeability of red cells by stopping ion-dependent ATPase and cholinesterase activity. Dacie and Lewis [Citation62] reported that intravascular hemolysis could be a major contributing factor to the decline in red cell number.
LAS induced significant elevations in serum levels of ALT and AST after 2 and 4 weeks from the beginning of treatment. Eight weeks after the beginning of treatment, there were no significant effects on these parameters. These findings are correlated with those observed by Vyas et al. [Citation54] where the activities of AST and ALT were significantly elevated by aspirin. Increased activity of AST and ALT is an indication of hepatic damage; [Citation63] this reflects that LAS has hepatotoxic effects. These toxic effects could result from the inhibition of prostaglandin synthesis induced by NSAIDs, which results in vasoconstriction and diminished organ perfusion, which plays a role in tissue abnormalities [Citation64]. In addition to compromising the cellular defense system against reactive molecule attacks, increased serum levels of ALT and AST seem to have profound impacts on the normal function of hepatocytes. The liver is well known as a primary target organ for the metabolism of drugs and hence more susceptible to toxicity. In these cases, liver toxicity might be a consequence of idiosyncratic metabolic reactions, as the toxic metabolites bind to cell proteins within the hepatocytes, leading to aberrations [Citation65].
Our results were in accordance with Prescott [Citation66], who stated that aspirin and salicylates have been recognized as potentially hepatotoxic. In addition, Ebong et al. [Citation67] observed a significant increase in the levels of ALT and AST levels upon aspirin exposure. According to Ingram [Citation68], the elevation of both transaminase enzymes is presumed to result from leakage, damage, or necrotic liver cells. These results are in agreement with Doxy [Citation69], who reported that levels of ALT and AST were increased following liver damage. These results agree with those of Tiefenbich and Wichner [Citation70], who reported an increase in liver transaminase levels; these enzymes show an early rise in almost all diseases of the liver and remain elevated for 2–6 weeks in the presence of the disease. Additionally, Bush [Citation71] reported that liver damage is associated with increases in serum levels of both AST and ALT. In two- and four-weeks’ post-administration, AST and ALT levels showed a pronounced increase at 80 mg/kg compared to 40 mg/kg, which indicates that the damage is dose-dependent.
The current study showed that injection of LAS induced significant elevations in serum levels of urea and creatinine after both 2 and 4 weeks from the beginning of treatment. This finding may be due the fact that the administration of NSAIDs to susceptible individuals might cause renal failure due to a reduction in glomerular filtration rate and renal plasma flow, as explained by Ejaz et al., [Citation72]. The mechanism of action of NSAIDs involves the prevention of vasodilator prostaglandin synthesis from the arachidonic acid metabolism pathway. This in turn causes vasoconstriction and a drop in glomerular capillary pressure, leading to a rapid decrease in the glomerular filtration rate, which is characterized by increased serum levels of creatinine and urea. Gaspari et al.[Citation73] reported that urea and creatinine are metabolic waste products, which are easily filtered via the glomeruli of the kidneys; therefore, their serum levels are commonly used as indicators of renal toxicity, as stated by Perrone et al.[Citation74] The effects of these drugs may be due to functional impairment of renal function by acetylsalicylic acid mediated by inhibition of prostaglandin synthesis from arachidonic acid. The elevation in urea and creatinine levels reflects the state of glomerular filtration and kidney damage, supported by Coles [Citation45]. Eight weeks after the beginning of treatment, there were insignificant effects on these parameters, indicating recovery from the adverse effects.
The results of the present study demonstrated that injection of LAS for two weeks induced a remarkable reduction in serum albumin level after 2 and 4 weeks from the beginning of the experimental period, which is consistent with the results of Karima et al.[Citation75] and Mendez et al.[Citation76], who reported that a significant decline in albumin production in the liver appears to be associated with a potential impairment of hepatocellular function stimulated by severe inflammation as well as subsequent sepsis. These consequences were confirmed by the histopathological findings in the examined liver and kidney, where congestion of both the central and portal veins and the detrimental alteration of the hepatocytes in the form of vacuolar and hydropic degeneration were observed. Eight weeks after LAS, the livers showed activation and enlargement of Von Kupffer cells with normal architecture. Moreover, the examined kidneys revealed large vesicular nuclei and pale basophilic cytoplasm in the regenerating tubules. These findings correlated and matched with the levels of AST, ALT, creatinine, urea, and other studied parameters, indicating the recovery from the adverse effects of LAS after 8 weeks from the beginning of its administration. It could be concluded that LAS is a beneficial drug for humans and must be used within therapeutic doses and for a specific period. It induced dose-dependent adverse effects on liver and kidney functions, complete blood count, and protein profiles. Recovery from these effects was dose-dependent.
Acknowledgements
The authors acknowledge Qassim University, Saudi Arabia, and Alexandria University, Egypt, for providing the facilities needed to conduct this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Park K, Bavry AA. Aspirin: its risks, benefits, and optimal use in preventing cardiovascular events. Cleve Clin J Med. 2013;80(5):318–326. doi:10.3949/ccjm.80a.12146
- Fuster V, Sweeny JM. Aspirin: a historical and contemporary therapeutic overview. Circulation. 2011;123(7):768–778. doi:10.1161/CIRCULATIONAHA.110.963843
- El-Maddawy ZK, El-Ashmawy IM. Hepato-renal and hematological effects of diclofenac sodium in rats. Global J Pharmacol. 2013;7(2):123–132.
- Kashef E, Sawy E, El-Ashmawy I, Massry E. Some pharmacological studies on nimesulide. Alex J Vet Sci 2013 Jan;38 (1):2014;38.
- Guo C-G, Leung WK. Potential strategies in the prevention of nonsteroidal anti-inflammatory drugs-associated adverse effects in the lower gastrointestinal tract. Gut Liver. 2019.
- Ahmed SA, Al-Lawati H, Jamali F. Dose-dependency of the cardiovascular risks of non-steroidal anti-inflammatory drugs. Inflammopharmacology. 2019;27(5):903–910. doi:10.1007/s10787-019-00621-5
- Li J, Zhong L, Duan L, et al. Non-steroidal anti-inflam-matory drugs caused duodenal stenosis. J Biol Regul Homeost Agents. 2019;33(4):1183–1185.
- Vyas A, Purohit A, Ram H. Assessment of dose-dependent reproductive toxicity of diclofenac sodium in male rats. Drug Chem Toxicol. 2019;42(5):478–486. doi:10.1080/01480545.2017.1421659.
- Ibtisham F, Chen J, Niu Y, et al. Effect of aspirin on reproductive profile of male Rat An-overview. Int J Res Dev Pharm Life Sci. 2016;5:5–12.
- El-Nakeeb A, El-Ashmawy I, El-Sawy A. Effect of meloxicam and tolfenamic acid on some reproductive aspects of male rats. Alexandria J Vet Sci. 2011;34(1):225–234.
- Ferreira S. Prostaglandins, aspirin-like drugs and analgesia. Nat New Biol. 1972;240(102):200–203. doi:10.1038/newbio240200a0
- Ferreira SH, Lorenzetti BB, Corrêa FM. Central and peripheral antialgesic action of aspirin-like drugs. Eur J Pharmacol. 1978;53(1):39–48. doi:10.1016/0014-2999(78)90265-0
- Hall SL, Lorenc T. Secondary prevention of coronary artery disease. Am Fam Physician. 2010;81(3):289–296.
- Würtz M. Aspirin in coronary artery disease: an appraisal of functions and limitations. Dan Med J. 2015;62(4):B5011-B.
- Roubille C, Martel-Pelletier J, Davy J-M, et al. Cardiovascular adverse effects of anti-inflammatory drugs. Anti-Inflammatory & Anti-Allergy Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry-Anti-Inflammatory and Anti-Allergy Agents). 2013;12(1):55–67. doi:10.2174/1871523011312010008
- Umezawa S, Higurashi T, Komiya Y, et al. Chemoprevention of colorectal cancer: past, present, and future. Cancer Sci. 2019;110(10):3018. doi:10.1111/cas.14149
- Manzano A, Pérez-Segura P. Colorectal cancer chemoprevention: is this the future of colorectal cancer prevention? Scientific World J. 2012;2012. doi:10.1100/2012/327341
- O'Brien CW, Juraschek SP, Wee CC. Prevalence of aspirin use for primary prevention of cardiovascular disease in the United States: results from the 2017 national health interview survey. Ann Intern Med. 2019.
- Ma N, Liu X-W, Yang Y-J, et al. Preventive effect of aspirin eugenol ester on thrombosis in κ-carrageenan-induced rat tail thrombosis model. PLoS One. 2015;10(7).
- Halvorsen S, Andreotti F, Jurriën M, et al. Aspirin therapy in primary cardiovascular disease prevention: a position paper of the European Society of Cardiology working group on thrombosis. J Am Coll Cardiol. 2014;64(3):319–327. doi:10.1016/j.jacc.2014.03.049
- McNeil JJ, Nelson MR, Woods RL, et al. Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med. 2018;379(16):1519–1528. doi:10.1056/NEJMoa1803955
- McNeil JJ, Wolfe R, Woods RL, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379(16):1509–1518. doi:10.1056/NEJM-oa1805819
- Vivas D, Martín A, Bernardo E, et al. Impact of intravenous lysine acetylsalicylate versus oral aspirin on prasugrel-inhibited platelets: results of a prospective, randomized, crossover study (the ECCLIPSE trial). Circ: Cardiovasc Interventions. 2015;8(5):e002281. doi:10.1161/CIRCIN-TERVENTIONS.114.002281
- Needs CJ, Brooks PM. Clinical pharmacokinetics of the salicylates. Clin Pharmacokinet. 1985;10(2):164–177. doi:10.2165/00003088-198510020-00004
- Ulrich CM, Bigler J, Potter JD. Non-steroidal anti-inflammatory drugs for cancer prevention: promise, perils and pharmacogenetics. Nat Rev Cancer. 2006;6(2):130–140. doi:10.1038/nrc1801
- Williams FM. Clinical significance of esterases in man. Clin Pharmacokinet. 1985;10(5):392–403. doi:10.2165/00003088-198510050-00002
- Higgs GA, Salmon JA, Henderson B, et al. Pharmacokinetics of aspirin and salicylate in relation to inhibition of arachidonate cyclooxygenase and antiinflammatory activity. Proc Natl Acad Sci USA. 1987;84(5):1417–1420. doi:10.1073/pnas.84.5.1417
- Libina V, Chaľka L, Kosheleva L, et al. Analgesic action and pharmacokinetics of lysine acetylsalicylate administered intramuscularly. Farmakol Toksikol. 1988;51(5):78–82.
- Koch HJ, Raschka C. Anthropometric data and acetylsalicylic acid pharmacokinetics. Int J Clin Pharmacol Ther. 2002;40(1):30–34. Epub 2002/02/12. PubMed PMID: 11837380. doi:10.5414/cpp40030
- Libina VV, Chaľka LA, Kosheleva LP, et al. [Analgesic action and pharmacokinetics of lysine acetylsalicylate administered intramuscularly]. Farmakol Toksikol. 1988; 51(5):78–82. Epub 1988/09/01. PubMed PMID: 3145214.
- Svensson BA, Karlsten R, Kristensen JD, Sottile A, Bennett A, Gordh T, Jr. Intrathecal injection of lysine acetylsalicylic acid in the rat: a neurotoxicological study. Acta Anaesthesiol Scand. 1993;37(8):799–805. Epub 1993/11/01. PubMed PMID: 8279257. doi:10.1111/j.1399-6576.1993.tb03812.x
- Baltazar MT, Dinis-Oliveira RJ, Guilhermino L, et al. New formulation of paraquat with lysine acetylsalicylate with low mammalian toxicity and effective herbicidal activity. Pest Manag Sci. 2013;69(4):553–558. Epub 2012/10/31. PubMed PMID: 23109273. doi:10.1002/ps.3412
- Silva VS, Gonçalves PP. Effect of lysine acetylsalicylate on aluminium accumulation and (Na(+)/K(+))ATPase activity in rat brain cortex synaptosomes after aluminium ingestion. Toxicol Lett. 2015;232(1):167–174. Epub 2014/12/03. PubMed PMID: 25455452. doi:10.1016/j.toxlet.2014.10.014
- Marissal J-P, Selke B, Lebrun T. Economic assessment of the secondary prevention of ischaemic events with lysine acetylsalicylate. Pharmacoeconomics. 2000;18(2):185–200. doi:10.2165/00019053-200018020-00008
- Bretagne J, Feuillu A, Gosselin M, et al. Aspirin and gastroduodenal toxicity. A double-blind endoscopic study of the effects of placebo, aspirin and lysine acetylsalicylate in healthy subjects. Gastroenterol Clin Biol. 1984;8(1):28–32.
- Brighton TA, Eikelboom JW, Mann K, et al. Low-dose aspirin for preventing recurrent venous thromboembolism. N Engl J Med. 2012;367:1979–1987. doi:10.1056/NEJMoa1210384
- Gurfinkel EP, Altman R, Scazziota A, et al. Fast platelet suppression by lysine acetylsalicylate in chronic stable coronary patients. Potential clinical impact over regular aspirin for coronary syndromes. Clin Cardiol. 2000;23(9):697–700. doi:10.1002/clc.4960230912
- Majluf-Cruz A, Chavez-Ochoa A, Majluf-Cruz K, et al. Effect of combined administration of clopidogrel and lysine acetylsalicylate versus clopidogrel and aspirin on platelet aggregation and activated GPIIb/IIIa expression in healthy volunteers. Platelets. 2006;17(2):105–107. doi:10.1080/09537100500438156
- Paget GE, Barnes JM. CHAPTER 6 - toxicity tests. In: Laurence DR, Bacharach AL, editors. Evaluation of drug activities. Academic Press; 1964. p. 135–166.
- Huang WD, Wang JZ, Lu YQ, et al. Lysine acetylsalicylate ameliorates lung injury in rats acutely exposed to paraquat. Chin Med J (Engl). 2011;124(16):2496–2501. Epub 2011/09/22. PubMed PMID: 21933594.
- Benjamin M. Outline cf veterinary clinical pathology. 3rd Edition. 3rd ed. Ames, USA: Iowa State University Press; 1978.
- Dacie J, Lewis S. Practical haematology. 6th ed London: Churchill Livingstone; 1984.
- Lubran MM. The measurement of total serum proteins by the Biuret method. Ann Clin Lab Sci. 1978;8(2):106–110. Epub 1978/03/01. PubMed PMID: 345944.
- Doumas BT, Biggs HG, Arends RL, et al. Determination of serum albumin. In: Cooper GR, editor. Standard methods of clinical chemistry. 7. New York: Academic press; 1972. p. 175–188.
- Coles E. Veterinary clinical pathology. EH Coles. 2nd ed. Philadelphia, USA: WB Saunders Company; 1974.
- Harries M. Carletons histopathological technique.Toronto (NY): Oxford Univ Press; 1989.
- SAS G, STAT User’s. Statistics, Version. Statistical Analysis System Institute, Cary, NC; 2001.
- Salvagno GL, Sanchis-Gomar F, Picanza A, et al. Red blood cell distribution width: A simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci. 2015;52(2):86–105. doi:10.3109/10408363.2014.992064
- Da Costa L, Suner L, Galimand J, et al. Diagnostic tool for red blood cell membrane disorders: Assessment of a new generation ektacytometer. Blood Cells, Mol Dis. 2016;56(1):9–22. doi:10.1016/j.bcmd.2015.09.001
- Agrawal R, Sherwood J, Chhablani J, et al. Red blood cells in retinal vascular disorders. Blood Cells, Mol Dis. 2016;56(1):53–61. doi:10.1016/j.bcmd.2015.10.003
- Al-Taei BS. Effect of aspirin on sperm specification and some hematological parameters in male albino white rat. J Biotechnol Res Center. 2014;8(4):34–38. doi:10.24126/jobrc.2014.8.4.377
- Adewusi E, Afolayan A. Safety evaluation of the extract from the roots of pelargonium reniforme Curtis in male wistar rats. African J Pharm Pharmacol. 2009;3(8):368–373.
- Oyedeji K, Bolarinwa A, Adeyemo C. Effect of aspirin on haematological and plasma biochemical parameters in male Albino rats. J Dent Med Sci. 2013;3(5):80–83.
- Vyas A, Ram H, Purohit A, et al. Adverse effects of subchronic dose of aspirin on reproductive profile of male rats. J Pharm (Cairo). 2016;2016.
- Miescher P. Blood dyscrasias secondary to non-steroidal anti-inflammatory drugs. Med Toxicol. 1986;1:57–70.
- Rainsford KD. Side effects and toxicology of the salicylates. In: Rainsford KD, editor. Aspirin and related drugs. London: Taylor & Francis; 2004. p. 367–554.
- Navratil L, Blehova Z, Drbohlavova H. Effect of continuous, long-term administrations of acetylsalicylic acid on hematological and hemocoagulation changes in the rat. Boll Chim Farm. 1992;131(10):363–368.
- Rainsford KD. Aspirin and the salicylates. London; Boston: Butterworths; 1984.
- Sarkar A, Chakraborti A, Saha U, et al. Effects of aspirin and paracetamol on ATPases of human fetal brain: an in vitro study. Indian J Exp Biol. 1989;27(9):802–804.
- Tomoda T, Takeda K, Kurashige T, et al. Acetylsalicylate (ASA)-induced mitochondrial dysfunction and its potentiation by Ca2 + . Liver. 1994;14(2):103–108. doi:10.1111/j.1600-0676.1994.tb00056.x
- Modi D, Merchant M. In vitro effects of aspirin and salicylate on erythrocytes: size and Na+/K+ ATPase activity. Indian J Pharmacol. 2003;35:27–31.
- Dacie J, Lewis S. Practical haematology. 7th ed New York: Churchill Livingstone; 1991.
- Tian Z, Liu H, Su X, et al. Role of elevated liver transaminase levels in the diagnosis of liver injury after blunt abdominal trauma. Exp Ther Med. 2012;4(2):255–260. doi:10.3892/etm.2012.575
- Purohit A, Daradka H. Effect of mild hyperlipidaemia on testicular cell population dynamics in albino rats. Indian J Exp Biol. 1999;37(4):396–398.
- Aprioku JS, Nwidu LL, Amadi CN. Evaluation of toxicological profile of ibuprofen in Wistar albino rats. Am J Bio Med Sci. 2014;6:32–40. doi:10.5099/aj140100032
- Prescott L. Hepatotoxicity of mild analgesics. Br J Clin Pharmacol. 1980;10(S2):373S–379S. doi:10.1111/j.1365-2125.1980.tb01825.x
- Ebong P, Eyong E, Udosen E. Effects of aspirin (acetylsalicylic acid) and Cataflam (potassium diclofenac) on some biochemical parameters in rats. Afr J Med Med Sci. 1998;27(3-4):243–246.
- Ingram LR. Liver function. In: Anderson SC, Cockayne S, editor. Clinical chemistry concepts and applications. Philadelphia: Saunders; 1993. p. 280–317.
- Doxey DL. Veterinary Clinical pathology. 1st ed. London: Baillière Tindall; 1971.
- Tiefenbach B, Wichner S. Dose dependence and mechanism of the acute effect of methamidophos on the immune system of the mouse. Z Gesamte Hyg. 1985;31(4):228–231.
- Bush B. Interpretation of laboratory results for small animal clinicians. Blackwell Scientific Publications Ltd; 1991.
- Ejaz P, Bhojani K, Joshi V. NSAIDs and kidney. J Assoc Physicians India. 2004;52:632–640.
- Gaspari F, Perico N, Matalone M, et al. Precision of plasma clearance of iohexol for estimation of GFR in patients with renal disease. J Am Soc Nephrol. 1998;9(2):310–313. doi:10.1681/ASN.V92310
- Perrone RD, Madias NE, Levey AS. Serum creatinine as an index of renal function: new insights into old concepts. Clin Chem. 1992;38(10):1933–1953. doi:10.1093/clin-chem/38.10.1933
- Karima R, Matsumoto S, Higashi H, et al. The molecular pathogenesis of endotoxic shock and organ failure. Mol Med Today. 1999;5(3):123–132. doi:10.1016/S1357-4310(98)01430-0
- Méndez-Sánchez N, Chavez-Tapia N, Uribe M. New molecular features of cholestatic diseases of the liver. Rev Invest Clin. 2003;55(5):546–556.
