Abstract
Poor wound healing is considered an obstacle in diabetics, which requires effective therapy. Our goal was to investigate the combined effect of mint and silver nanoparticle hydrogel films as wound-healing agents in diabetic rats. Thirty rats were arranged into five groups. The hydrogel films were prepared through an eco-friendly method, excluding toxic solvents and diluents. Gel 1 and fucidin showed complete wound-healing effect on the 22nd day, while Gel 2 showed a faster effect on the 16th day, all compared to group 1 which healed in 25 days. Diabetic rats in group 2 healed beyond 25 days. Moreover, Gel 1 and Gel 2 decreased the fasting blood glucose. Gel 2 enhances wound healing in diabetic rats via multiple mechanisms of action, possibly due to the ability of mint and silver nanoparticles to sustain their concentration at the wound site with limited toxicity.
Abbreviations: CO: castor oil; CS: corn starch; DIW: deionized water; H: hydrogel film; H-MME: hydrogel film developed with CS, PVA, CO and MME; H-SMME: hydrogel film developed with CS, PVA, CO and MME/ SNP; MME: methanolic mint extract; PVA: polyvinyl alcohol; SNP: silver nanoparticles; STZ: streptozotocin
1. Introduction
Diabetes mellitus (DM) is considered as one of the most common metabolic disorders, usually characterized by high blood glucose level or “hyperglycemia”. In diabetic patients, poor wound healing and diabetic foot syndrome (DFS) continue to be major health problems. Poor management or neglecting wounds in diabetics can lead to DFS, bad ulcerations and amputations [Citation1]. From an epidemiological point of view, about 15% of diabetics had amputations, and these patients have high levels of mortality [Citation2]. Moreover, the risk of poor wound healing in diabetic patients increases in parallel with the duration of diabetes and age [Citation3]. Chronic complications caused by diabetes can result in severe disability, poor quality of life as well as high financial costs. Although there are protocols for standardized care in wound healing, the physiological impairments overcast healing progression in diabetics [Citation3]. Wounds and ulcers in diabetics have long-term treatment durations that can be very costly. Applying effective therapies that can prevent the development of wounds and accelerate healing rates once wounds develop is essential.
Using medicinal plants as a remedy is trusted in many cases, and mint is regarded as one of the important and cheap herbal products [Citation4]. Several medicinal plants, especially in rural areas and developing countries, are still used for wound treatment but may lack scientific studies that demonstrate their efficacy [Citation5]. Li et al. used neferine as a treatment, which is a compound purified from a traditional non-toxic medicinal plant widely used in China and India [Citation6]. In that study, neferine showed promising wound-healing activity in diabetic rats via improved contraction, epithelialization and modulation of inflammatory mediators of the wound.
Regarding the therapeutic agents of wound healing, silver nanoparticles were found to have a wide range of anti-microbial and anti-inflammatory properties, which can help increase the rate of wound closure and reduce scar appearance after healing [Citation7]. Moreover, mint is one of the important and cheap herbal products, which can easily be grown in Saudi Arabia, and has many useful medicinal properties [Citation8]. In a study, mentha “mint” oil was found to be effective in promoting healing in wounds infected with Candida albicans in Wistar Rats [Citation9].
However, our literature survey revealed that despite the excellent properties of medicinal herbs such as mint, no study has investigated the properties of combining mint with silver nanoparticles as wound-healing agents.
We have previously made non-toxic methanolic mint extract- (MME) and silver nanoparticles (SNP)-loaded hydrogel films [Citation10]. The synthesis, spectral analysis, morphology, thermal stability, bio-degradation potential and antibacterial properties of MME- and SNP-loaded hydrogel films have been described in that earlier manuscript [Citation10]. Therefore, the main goal of the present work is to investigate the wound-healing effect of mint and silver nanoparticle hydrogel films in diabetic rats.
2. Materials and methods
2.1. Chemicals
All chemicals and reagents, including (Corn starch (CS: C6H10O5)n; MW (162.14)n; Holyland, Saudi Arabia), polyvinyl alcohol (PVA; MW 140,000; HIMEDIA, Mumbai, India), silver nitrate (SV; extra pure from Scharlau Chemie S.A.; Sentmenat, Spain), castor oil (CO; Loba Chemie, Mumbai, India), glycerol (GL; Sigma-Aldrich, Germany), Tween 80 (TW; Sigma- Aldrich, Germany), glutaraldehyde (GL; Loba Chemie, Mumbai, India) and ethanol (AnalaR Normapur, France) were used as received. For the preparation of hydrogel films, deionized water (DIW) was used. Citrate, diethyl ether and streptozotocin (STZ) were purchased from Sigma, USA. Haematoxylin and eosin were purchased from Biorad, USA. Paraffin was purchased from Leica, Germany. Biochemical assay kits were purchased from Siemens Health care diagnostics (Newark, USA).
2.2. Plant material
The methanolic Mentha piperita leaves’ extract (MME) was obtained from freshly plucked leaves of a mint plant (Family: Lamiaceae), grown in Alahmadi farm in Madina Al-Monawwarah, Saudi Arabia, December 2020. The plant was identified by Dr Kadry Abdel Khalik, Botany Department, Faculty of Applied Sciences, Umm Al-Qura University.
2.3. Preparation of hydrogel films
Hydrogel (H) films were prepared from PVA, CS, CO and biosynthesized SNP, using GL as a crosslinker. The methanolic mint extract (MME) of leaves plucked from the locally grown M. piperita L. plant was used for the biosynthesis of SNP. The preparation of MME, biosynthesis of SNP and the formulation of hydrogel films have been described in our previous manuscript [Citation10]. MME-containing SNP has been termed as MME/SNP. The two hydrogel films used in the present study have been termed as follows:
1-Gel 1: H-MME (hydrogel films prepared from CS, PVA, CO and MME)
2-Gel 2: H-SMME (hydrogel films prepared from CS, PVA, CO and MME/SNP)
2.4. In vivo study
2.4.1. Preparation of rats and induction of diabetes Type I
Ethical clearance was obtained for the present study and was approved by the IRB committee at the College of Medicine, Umm Al-Qura University: HAPO-02-K-012-2024-0Z-547. Thirty male Sprague Dawley albino rats weighing 290–310 g were marked in their tails and arranged in five groups (n = 6, Table ). These rats were used for testing the wound-healing effects of the different prepared films on the acute diabetic wound-healing model in accordance with the previous reports [Citation11–15]. Rats were kept individually and were acclimatized for one week, with a daily check of their water (ad libitum) and adjusted atmosphere (25 °C). Diabetes insipidus (Type I) was induced by a single intravenous injection of STZ (45 mg/kg rat body weight, prepared in 0.15 M citric acid and 0.25 M sodium phosphate, pH 4.65) in fasting rats. Glucose strips (BeneCheck, Germany) were used to monitor the blood glucose level using blood samples obtained from the tail vein 72 h after STZ injection. All experiments were conducted according to the guidelines of the National Institute of Health for the Care and Use of Laboratory Animals.
Table 1. Description of the five wounded rat groups (n = 6/group).
2.4.2. Induction of wound and determination of the wound-healing effect
At the beginning of the second week, fasting glucose level was redetermined to confirm that rats in groups 2–5 had become diabetic. Then, each rat (groups 1-5) was anaesthetized with diethyl ether. The dorsal flank skin of each rat was shaved using a clipper, followed by disinfection with 70% isopropyl alcohol. Full-thickness wounds up to 20 mm in diameter were created according to a pervious method [Citation16]. Wounds of both normal and diabetic rats were dressed using standard commercial films. Either CS/PVA, Gel 1, Gel 2 or fucidin were applied daily over the wound area, fixed with film dressing, and then tied up with the help of a hypoallergenic elastic adhesive. Fucidin was used because of its antimicrobial and wound-healing effects. The hypoallergenic adhesives were porous, air permeable with woven edges. Wound dressings were changed twice per week. Dimension of each wound was determined every third day in millimetres until complete healing occurred. Additionally, the wound decrease percentage was calculated compared to the control of the same group.
2.4.3. Determination of fasting-blood glucose and biochemical profile
Fasting blood glucose was determined for each rat in all groups every third day using strips (BeneCheck, Germany). Additionally, kidney markers, liver markers, lipid and protein profiles were assessed for all groups on the 1st, 13th and 25th days. On the final day of the experiment, blood was collected from heart puncture into 2-mL containers and centrifuged (5702/R, Eppendorf, Hauppauge, USA) at 1500 g at 4◦C for 10 min. Plasma concentrations of liver, kidney and lipid profiles were assessed (Randox, Crumlin, U.K.) using a spectrophotometer (GENESYS 10 Bio UV-Vis Spectrophotometer, Thermo Fisher Scientific Inc., Waltham, MA, USA).
2.4.4. Determination of the mRNA expression patterns of wound-related genes
The real-time RT–PCR platform (Applied Biosystems 7500 Fast Real-Time PCR System) was used to quantify the mRNA expression levels of the following genes that are related to the wound-healing process: WNT4, MMP9, TGFB1, 5srRNA and CTNNB1 genes in all tested rats on days 1, 13 and 25 as previously reported [Citation17,Citation18]. The RNA was extracted from the blood samples using a kit (Ambion, Austin, TX, USA). Then, the quantification and purity of RNA were confirmed via Nano-Drop (Thermo scientific, MA, USA). The extracted purified RNA was stored at −70°C till being furtherly processed for the synthesis of complementary DNA (cDNA) using High-Capacity cDNA Reverse Transcription Kit with RNase Inhibitor (Applied Biosystems, MA, USA). Real-time PCR analyses of cDNA sample were performed using the SYBR Green Real-Time PCR Master Mix Kit (Toyobo, Japan) under the following conditions: 5 s at 95°C, 15 s at 60°C, 40 cycles of 30 s at 60°C and 60 s at 72°C. The primers used in PCR were obtained from Sangon Biotech Co. Ltd. (Shanghai, China), and the sequences of the studied genes are listed in Table . The reaction mixture comprises 9 µL of qPCR Master Mix (EURx Company, Gdansk, Poland), 10 ng of cDNA solution and 20 µL of all primers. The 2-ΔΔCt formula was used to calculate the relative gene expression. The mRNA expression levels were normalized to the reference gene of β-actin of the same sample against controls.
Table 2. Sequence of WNT4, MMP9, TGFB1, 5srRNA, CTNNB1, and actin primers.
2.4.5. Histopathological study of rat wounds
Wound tissue samples were fixed in 10% formalin, and paraffin-embedded blocks were prepared according to a previous method [Citation19]. Sections of 5-μm thickness from each tissue block were stained with haematoxylin and eosin and were subsequently examined with a 100× objective on a Leica DMi8 microscope (Leica Microsystems, Wetzlar, Germany) and the images were acquired from 10 different fields per section on days 1, 13 and 25. The wound tissues were differentiated according to their histopathological features into Grade I (normal control): thick epidermis, numerous layers of healed tissue with minimal unhealed tissue. Grade II (Gel 1 and Gel 2): thick epidermis, multiple layers of healed tissue but the unhealed tissue is considerable. The less unhealed tissue, the more effective the Gel. Grade II (fucidin): the epidermis is less thick, unhealed but is more than the healed tissue. Grade IV (Diabetic): the thin epidermis and huge amount of immature granulation tissue.
2.5. Statistical analysis
Statistical differences were determined using one-way ANOVA with Tukey’s posthoc multiple comparison test. p < 0.05 (*) and p < 0.01 (**) were regarded as significant.
3. Results
3.1. Effect of hydrogel films on wound dimension
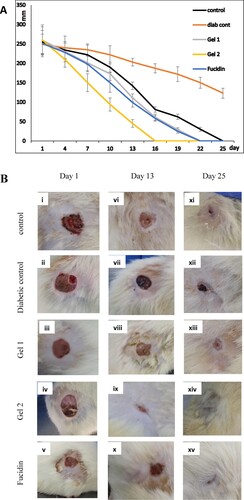
The wound of group 1 (non-diabetic control) healed in 25 days, while group 2 (diabetic control) showed complete healing beyond the 25th day (i.e. 30th day). Group 3 (Gel 1) and group 5 (fucidin) showed similar complete healing effects on the 22nd day. Interestingly, group 4 (Gel 2) was 6 and 9 days faster in wound healing compared to group 5 and group 3, respectively (Figure (A, B) and supplementary Table S1).
3.2. Effect of hydrogel films on fasting blood glucose
The fasting blood glucose remained high in the diabetic control group 2 compared to the control group 1 until the 25th day (83 mg/dL for group 1 and 366 mg/dL for group 2). However, both Gel 1 and Gel 2 decreased the fasting blood glucose levels by 7% and 45%, respectively, all compared to diabetic control, which had a marginal decreasing effect (Table ). Gel 2 exhibited the best fasting blood glucose decreasing effect.
Table 3. Fasting blood glucose (mg/dL) for the five rat groups.
3.3. Biochemical profiles
The biochemical profiles of all groups were performed on the 1st, 13th and 25th days (Tables ). Profiles of the 1st day were performed for comparison. On the 13th day, the creatinine and urea increased in group 2, while it did not show any significant change in group 3, group 4 and group 5. SGOT and SGPT increased in all groups compared to the control group, except for group 4 treated with Gel 2, which caused the least amount of increase. Moreover, the cholesterol increased in all groups, again except in group 4 where it remained the same possibly due to the effect of Gel 2. The triglyceride levels increased only in the diabetic group 2 and group 3 compared to the normal range of group 1, while no change happened in group 4 and group 5. Moreover, Gel 2 caused a slight increase in LDL, while causing an increase in HDL. However, the protein levels remained unchanged. Gel 2 was found to cause the least increase of SGOT, SGPT, cholesterol, triglycerides and LDL, while it causes an increase in HDL. On the 25th day, both Gel 1 and Gel 2 had decreased creatinine levels, but that decrease was more significant for Gel 2 (by three folds) compared to the control. Urea level only increased in group 2. SGOT and SGPT levels were maintained by Gel 2. Additionally, Gel 2 caused cholesterol, TG and LDL levels to remain similar to control group 1. Gel 2 also caused a significant increase in HDL. The protein level decreased in the diabetic group 2 while it remained similar to group 1 by Gel 1, Gel 2 and fucidin. Gel 2 was found to maintain the level of SGOT, SGPT, cholesterol, triglycerides and LDL compared to control group 1, while it caused a significant increase in HDL.
Table 4. Biochemistry profile for all groups on day 1.
Table 5. Biochemistry profile of all groups on day 13.
Table 6. Biochemistry profile of all groups on day 25.
3.4. Real-time-PCR analysis
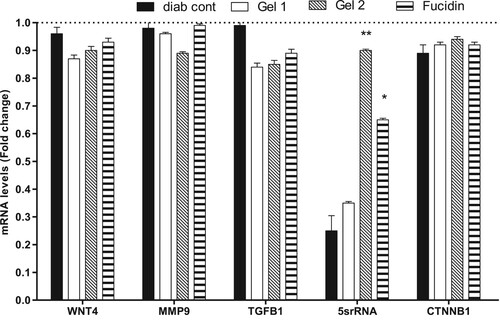
The real-time PCR analysis (RT–PCR) was performed for samples from different groups on the 25th day. Gel 1, Gel 2 and fucidin have caused a non-significant decrease of the mRNA amount of WNT4, MMP9, TGF B1 and CTNNB1 genes in the relevant rat groups. However, Gel 2 followed by fucidin, respectively, have caused a significant increase of 5srRNA expression compared to the diabetic control group (G2, Figure ).
Figure 2. The expression of WNT4, MMP9, TGFB1, 5srRNA and CTNNB1 in different rat groups on day 25. Data are represented as mean ± SD (n = 3, two independent experiments). The results are expressed as fold-change compared to the untreated group (1-fold change: G1). The raw delta-Ct values (the difference between CT values obtained for the gene of interest and the housekeeping gene) were converted into relative expression levels (fold-change) using the formula 2−ΔΔCt. Statistical differences, compared to untreated control cells, were assessed using a one-way ANOVA with Tukey’s posthoc multiple comparison test. p < 0.05 (*) and p < 0.01 (**) were taken as significant.
3.5. Histopathological study of rat wounds
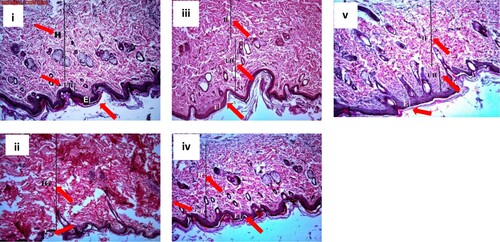
The normal control tissue of group 1 (grade I, Figure .i) was found to have a thick epidermis and multiple layers of compact collagen fibres with minimal unhealed tissue. On the other hand, the diabetic tissues of group 2 (grade IV, Figure .ii) showed a marked thinning of the epidermis together with interrupted inconsistent layers of immature granulation tissue. Groups 3, 4 and 5 treated with Gel 1, Gel 2 and fucidin (Figure .iii, 3.iv and 3.v, respectively) showed improvement of the histopathological picture of the wound tissues, which is suggested by increased epidermal thickening and increased amounts of collagen fibres with reduced amounts of unhealed tissue compared to diabetic tissues of group 2 and control group 1. The most significant result was observed with Gel 2 (grade II, Figure .iv), followed by Gel 1 (grade II) which showed a better histopathological picture than fucidin (grade III).
Figure 3. Sections of wound tissues stained with haematoxylin and eosin. i: control group 1, ii: diabetic control group 2, iii: Gel 1 group 3, iv: Gel 2 group 4, v: Fucidin group 5. Red arrows: E: epidermis, H: healed tissue, UH: unhealed tissue, IG: immature granulation tissue. The thickness of each layer is indicated by a vertical line. The diabetic tissue has only immature granulation tissue.
4. Discussion
Poor wound healing is a major health problem in diabetics that requires proper handling and therapy [Citation3]. Mint is regarded as an important medicinal plant with many proven uses. The present study aims to investigate the wound-healing effect of mint and silver nanoparticle hydrogel films and to confirm their application suitability as dressing agents for wound healing in diabetic rats. In a previous study, conductive hydrogels were used as antimicrobial wound-healing agents in rats. They are based on glycidyl methacrylate functionalized quaternized chitosan (QCSG), gelatin methacrylate (GM) and graphene oxide (GO), all of which showed promising wound-healing and repair properties [Citation20].
Diabetes and wound-healing possess have a complex relationship due to the ability of different bodies to recover. Skin is the largest protective barrier that performs a wide range of protection from microorganisms, heat and deep injuries [Citation21]. Skin is formed from multiple layers and when injured with a wound, the healing process is controlled by three mechanisms: inflammation, proliferation and the remodelling process. The proliferation step is characterized by angiogenesis, deposition of collagen, formation of granulation tissue, epithelialization and contraction of the wound [Citation21]. Therefore, wound contraction is considered one of the essential factors to study the duration of wound healing. In the present study, we found a significant difference between the controls and diabetic rats treated with Gel 2, where the contraction rate was faster in rats treated with Gel 2, and full healing occurred after 16 days, compared with 25 days for the control group. This may possibly be explained by the normal enhanced activity of fibroblasts during the angiogenesis process (Carleton’s, 1980). Additionally, Gel 1 and Gel 2 were found to decrease the fasting blood glucose levels by 7% and 45%, respectively, all compared to the diabetic control.
Diabetes can be associated with elevated levels of lipids [Citation22]. Our results showed a significant elevation of the following in the diabetic control group 2: SGOT, SGPT, cholesterol, triglycerides and LDL. After applying Gel 2, it was found to maintain the levels of SGOT, SGPT, cholesterol, triglycerides and LDL compared to control group 1, while it caused a significant increase in HDL. These useful effects of the mint could be attributed to its enhancement of insulin sensitivity, which can stimulate the metabolism of glucose inside the adipocytes. Fatty acids play an essential part in the development of insulin resistance, which is a major risk factor for diabetes. The enhancement of insulin sensitivity by mint can lead to a decrease in the lipids level. This observation is parallel with the evidence that most hypoglycaemic plants have the potential for the amelioration of diabetic lipid metabolism anomalies [Citation23,Citation24].
Certain conditions can impair the wound-healing process, such as weight loss [Citation25]. In our present study, the weight of rats in the diabetic control group decreased from 310 to 265 g. This may explain the delay of diabetics in wound healing as wounds that do not heal belong to patients with increased protein losses and depletion of body mass. This pathological state may be a result of the intake of energy and protein being inadequate for meeting the body’s needs [Citation26]. However, Gel 1, Gel 2 and fucidin resulted in maintenance of weight in groups 3, 4 and 5, respectively.
The wound-healing process has been attributed to the elevation of collagen and angiogenesis that can afford nutrients and enhance granulation of the tissue. Imbalance in the angiogenesis process and collagen formation in diabetes was found to be a risk factor in delaying wound healing [Citation27]. Our histopathological results in this study showed a well-organized collagen fibre, increases in the number of fibroblast cells and the formation of new blood vessels after wound treatment by Gel 1 and Gel 2 in diabetic rats compared to diabetic control rats. This enhancement of wound contraction happened through improvement in the epithelization step. Evidence has shown that topically administered drugs may promote quicker wound contraction and good overall healing [Citation6]. Moreover, a possible mechanism for the improved healing by Gels 1 and 2 could be due to the ability of mint and silver nanoparticles to sustain the concentration at the site of injury with limited potential for systemic absorption and toxicity. Comparable findings were reported in another study in which topical application of Moringa oleifera aqueous extract enhanced wound healing in diabetic rats through the enhancement of wound contraction [Citation28]. Moreover, these findings are in accordance with Kant et al. [Citation29] who proved that curcumin enhanced wound healing by stimulating fibroblast proliferation and promoting new blood vessel formation in diabetic rats.
To further examine the possible molecular mechanism underlying the response of hydrogel films to the wound-healing process, we examined gene expression using the real-time polymerase chain reaction. We found that Gel 2 (p < 0.01: **) followed by fucidin (p < 0.05: *) have caused a significant increase of 5srRNA expression compared to the diabetic control. MicroRNAs (miRNAs) are key regulators of the inflammatory response and tissue repair, acting through translational processing of target mRNAs. Recently, the importance of miRNA in skin wound healing of mice and humans has been identified. Moreover, 5sRNA is now considered an integral component that enhances protein synthesis by stabilization of the ribosomal structure [Citation30]. During the wound-healing process, there is coordination in the regulation of several target genes by multiple transcription factors, and these target genes can change over time to allow excellent integration of external signals to develop a balanced physiological output [Citation31]. Researchers have previously found that the ability of multiple transcription factors to bind to these regulatory aspects in many cell types [Citation32], or in response to stimuli [Citation33], is often investigated by pre-existing genome-wide chromatin accessibility. Transcriptional networks have remarkable cell-type specificity [Citation31], and even across cell types that have similar chromatin accessibility, a subset of transcription factors have sequence-specific binding differentiation [Citation34]. Moreover, evidence for the possible role of DNA methylation in the regulation of wound healing was provided by a recent study on the skin-specific long non-coding RNA (lncRNA) called WAKMAR1, which is expressed in leading-edge keratinocytes and promotes migration [Citation35]. Understanding the process of transcriptional regulation in wound healing remains a big and complex challenge. However, recent advances in single-cell analysis, including RNA profiling (scRNA-seq) [Citation36], DNA binding protein mapping (ChIP-seq) [Citation37], methylation profiling (snmC-seq2) [Citation38], chromatin accessibility (scATAC-seq) [Citation39] and spatial positioning [Citation40], along with new computation methods for integrating this complex data [Citation41,Citation42], have opened up new ways to investigate the effect of multiple transcriptional states of individual cells during the wound-healing process.
5. Conclusion
This study showed that the wound-healing process was slow in diabetic rats. On the other hand, hydrogel film with mint and loaded silver nanoparticles (Gel 2) significantly improved wound-healing time in diabetic rats. The hydrogel films were prepared through eco-friendly and benign methods, obviating the use of toxic solvents, diluents, surfactants and stabilizers, utilizing with application suitability as wound dressings in diabetic wounds. The beneficial effect of mint and silver hydrogel film for diabetic rats (Gel 2) could be due to its multi-potent properties that include improving blood glucose levels and enhancing the lipid profile. The results of the present study were further confirmed through RT-PCR and histopathological findings. However, investigating the antioxidant and anti-inflammatory effects of mint and silver nanoparticles could be interesting for future studies.
Ethical approval
Ethical approval was obtained from IRB committee at College of Medicine, Umm Al-Qura University, approval No. (HAPO-02-K-012-2024-0Z-547).
Supplemental Material
Download MS Word (13.5 KB)Acknowledgements
The authors would like to thank the Deanship of scientific research at Umm Al-Qura University for the continuous support. This work was supported financially by the Deanship of Scientific Research at Umm Al-Qura University to Dr. Mariam Mojally (Grant code:19-MED-1-03-0008).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Alkhudhayri, S, Sajini, R, Alharbi, B, et al.. Investigating the beneficial effect of aliskiren in attenuating neuropathic pain in diabetic Sprague-Dawley rats. Endocrinol Diabetes Metab. 2021;4(2):e00209.
- Alexiadou K, Doupis J. Management of diabetic foot ulcers. Diabetes Ther. 2012;3(1):1-5.
- Saluja S, Anderson SG, Hambleton I, et al. Foot ulceration and its association with mortality in diabetes mellitus: a meta-analysis. Diabet Med. 2020;37:211–218.
- Hussein AH, Alsubhi WF, Alharbi HA, et al. Awareness of diabetic foot among medical students at Umm Al-Qura university: a cross-sectional study. International Journal of Medicine in Developing Countries. 2020;4(10):1–6.
- Alqathama A, Alluhiabi G, Baghdadi H, et al. Herbal medicine from the perspective of type II diabetic patients and physicians: what is the relationship? BMC Complement Med Ther. 2020;20(1):1–9.
- Li J, Chou H, Li L, et al. Wound healing activity of neferine in experimental diabetic rats through the inhibition of inflammatory cytokines and nrf-2 pathway. Artif Cells Nanomed Biotechnol. 2020;48:96–106.
- El-Aassar MR, Ibrahim OM, Fouda MMG, et al. Wound healing of nanofiber comprising polygalacturonic/hyaluronic acid embedded silver nanoparticles: In-vitro and in-vivo studies. Carbohydr Polym. 2020;238:116175.
- Alqethami A, Aldhebiani AY, Teixidor-Toneu I. Medicinal plants used in Jeddah, Saudi Arabia: a gender perspective. J Ethnopharmacol. 2020;257:112899.
- Baali F, Boumerfeg S, Boudjelal A, et al. Wound-healing activity of Algerian Lavandula stoechas and Mentha pulegium extracts: from traditional use to scientific validation. Plant Biosyst Int J Dealing Aspects Plant Biol. 2020;(28):1–13.
- Mojally M, Sharmin E, Obaid NA, et al. Polyvinyl alcohol/corn starch/castor oil hydrogel films, loaded with silver nanoparticles biosynthesized in Mentha piperita leaves’ extract. J King Saud Univ Sci. 2022;34:101879.
- Muhammad AA, Arulselvan P, Cheah PS, et al. Evaluation of wound healing properties of bioactive aqueous fraction from Moringa oleifera Lam on experimentally induced diabetic animal model. Drug Des Devel Ther. 2016;(10):1715–1730.
- Belachew TF, Asrade S, Geta M, et al.. In Vivo evaluation of wound healing and anti-inflammatory activity of 80% methanol crude flower extract of Hagenia abyssinica (Bruce) J.F. Gmel in mice. Evid Based Complementary Altern Med. 2020. Article ID: 9645792.
- Akbar MU, Zia KM, Akash MSH, et al. In-vivo anti-diabetic and wound healing potential of chitosan/alginate/maltodextrin/pluronic-based mixed polymeric micelles: curcumin therapeutic potential. Int J Biol Macromol. 2018;120:2418–2430.
- Sato AC, Bosch RV, Will SE, et al. Exploring the in vivo wound healing effects of a recombinant hemolin from the caterpillar Lonomia obliqua. J Venom Anim Toxins Incl Trop Dis. 2016;22:10–36.
- Nasrabadi HT, Ebrahimi T, Banadaki, Sh D, et al. Study of cutaneous wound healing in rats treated with Lactobacillus plantarum on days 1, 3, 7, 14 and 21. Afr J Pharmacy Pharmacol. 2011;5:2395–2401.
- Abdalla AN. Antimicrobial and wound healing activity of some Sudanese medicinal plants. Doctoral dissertation, M. Sc. thesis. University of Khartoum; 2004.
- Abdalla AN, Qattan A, Malki WH, et al. Significance of targeting VEGFR-2 and cyclin D1 in luminal-A breast cancer. Molecules. 2020;(2):10–25.
- Abdalla AN, Abdallah ME, Aslam A, et al. Synergistic anti leukemia effect of a novel Hsp90 and a pan cyclin dependent kinase inhibitors. Molecules. 2020;(3):15–25.
- Carleton HM. Carleton's histological technique. Ulster Med J. 1967;36:172–172.
- Liang Y, Chen B, Li M, et al. Injectable antimicrobial conductive hydrogels for wound disinfection and infectious wound healing. Biomacromolecules. 2020;21:1841–1852.
- Driskell RR, Jahoda CA, Chuong CM, et al. Defining dermal adipose tissue. Exp Dermatol. 2014;23:629–631.
- Raziyeva K, Kim Y, Zharkinbekov Z, et al. Immunology of acute and chronic wound healing. Biomolecules. 2021;11:667-700.
- Zhou Y, Chi J, Lv W, et al.. Obesity and diabetes as high‐risk factors for severe coronavirus disease 2019 (Covid‐19). Diabetes/Metab Res Rev.. 2021;37(2):e3377.
- Daisy P, Saipriya K. Biochemical analysis of Cassia fistula aqueous extract and phytochemically synthesized gold nanoparticles as hypoglycemic treatment for diabetes mellitus. Int J Nanomed. 2012;7:1189–1202.
- Wang XF, Li ML, Fang QQ, et al. Flexible electrical stimulation device with chitosan-vaseline(R) dressing accelerates wound healing in diabetes. Bioact Mater. 2021;6:230–243.
- Collins N. Protein-energy malnutrition and involuntary weight loss: nutritional and pharmacological strategies to enhance wound healing. Expert Opin Pharmacother. 2003;4:1121–1140.
- Singh S, Young A, McNaught C-E. The physiology of wound healing. Surgery (Oxford). 2017;35:473–477.
- Chokpaisarn J, Chusri S, Amnuaikit T, et al. Potential wound healing activity of Quercus infectoria formulation in diabetic rats. PeerJ. 2017;5:e3608.
- Kant V, Gopal A, Kumar D, et al. Curcumin-induced angiogenesis hastens wound healing in diabetic rats. J Surg Res. 2015;193:978–988.
- Herter EK, Xu Landen N. Non-Coding RNAs: new players in skin wound healing. Adv Wound Care (New Rochelle). 2017;6:93–107.
- Aragona M, Dekoninck S, Rulands S, et al. Defining stem cell dynamics and migration during wound healing in mouse skin epidermis. Nat Commun. 2017;8:14684.
- Joost S, Jacob T, Sun X, et al. Single-cell transcriptomics of traced epidermal and hair follicle stem cells reveals rapid adaptations during wound healing. Cell Rep. 2018;25:585–597.e7.
- Dekoninck S, Blanpain C. Stem cell dynamics, migration and plasticity during wound healing. Nat Cell Biol. 2019;21:18–24.
- Boyle AP, Song L, Lee BK, et al. High-resolution genome-wide in vivo footprinting of diverse transcription factors in human cells. Genome Res. 2011;21:456–464.
- Samstein RM, Arvey A, Josefowicz SZ, et al. Foxp3 exploits a pre-existent enhancer landscape for regulatory T cell lineage specification. Cell. 2012;151:153–166.
- Li D, Kular L, Vij M, et al. Human skin long noncoding RNA WAKMAR1 regulates wound healing by enhancing keratinocyte migration. Proc Natl Acad Sci USA. 2019;116:9443–9452.
- Ransohoff JD, Wei Y, Khavari PA. The functions and unique features of long intergenic non-coding RNA. Nat Rev Mol Cell Biol. 2018;19:143–157.
- Di Ruscio A, Ebralidze AK, Benoukraf T, et al. DNMT1-interacting RNAs block gene-specific DNA methylation. Nature. 2013;503:371–376.
- Tang F, Barbacioru C, Wang Y, et al. mRNA-Seq whole-transcriptome analysis of a single cell. Nat Methods. 2009;6:377–382.
- Ai S, Xiong H, Li CC, et al. Profiling chromatin states using single-cell itChIP-seq. Nat Cell Biol. 2019;21:1164–1172.
- Grosselin K, Durand A, Marsolier J, et al. High-throughput single-cell ChIP-seq identifies heterogeneity of chromatin states in breast cancer. Nat Genet. 2019;51:1060–1066.
- Luo C, Rivkin A, Zhou J, et al. Robust single-cell DNA methylome profiling with snmC-seq2. Nat Commun. 2018;9:3824.