?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Nigella sativa L. is a fragrant plant that belongs to the Ranunculaceae family. It is commonly used as a form of traditional medicine in Middle-Eastern countries. In this study, Nigella seed oil from Saudi Arabia was extracted using the cold press and Soxhlet methods to investigate the effects of solvents polarities on yield and antioxidant properties. The analysis revealed that the highest yield (40%) of oil was obtained with ethanol solvent. The aqueous methanol extract the highest level of total phenolic compounds (239 mg GAE/g). Moreover, the methanol extract showed the most elevated 2,2-Diphenyl-1-picrylhydrazyl radical rummaging assay, at 59%. The ferric reducing and antioxidant power activities of the oils extracted with ethanol and tetrahydrofuran were 13,199 and 13,326 mmol Fe2+/100 g dry weight, respectively. Thus, the findings show that a high level of natural antioxidants can be derived from Nigella sativa oil extracted by ethanol solvent.
1. Introduction
Nigella sativa L. has been widely recognized for its medicinal properties for centuries. However, its potential benefits are continually increasing at a rapid pace and already covering other industries beyond the traditional realm of high tech [Citation1]. Mindfulness and anticipation of this healthy flora are unabated as a result of the well-known defensive properties of Nigella sativa, as well as the intense perfume of this plant. Nigella sativa is a plant member of the Ranunculaceae family of plants. It is indigenous to the Mediterranean region, where its seed, black cumin, is commonly used as a seasoning in various foods as a condiment.
Because of the active ingredients in Nigella seeds, food supplemented with N. Sativa tends to survive longer and preserve its nutritious content, and the seeds also help in preventing the formation of toxins. Consequently, herbal therapies containing N. Sativa are used for liver and kidney difficulties, rejuvenation, cerebral agony, influenza, asthma, and diuretic purposes in nations such as China, Saudi Arabia, and the majority of countries in the Asia Pacific area. Through education, the World Health Organization supports the efforts of communities throughout the world that desire to rely on Nigella sativa as an alternative health cure by offering insights into the plant’s qualities that will enable the development of new restorative healthcare programmes [Citation2]. It is thymoquinone, one of the bioactive compounds found in oil extracted from Nigella seeds, that has piqued the curiosity of researchers, and it is the critical component of interest. This is because thymoquinone’s defensive mechanism not only enhances a person’s physical well-being but it can also aid in the prevention of cancer due to its ability to diminish the presence of free radicals in the body [Citation3,Citation4]. It is well-known that free radicals are harmful to the body. They build up over time and can be exacerbated by confident lifestyle choices, and they act as toxins that harm the body by increasing the risk of heart disease, liver and kidney failure, skin aging, and tumour formation [Citation5]. Antioxidants, which are molecules that can prevent the propagation of severe responses by scavenging free radicals, are required to counteract these effects. Since N. sativa has outstanding antioxidant properties, this study will explore the effects of several solvents with varying polarity on the yield extracted to evaluate specific strategies that may allow the maximal transfer of phytochemicals during the extraction of N. an oil. To maximize oil yield, a close attention must be paid to the transfer of the plant’s phenolic mixture during extraction as this blend is critical to the oil’s therapeutic effects [Citation6,Citation7]. Oil quality is determined by the phenolic compounds that give it its flavour, aroma, and therapeutic properties. Because of this, one must learn all possible features about plant phenols. Selecting the proper solvent for extraction is critical since heat during solvent extraction has a detrimental effect on bioactive ingredients. As a result, the selection of appropriate solvent is essential when trying to isolate active bioactive chemicals like those found in N. sativa [Citation8]. For this study, the factors for optimal oil yield and quality were narrowed down to address the issues with simplifying the extraction process [Citation9,Citation10]. Cold pressing is a standard extraction method in the N. sativa industry, and most manufacturers use it mechanically because of its bioactivity safety; no degradation of the bio components can happen during this procedure. Cold pressing is a standard extraction method in the N. sativa industry, and most manufacturers use it mechanically. Biological safety and no degradation of the bio components make this procedure as a top choice. Unfortunately, the low oil production and the limited amount of antioxidant chemicals recovered are the principal downsides of this approach [Citation6,Citation11]. Consequently, different approaches must be investigated, such as the Soxhlet method. However, despite earlier studies, there is no information about N. sativa in Saudi Arabia. As far as the author knows, there is no correlation between oil yield and the polarity of the solvent. Therefore, it is necessary to investigate how different solvent polarities affect the yield and antioxidant characteristics of N. sativa oil.
2. Experimental
2.1 Materials
The dried Nigella sativa seed oil from Saudi Arabia, Figure , was used in the extraction process. The seeds were ground into the powdered form using an electrical grinder, and the powder was stored for mechanical compression. The spectrophotometer (SPECTRO star NANO, BMG Lab Tech, Germany) was used to quantify the results. The seed powder of all samples was homogenized using the rotator machine at (20000 rpm) for mixing, and then a vacuum evaporator from Khera Co (serial number, company name, and location) was used for effective solvent recovery.
To evaluate the effects of various solvents on Nigella sativa seed oil extract. The Soxhlet extraction method was carried out using different polarities of solvents, including hexane, methanol, ethanol, dichloromethane (CH2Cl2), and tetrahydrofuran (THF). Each of these solvents was classified as a high-performance liquid chromatography (HPLC) grade and obtained from Sigma Aldrich.
In addition, chemical materials used in this study included Gallic acid, Trolox, 2,2′-Diphenyl-1-picrylhydrazil (DPPH), Quercetin, Ferrous sulfate, and 2,4,6-Tripyridyl-S-Triazine (TPTZ). Moreover, Folin-Ciocalten reagent, Sodium Hydrogen Carbonate, sodium hydroxide, HCl, sodium nitrite, and aluminum chloride were also obtained from Sigma Aldrich. Furthermore, twofold refined deionized water was utilized during reagent preparation accordingly.
2.2. Oil extraction
2.2.1. Cold press
As described in Figure C, the seeds of Nigella sativa were mechanically squeezed at room temperature (25°C) without heat treatment. The compressed seeds were left overnight at room temperature (25°C) to isolate oil from their strands. The oil was then filtered using Whatman No.4 filter paper and a glass funnel.
2.2.2. Solvent extraction
A Soxhlet apparatus was set up to extract the oil from the powdered Nigella sativa seeds according to Alrashidi et al. [Citation12] mothed, with slight modification. The extraction was carried out individually, using six solvents with different polarities. The solvents were classified according to their polarity; from low (hexane and THF) to medium (ethanol and dichloromethane) and high (methanol and aqueous methanol 1:1). Finlay, all solvent-containing samples were extracted using the Soxhlet apparatus. The final supernatants were collected in vials as presented in Figure C.
2.3. Total phenolic content (TPC)
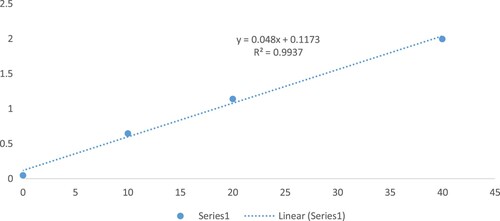
As phenolic compounds could dissociate the Folin-Ciocalten reagent under essential conditions, this reduction process provides an explicit method to measure the TPC [Citation13]. The reaction was initiated using 75 μL of Nigella sativa oil extract, 500 μL Folin-Ciocalteu reagent, 1.5 mL of 0.2 g/mL sodium carbonate, and 1.5 mL of pure water. The absorbance of the samples was measured spectrophotometrically at 765 nm after 2 h of reaction at ambient temperature. The TPC was calculated using Gallic acid as the standard. The results were expressed as Gallic acid equivalents (mg GAE/g sample). A standard calibration curve of Gallic acid was separately obtained by plotting the absorbance versus concentration (0–40 mg/mL). The entire procedure was carried out in triplicate, and the results were averaged [Citation13]. The details of the calculations were presented in (Appendix 1).
2.4. 2,2′-Diphenyl-1-Picrylhydrazil (DPPH) assay
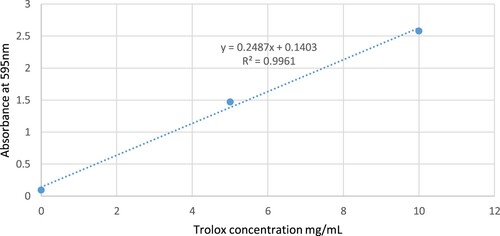
The capacity of the Nigella sativa extracts to scavenge the stable free radical DPPH reflects the antioxidant activity of the oil [Citation14]. About 5 µL of an oil sample is diluted with 200 µL methanol and then mixed with 100 µL of DPPH solution (300 µM). The absorbance was measured at the following times: 5 min, 1, and 18 h, because response pace was delayed for particular examples. The percentages of DPPH radical scavenging were determined from the absorbance of the control and the sample (As) utilizing Equation (1). Gallic acid corrosive activity was utilized as the positive control. The cell reinforcement limit was evaluated using Trolox and Trolox proportionate (400 mM stock arrangement in methanol). Trolox demonstrated linearity from 20 µL to 320 µL (R2 = 0.99).
(1)
(1)
Where A conc is defined as absorbance of the control, and A sample is defined as the absorbance of the sample. The details of the calculations were described in (Appendix 2).
2.5. Ferric Reducing Antioxidant Power (FRAP) assay
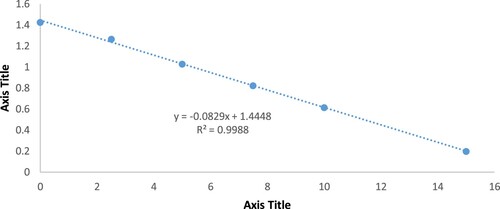
Ferric 2,4,6-tripyridyl-s-triazine oxidant (Fe (III) TPTZ) with a redox ability of 0.77 V was used. At low pH, the antioxidant components in the Nigella sativa oil extract reduce Fe (III) TPTZ complex into its ferrous structure (serious blue shading) that can be observed by estimating the adjustment in assimilation at 596 nm. The adjustment in absorbance was legitimately identified with the consolidated or all-out decreasing intensity of the electron giving cancer prevention agents present in the response blend. The technique was adjusted, and the response was assessed at the start point, at four min, and 10 min. The reagent of FRAP was naturally arranged by mixing 200 ml of acetic acid derivation support (300 mM, pH 3.6), 20 ml of TPTZ arrangement (10 mM), 20 ml of FeCl3 (20 mM), and 24 ml refined water. The straw shaded arrangement acquired was kept at 37°C until utilization. In the spectrophotometer cuvette, 2 ml of the newly arranged reagent of FRAP was added with 30 µL of oil sample and 70 µL methanol. Absorbance was measured at 596 nm promptly after 30 min interims against a specific reagent. Regarding standard bend FeSO4 arrangement weakening from 0.2 mM to 1.6 mM, the focus was set up from the stock and prepared comparably [Citation11]. The results were expressed as micro- moles of Fe2+ equivalents per gram of sample (µmol Fe2+/100g sample) using a calibration curve of Fe2+ with a linearity range of 0–10 µM (R2 > 0.99). The details of the calculations were described in (Appendix 3).
2.6. Statistical analysis
Nigella seeds were extracted using cold press and Soxhlet extraction with: hexane, ethanol, dichloromethane, THF, methanol, and aqueous methanol. The experiments were performed in triplicate. Data obtained from the TCP, DPPH, and FRAP assays were subjected to a one-way analysis of variance (ANOVA) to determine the significance of differences among the samples. The significance of difference among the mean values was determined using Tukey’s test. A probability of p < 0.05 was considered significant. All the measurements are reported as the mean ± standard deviation of triplicate analysis. All the analyses were conducted using the statistical software MINITAB 15 (Minitab Inc., state college, PA, USA).
3. Results and discussion
3.1. Ultraviolet (Yu et al.)/ Vis scan for Nigella sativa seed oil extract
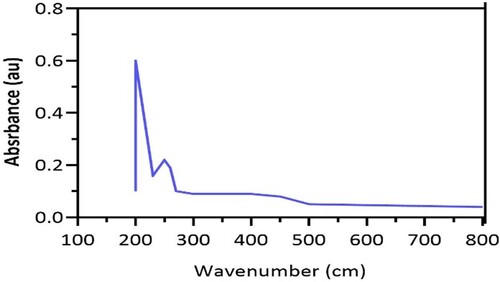
Figure shows UV absorption of Nigella sativa seed obtained from Saudia Arabia. The maximum absorbance was observed at 265 nm. Therefore an apparent analytical parameter for Nigella sativa seed obtained Ismail et al. [Citation15] investigated Nigella sativa oil microparticles quantification reported that the absorbance was approximately higher (271 nm).
3.2. Oil yield
The Nigella sativa seeds were either mechanically compressed or powdered and subjected to solvent extraction. The difference in oil yield was noticeable depending on the extraction method and used solvents of varying polarities (Table ). The findings thereby highlight the preeminent effect of using ethanol as a solvent. This corroborates the findings of Alrashidi et al. [Citation12] where the Nigella sativa oil yield was 28–40%. As shown in Table , a satisfying 40.16% oil yield using ethanol suggests the superior ability of ethanol to extract lipid components from the seed. Using mixed solvents harmed Nigella sativa oil yield.
Table 1. The yield and antioxidant activities of Nigella sativa seed oil using TPC, DPPH, and FRAP assays.
3.3. Total phenolic content (TPC) in the Nigella Sativa seeds oils
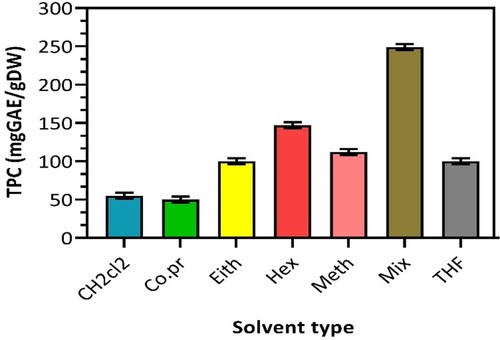
Phenolic substances and peroxide esteem are regularly utilized as markers to screen the nature of seed oils. Most phenolics show high levels of antioxidant activity. Therefore, the benefit of the oil is highly reflected by the phenolic substances extracted from the seed. Figure exhibits a straightforward overview of the TPC established using various solvents during extraction and the TPC obtained from mechanical pressing.
Regardless of the high volume of oil yield from using ethanol as a solvent, the TPC is somewhat lower than expected (107 mg GAE/g). On the other hand, aqueous methanol (1:1) proved to boost the capacity to extract phenolic compounds from the seeds (239 mg GAE/g). As shown in Figure , the highest level of TPC is 239 mg GAE/g as it is believed that aqueous methanol (1:1) directly correlates with the level of unsaponified compounds [Citation16]. In general, as the bioactive substances in the seeds are attracted to the polar solvents, it was expected that the TPC would increase with the polarity of the solvents because flavonols are commonly dissolvable in polar solvents. However, it may not seem simple as in this case, most probably due to the nature of each component’s polyphenolic profile and behaviour. The mechanical compression technique gave the least TPC (51 mg GAE/g) in comparison, where no solvent is involved. Nevertheless, it is essential to highlight that solvent extraction effectively isolates rich phenolic compounds from Nigella sativa seeds. This manifests the practicality of logistics whereby oil oxidation is not a significant drawback and reveals and reveals its extraordinary cancer and various disease prevention properties [Citation3,Citation16].
3.4. Antioxidant activities of Nigella sativa oil
Nigella sativa seed oil has gained a spotlight from the research community for the luxury of its natural bioactive components such as thymoquinone, 2-decenal, 2,4-decadienal, thymol, longifolene, and indole [Citation12]. Of the vast profile of phytochemicals found in Nigella sativa, thymol and thymoquinone are the essential components with good antioxidant capacities [Citation17]. Thymoquinone, structurally related to Tert-butyl hydroquinone (TBHQ), is formidable in reducing free radicals, exhibiting strong in vitro antioxidant potential [Citation18]. This oxidation-reduction relationship is highly convenient, hence, allowing widespread use of DPPH and FRAP to elucidate the antioxidant potential of phenolic compounds. The antioxidant potential of Nigella sativa is quite comparable to that of Trolox, a recognized antioxidant standard compound that is beneficial in antioxidant therapy.
3.4.1. DPPH radical scavenging activity
DPPH assay tests have clearly distinguished the radical scavenging ability of Nigella sativa oil extracted from its respective solvents, as shown in Figure . Based on the electron transfer assay, the scavenging activity of various Nigella sativa seed oil extractives is expressed as the percentage inhabitation. It seems that the extractable compounds from high polarity solvents are superior in radical scavenging activity. Nadaf et al. [Citation17] reported a similar pattern, whereby the percentage inhibition of the Nigella sativa can be arranged in the order of methanol (75%), aqueous methanol (75%), and acetone (55%). This explains the highest percentage of inhibition of methanol extractives (59%); however, the initial presumption on using an aqueous solvent to obtain oil with the higher antioxidant property was not proved. It was found that the dissolvable aqueous methanol extract produced the lowest DPPH radical searching action (16%). This is comparable with the scavenging activity of blending Nigella sativa oil and corn oil (12–22%), as reported by Solati and Sham [Citation19].
Figure 4. Effect of different solvents on inhibition of Nigella sativa seed oil using 2,2-Diphenyl-1-Picrylhydrazyl (DPPH).
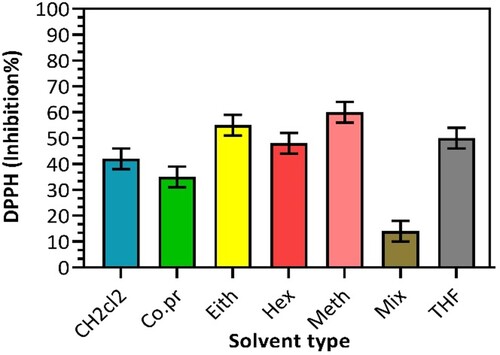
Concerning the TPC data in Figure , it is noteworthy to mention that the high TPC of the mixed solvent extractives could indicate a high measure of polyphenolic constituents present in the Nigella sativa seed oil that goes about as accessible as foragers. Therefore, the inhabitation percentage of antioxidants in the mixed solvent extractives became the lowest (Figure ). Moreover, it is vital to consider thymol and thymoquinone in the extractable compounds since they are highly valued as vital antioxidant compounds [Citation20]. A functional annotation to explain the results in Figure is the lower content of thymol; thymoquinone is the extracted oil concerning the solvent used in the extraction process.
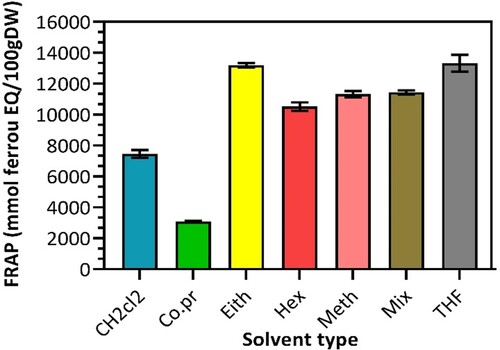
3.4.2. Ferric reducing antioxidant power (FRAP)
The FRAP assay provided another standpoint on the antioxidant power of the oils extracted by their respective solvents. It shows that oil compounds extracted with THF give the highest ferric reducing ability (13326 mmol Fe2+/100 g dry weight), which is significant enough compared with its result from the DPPH assay test (Figure ). Other samples showed ferric reducing activities ranging between 3091 and 13199 mmol/100gDW (Table ). These results exceeded the FRAP values of Nigella sativa oil obtained from supercritical extraction (538.67 mmol/100 mL) [Citation21].
The effect of thymoquinone is more evident with the FRAP assay test, shown by the significant elevation of antioxidant activity [Citation21]. Seeing the data in Table and Figure , most of the solvents used in this study are substantially effective for extracting antioxidant-rich compounds from the Nigella sativa oil of Saudi Arabia. It is crucial to ensure the extracted oil is highly nourished with antioxidants like thymoquinone as it is far more potent than α-tocopherol [Citation18]. Meanwhile, the difference in ferric reduction activity by the mechanically compressed oil is very distinguishable from solvent extraction.
3.5. Statistical analysis
ANOVA is a statistical technique that subdivides the total variation in a set of data into parts associated with specific sources of variation to test an assumption on the model’s parameters. ANOVA results for the TCP, DPPH, and FRAP experiments are shown in Table . The F-values were 1053, 268, and 766, respectively, inferring that the assumption was significant. Moreover, the associated values of P lower than 0.0001 indicated that assays results are statistically significant.
Table 2. ANOVA results for TPC, DPPH, and FRAP.
4. Conclusions
The study also showed that the Soxhlet extraction method using ethanol is the best method for extracting oil from Nigella seeds 40% compared to cold pressing (22%). Moreover, the presence of phenolic substances in the methanolic-extracted oil is highly noticeable in the DPPH assays (59%) due to the polarity of the solvents. Thus, favouring this method in terms of quality and quantity of the extracted oil. The food products and pharmaceutical industries, Nigella sativa methanolic and aqueous methanolic extract were used as additives with appropriate antioxidant properties. The data from this study is thought to be the reference for future studies.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Farooqui Z, Ahmed F, Rizwan S, et al. Protective effect of Nigella sativa oil on cisplatin induced nephrotoxicity and oxidative damage in rat kidney. Biomed Pharmacother. 2017;85:7–15.
- Koshak A, Koshak E, Heinrich M. Medicinal benefits of Nigella sativa in bronchial asthma: a literature review. Saudi Pharm J. 2017;25(8):1130–1136.
- Attree R, Du B, Xu B. Distribution of phenolic compounds in seed coat and cotyledon, and their contribution to antioxidant capacities of red and black seed coat peanuts (Arachis hypogaea L.). Ind Crops Prod. 2015;67:448–456.
- Orhon ZN, Uzal C, Kanter M, et al. Protective effects of Nigella sativa on gamma radiation-induced jejunal mucosal damage in rats. Pathol Res Pract. 2016;212(5):437–443.
- Dajani E, Shahwan T, Dajani N. Overview of the preclinical pharmacological properties of Nigella sativa (black seeds): a complementary drug with historical and clinical significance. J Physiol Pharmacol. 2016;67(6):801–817.
- Azwanida N. A review on the extraction methods use in medicinal plants, principle, strength and limitation. Med Aromat Plants. 2015;4(196):2167–0412.
- Yu M, Gouvinhas I, Rocha J, et al. Plasma Hsp90 levels in patients with systemic sclerosis and relation to lung and skin involvement: a cross-sectional and longitudinal study. Sci Rep. 2021;11(1):1–14.
- Krishnaveni M, Saranya S. Phytoconstituent analysis of Nigella Sativa seeds using analytical techniques. Bull Environ Pharmacol Life Sci. 2016;5(3):25–38.
- Iqbal MS, Ghafoor A, Ullah I, et al. Quantification and compositional diversity of fatty acid methyl esters profile in Nigella sativa L. germplasm. J Am Oil Chem Soc. 2014;91(11):1975–1986.
- Mohammed NK, Manap A, Yazid M, et al. The effects of different extraction methods on antioxi’dant properties, chemical composition, and thermal behavior of black seed (Nigella sativa L.) oil. Evidence-Based Complement Altern Med. 2016;2016:1–10.
- Kiralan M, Özkan G, Bayrak A, et al. Physicochemical properties and stability of black cumin (Nigella sativa) seed oil as affected by different extraction methods. Ind Crops Prod. 2014;57:52–58.
- Alrashidi M, Derawi D, Salimon J, et al. An investigation of physicochemical properties of Nigella sativa L. Seed oil from Al-Qassim by different extraction methods. J King Saud Univ Sci. 2020;32(8):3337–3342.
- Hassanien MF, Assiri AM, Alzohairy AM, et al. Health-promoting value and food applications of black cumin essential oil: an overview. J Food Sci Technol. 2015;52(10):6136–6142.
- Mohammed NK, Tan CP, Abd Manap Y, et al. Process conditions of spray drying microencapsulation of Nigella sativa oil. Powder Technol. 2017;315:1–14.
- Ismail A, Doolaanea A, Mohamed F, et al. Method development and validation using UV spectrophotometry for Nigella sativa oil microparticles quantification. J Appl Pharm Sci. 2015;082–088. doi:10.7324/japs.2015.50915.
- Amina B. Toxicity and anti-oxidant activity of the essential oil of Nigella sativa. Der Pharmacia Lettre. 2016;8(15):245–249.
- Nadaf N, Gawade S, Muniv A, et al. Exploring anti-yeast activity of Nigella sativa seed extracts. Ind Crops Prod. 2015;77:624–630.
- Lim TK. Edible medicinal and non-medicinal plants: volume 5, fruits. Vol 5. Springer; 2013. doi:10.1007/978-94-007-4053-2.
- Solati Z, Baharin BS, Bagheri H. Antioxidant property, thymoquinone content and chemical characteristics of different extracts from Nigella sativa L. seeds. J Am Oil Chem Soc. 2014;91(2):295–300.
- Karacabey E. Optimization of microwave-assisted extraction of thymoquinone from nigella sativa L. Seeds. Maced J Chem Chem Eng. 2016;35(2):209–216.
- Rohman A, Lukitaningsih E, Rafi M, et al. Nigella sativa oil: physico-chemical properties, authentication analysis and its antioxidant activity. Food Res. 2019;3(6):628–634.