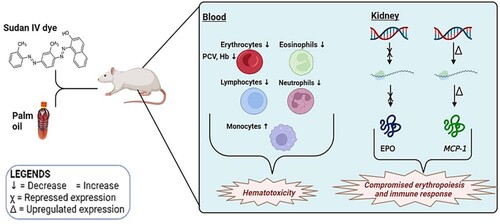
ABSTRACT
Palm oil (PO), the most consumed edible oil, is persistently adulterated with Sudan IV dye (S4D). We investigated the potential hematological derangements occasioned by ingesting S4D-adulterated PO. Thirty male albino rats were grouped into five (n = 6); Control, PO (10%), PO + S4D (100 mg), PO + S4D (250 mg), and S4D (250 mg). Rats received unadulterated or S4D-adulterated PO through diet for 21 days. Exposure to S4D (alone and via adulterated PO) provoked significant (p < 0.05) reductions in the packed cell volume, hemoglobin, red and white blood cell counts, neutrophils, lymphocytes, and eosinophils, while monocyte population was significantly (p < 0.05) elevated. The erythropoietin gene expression was downregulated, while monocyte chemoattractant protein-1 was upregulated in the kidneys of S4D-exposed groups compared to control and PO-alone groups. Notably, these hematological parameters were not significantly different (p > 0.05) in the unadulterated PO-exposed rats compared to the control. These results reveal the potential hematotoxic and immunotoxic effects of adulterating PO with S4D.
1. Introduction
Despite human efforts to control it, the world’s population has continued to rise, and there is the ever-present demand for food, among other amenities [Citation1]. Food vendors, in a bid to make their food products more attractive, increase the product’s shelf-life, improve their nutritional flavor, or make them taste better, add several natural or synthetic compounds to the food products. Such compounds, introduced into foods to produce a technical effect, are called food additives. These additives may be intentionally added (as are most food additives) or indirectly added during production (while packaging, storing, or transporting) [Citation2,Citation3].
However, end-users and researchers continue to question the need and safety of these additives, given the various reports of attendant adverse effects. For example, monosodium glutamate has been implicated in testicular damage [Citation4]), neurodegenerative diseases [Citation5], and metabolic syndrome [Citation6]. Higher risks of various cancers have been associated with higher intakes of nitrites and nitrosamine (found in processed meat and other similar products as a preservative). In addition, hepato-renal dysfunction and oxidative stress have been reported following exposure to tartrazine and carmoisine - typical examples of azo food dyes [Citation7].
Probably because color is the most noticeable attribute of food products and can be easily assessed without necessarily having to purchase the product, food vendors and industries employ several dyes to improve color, thereby boosting sales [Citation8]. Palm oil (PO) is one food item whose color is essential to sales. PO, edible oil derived from the mesocarp of oil palm fruits, is the most consumed plant oil globally. The world consumed over sixty-three million tons of PO in 2016, compared to under a million tons in 1970. This value is expected to triple by 2050 [Citation9]. Found in a wide range of products (foods, biofuel, and even cosmetics), the intense red color of PO is typically used as a measure of its quality [Citation10]. Regrettably, many markets add colorants to enhance the color of PO, despite the health issues associated with these colorants. The primary colorant in PO is Sudan IV dye (S4D) [Citation11,Citation12].
Sudan IV dye (C24H2ON4O) is an azo dye used as a colorant in industrial products, such as shoe polish, petrol, soaps, plastics, oil, and printing inks [Citation13]. It has been ruled unsafe under the 1995 Colors in Food Regulations and is an illegal food dye. But owing to the intense red-orange color and inexpensiveness of S4D, it has been deliberately added to foods products, such as palm oil, chili powder, egg products, and poultry meat, as coloring agents [Citation13]. The rise in the use of palm oil, particularly in homemade and ready-to-eat meals, coupled with its bright reddish color, make it a prime target for adulteration with S4D; an incident that has, unfortunately, continued to escalate [Citation11,Citation12]. In Washington DC, the United States, a survey by Genualdi et al. [Citation11] observed S4D as the principal azo-dye in PO, with concentrations reaching 24 µg/ml, as against the EU detection limits of <1.2 µg/ml. Another study, this time in Western Africa, confirmed the adulteration of PO samples with S4D, previously reported by the Food and Drug Authorities of Ghana [Citation12]. Even in Nigeria, the adulteration of PO with S4D is rampant and now considered normal [Citation12].
Nevertheless, few studies have attempted to assess the in vivo effects of the adulteration of PO with S4D. To the best of our knowledge, no study has reported its adverse effects on haematological parameters using a murine model.
2. Methodology
2.1. Test materials and chemicals
Sudan IV dye was a product of NICE Chemicals Pvt. Ltd. (Kochi, Kerala, India), while unadulterated palm oil was obtained directly from the oil mill in Abeokuta, Ogun State, Nigeria. TRIzol reagent and DNAse-I enzyme were products of ThermoFisher Scientific (Waltham, Massachusetts, USA). Gene-specific primers were synthesized by ShineGene Bio-Technologies, Inc. (Xuhui District, Shanghai, China). All other chemicals, unless otherwise specified, were products of the British Drug House Chemicals Limited (Poole Dorset, England).
2.2. Experimental animals and study design
The departmental Animal Ethical Committee approved this study (FUNAAB/CBS/BCH/20150805). Thirty male Wistar rats (weighing 150–200 g; 8–9 weeks old) were procured from the animal house of the College of Veterinary Medicine, Federal University of Agriculture, Abeokuta, Nigeria, housed in the departmental animal house and allowed free access to chow and water. The diet consisted of carbohydrates (65%), protein (20.3%), fat (5%), fiber (5%), vitamins mixture (1%), and mineral mixture (3.7%), according to Amin et al. [Citation7]. Rats were acclimatized for one week and then randomly distributed into five groups (n = 6): Control, palm oil (PO), PO + S4D (100 mg), PO + S4D (250 mg), and S4D (250 mg). Control rats received standard chow; PO groups received feed supplemented with PO (10%); PO + S4D (100 mg) and PO + S4D (250 mg) groups received chow supplemented with PO (10%) containing 100 mg or 250 mg of S4D per liter of PO, respectively; the S4D (250 mg) group received chow supplemented an equivalent amount of the 250 mg of S4D per liter of PO (albeit without PO). These doses of S4D were based on the previously reported detection levels of S4D in PO [Citation11], while the 10% PO used was in line with the recommendation that saturated fats (which palm oil is abundant in) should not contribute more than 10% of the total calorie intake [Citation14]. The study duration was 21 days.
2.3. Sacrifice and sample collection
After 21 days, rats were fasted overnight, anesthetized with 100 mg/kg Ketamine and 7 mg/kg Xylazine (i.p.), and euthanized. Blood (1 mL) was collected via retro-orbital plexus into EDTA tubes for hematological analysis. Small portions of the left kidney were also preserved in TRIzol reagent for gene expression analyses.
2.4. Hematological analysis
Hematological profiles, including red blood cell (RBC) count, white blood cell (WBC), hemoglobin, and packed cell volume (PCV), as well as WBC differentials, were determined in the blood samples using an automated hematological analyzer (Model ACT-8; Beckman Coulter Electronics, Brea, California, USA).
2.5. Genes profiling
The expressions of genes coding for erythropoietin (EPO) and monocyte chemoattractant protein-1 (MCP-1) were assessed, relative to the house-keeping gene β-actin, using the reverse transcriptase-polymerase chain reaction technique. Total mRNA was isolated from the kidney tissues using TRIzol and purified by DNAse-I treatment. Reverse transcription of the purified (DNA-free) mRNA to cDNA was done using ProtoScript® First Strand cDNA Synthesis Kit (New English Biolabs (NEB), Ipswich, Massachusetts, USA). cDNA was amplified via a polymerase chain reaction, using OneTaq® 2X Master Mix (NEB) with gene-specific primers (Table ). Representative snapshots of reverse transcription-polymerase chain reaction-agarose gel electrophoresis were taken, and the band density (Image-J) [Citation15] was plotted as a bar graph (mean ± SEM, n = 6 per group).
Table 1 Gene-specific primers
2.6. Statistical analysis
The data obtained are as mean ± S.E.M. Differences between groups were compared with one-way Analysis of Variance (ANOVA) and heterogeneity occurred, means were separated using Bonferroni test, with p < 0.05 considered significant. All analyses and graphs were plotted using Graph Pad Prism (Version 6.0).
3. Results and discussion
The most relevant transport route within the body is the blood, which plays a vital role in chemical toxicity. Therefore, hematological assessments are critical aspects of toxicology. Unsurprisingly, repressed hematopoiesis, hemoglobin denaturation, and increased destruction of blood cells often result from exposure to a wide range of toxic agents, including food colorants [Citation16]. The adulteration of palm oil (PO) with Sudan IV dye (S4D) is now a common practice, particularly in Western Africa, some parts of Asia, and even the United States [Citation11,Citation12]. The persistent inclusion of several food additives into food products, despite the alleged health implications associated with the use of food additives, calls for scientific scrutiny to ascertain these implications and help promulgate policies that will limit their use [Citation3,Citation12]. This study elucidates the hematological changes elicited by the ingestion of S4D-adulterated PO.
The effects of sub-acute exposure to S4D-adulterated PO on the PCV and Hb of experimental rats are depicted in Figure A and B. Exposure to S4D-adulterated PO provoked significant (p < 0.05) reductions in the PCV and Hb compared to the control group. In contrast, exposure to unadulterated PO had no significant (p > 0.05) effect on the PCV and Hb of the experimental rats. Figure C and D show the impact of S4D-adulterated PO ingestion on RBC and WBC counts, which revealed significant (p < 0.05) depletions of these blood cell counts in rats exposed to S4D, via PO (both doses) and alone (250 mg). On the other hand, rats exposed to unadulterated PO had RBC, and WBC counts statistically at par with those of the control group.
Figure 1 Effects of exposure to Sudan IV dye (S4D)-adulterated palm oil (PO) on (A) packed cell volume (PCV), (B) hemoglobin concentration (Hb), (C) red blood cell (RBC) counts, and (D) white blood cell (WBC) counts in the blood of control and experimental rats. Bars (mean ± S.E.M. of six rats n = 6) bearing * are significantly different from the control group at p < 0.05 (Bonferroni test).
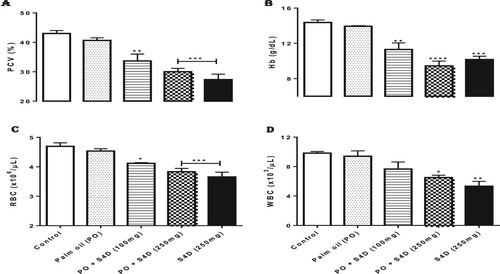
These results suggest the ability of S4D to affect the hemato-physiology of exposed rats. Similar results have been observed in mice fed a mixture of tartrazine, carmoisine, and brilliant blue [Citation17], in female Swiss mice fed tartrazine [Citation18], and in rats fed with a mixture of Sunset Yellow, azorubine, and their degradation products [Citation19]. These authors all reported decreased hematological parameters in animals exposed to various other azo dyes. Sharma et al. [Citation18] attributed the dye (tartrazine was used in their study)-induced decrease in Hb content to inhibited iron absorption by developing RBCs, thereby preventing Hb synthesis and contributing to reduced PCV. A separate experiment by Parmar and Shah [Citation20] evaluated the hematological parameters following exposure to another azo dye (Acid Red-97) and observed similar depletion of hematological parameters, which was attributed to the deficiency of iron and its decreased utility.
Furthermore, careful evaluation of the biotransformation of S4D may provide another perspective. The biotransformation of S4D and other azo dyes follow several oxidative routes involving azoreductases, cytochrome P450 monooxygenases, and peroxidases. Regardless of the pathway, reactive species, such as O2•-, •OH, and H202, are invariably produced [Citation13,Citation21,Citation22]. Indeed, Zhang et al. [Citation13] demonstrated increased release of these reactive species by HepG2 cells exposed to S4D, after just one hour, while An et al. [Citation21] reported a dose-dependent increase in reactive species production by HepG2 cells exposed to Sudan I dye compared to the positive control. Therefore, it would seem probable that the S4D-increased production of these reactive species could have mediated an oxidative attack on these blood cells, which may be involved in the depletion of hematological parameters observed. In addition, the reduced RBCs and Hb content may suggest that the S4D-exposed rats suffered from hypochromic microcytic anemia (a condition of low levels of red blood cells that are both smaller and paler due to reduced Hb) [Citation20], while the depletion in the WBC counts may indicate the immunosuppressive capacity of S4D.
Figure presents the effects of ingestion of S4D-adulterated PO on WBC differentials in the blood of experimental and control rats. While the population of neutrophils was lower in the PO + S4D (100 mg), PO + S4D (250 mg), and S4D (250 mg) groups, the decrease was only significant (p < 0.05) in the S4D (250 mg) group. Significant (p < 0.05) decrease in the lymphocytes population was observed in the PO + S4D (250 mg) and S4D (250 mg) groups, while all S4D-exposed groups had a significantly lower amount of eosinophils when compared to the control group. These results further accentuate the immunosuppressive capacity of S4D. Neutrophils are the most abundant granulocytes (about 40–70% of WBCs), while the eosinophils and basophils make up the remaining granulocytes present in the blood. These granulocytes are a vital component of the innate immune response in their fight against various infections, such as bacterial and multicellular parasites [Citation23]. Lymphocytes, the main WBC found in the lymph, are the primary mediators of adaptive immunity and include both T- and B-cells [Citation24]. Thus, a reduced lymphocyte population would invariably compromise innate and adaptive immunity.
Figure 2 Effects of exposure to Sudan IV dye (S4D)-adulterated palm oil (PO) on (A) neutrophils, (B) lymphocytes, (C) monocytes, and (D) eosinophils counts in the blood of control and experimental rats. Bars (mean ± S.E.M. of six rats n = 6) bearing * are significantly different from the control group at p < 0.05 (Bonferroni test).
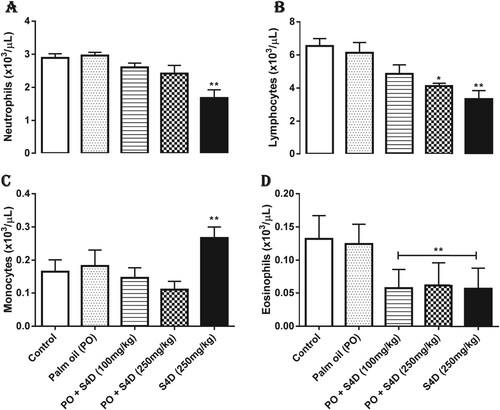
Interestingly, the monocyte population was significantly (p < 0.05) increased in the S4D (250 mg) group compared to other experimental groups. Monocytes are the largest WBCs and are specialized in the phagocytosis of foreign antigens. They mature into macrophages that are vital mediators of inflammation [Citation25]. Therefore, their increased population may connote the initiation of an inflammatory response following ingestion of S4D. The increased production of reactive species during the metabolism of S4D may have obligated the increase in the monocyte population, since oxidative stress and inflammation are tightly-linked events [Citation26].
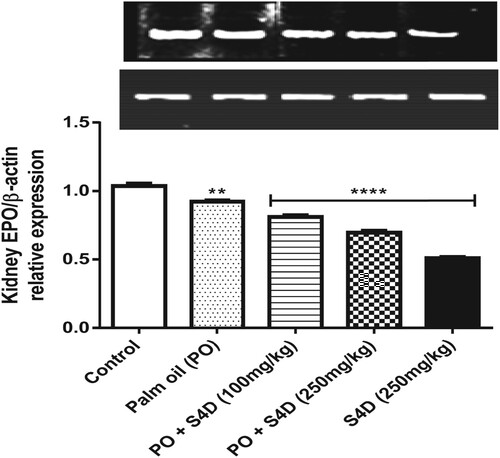
Figure depicts the effects of ingestion of S4D-adulterated PO on the expression of the gene coding for EPO, relative to β-actin, in the kidneys of experimental and control rats. EPO is a glycoprotein hormone secreted mainly by the kidney and is crucial for RBC production (erythropoiesis) in the bone marrow. EPO exerts its effects by protecting RBC progenitors and precursors, resident in the bone marrow, from necrosis or apoptosis. Moreover, to make up for normal RBC turnover, EPC is constitutively expressed, albeit at minimal levels [Citation27]. The relative expression of EPO was significantly (p < 0.05) downregulated in the groups that consumed S4D, via PO (both doses) and alone (250 mg) compared to the control. Also, the PO alone group showed significantly (p > 0.05) decreased EPO gene expression compared to the control. This repression of EPO expression by S4D has not been reported before and may contribute to the observed decrease in PCV, Hb, and RBC count. Therefore, not only did S4D deplete the hematological parameters, it could have also mediated a mechanism that leads to reduced erythropoiesis, possibly via inhibition of EPO secretion.
Figure 3 Effects of exposure to Sudan IV dye (S4D)-adulterated palm oil (PO) on the expression of erythropoietin (EPO), relative to β-actin, in the renal tissues of control and experimental rats. Bars (mean ± S.E.M. of six rats n = 6) bearing * are significantly different from the control group at p < 0.05 (Bonferroni test).
Besides its erythropoietic role, EPO is a crucial regulator of immune homeostasis, protecting tissues against pathogen invasion, injury, oxidative damage, and hypoxia. In response to these stimuli, transcription factors (TFs), such as hypoxia-inducible factors, are activated. These TFs bind to the enhancer region of the EPO gene and upregulate its expression [Citation28]. EPO then binds to tissue-protective receptors, thereby inhibiting immune and inflammatory responses, rescuing cells, and attenuating the spread of injury [Citation27]. Therefore, repression of its production may delay the resolution of immune and inflammatory responses, eventually leading to cell death [Citation27], and might have contributed to the depleted blood cell counts observed in the S4D-exposed groups.
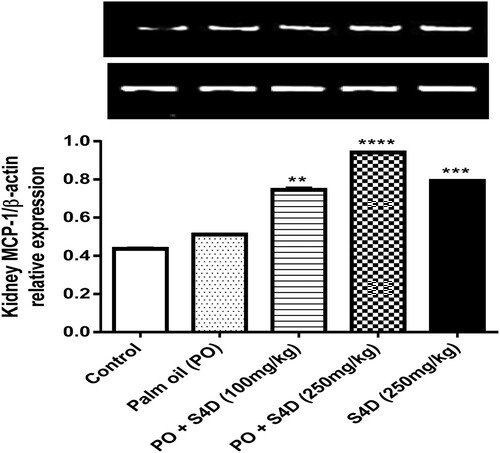
Conversely, as shown in Figure , the relative expression of MCP-1 was significantly (p < 0.05) upregulated in the kidneys of all the groups that ingested S4D compared to the control group. In contrast, the group that consumed unadulterated PO showed no significant (p < 0.05) difference. MCP-1, an inducible cytokine belonging to the CC class of chemokines, is predominantly produced by monocytes and matured monocytes (i.e. macrophages or dendritic cells) to recruit other immune cells to the sites of inflammation elicited by a xenobiotic or infection [Citation29]. Also, MCP-1 regulates monocyte release from the bone marrow and creates a chemokine gradient that directs monocytes to inflamed tissue. Besides, the increased monocyte population (a prevailing event in inflammatory response) may also contribute to the elevated MCP-1 expression, as they are major secretors of MCP-1 [Citation30]. Thus, the upregulated MCP-1 expression may suggest the involvement of immune response activation and inflammation in S4D-provoked toxicity.
Figure 4 Effects of exposure to Sudan IV dye (S4D)-adulterated palm oil (PO) on the expression of monocyte chemoattractant protein-1 (MCP-1), relative to β-actin, in the renal tissues of control and experimental rats. Bars (mean ± S.E.M. of six rats n = 6) bearing * are significantly different from the control group at p < 0.05 (Bonferroni test).
4. Conclusion
This study is the first to provide scientific data on the hematologic effects of adulterating PO with S4D. We conclude that ingestion of S4D-adulterated PO may be associated with hematotoxic and possibly immunotoxic changes (Figure ), although more research is warranted to elucidate the precise mechanism(s) involved. Nevertheless, we recommend that consumers and manufacturers of food products be sensitized to the health hazards implicated in the adulteration of PO with S4D.
Conflicts of interest
The authors declare nothing.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Crist E, Mora C, Engelman R. The interaction of human population, food production, and biodiversity protection. Science. 2017;356(6335):260–264.
- FDA (Food and Drug Administration). (2018). Food ingredients and packaging terms. FDA Centre for Food Safety and Applied Nutrition. [accessed 2021 Mar 6]. https://www.fda.gov/food/food-ingredients-packaging/food-ingredient-packaging-terms.
- WHO (World Health Organization). (2020). Food additives. WHO fact sheets. [accessed 2021 Mar 8]. https://www.who.int/news-room/fact-sheets/detail/food-additives.
- Hamza RZ, Diab AEAA. Testicular protective and antioxidant effects of selenium nanoparticles on monosodium glutamate-induced testicular structure alterations in male mice. Toxicol Rep. 2020;7:254–260.
- Hussein UK, Hassan NEHY, Elhalwagy ME, et al. Ginger and propolis exert neuroprotective effects against monosodium glutamate-induced neurotoxicity in rats. Molecules. 2017;22(11):1928.
- Bautista RJH, Mahmoud AM, Königsberg M, et al. Obesity: pathophysiology, monosodium glutamate-induced model and anti-obesity medicinal plants. Biomed Pharmacother. 2019;111:503–516.
- Amin KA, Hameid II HA, Abd Elsttar AH. Effect of food azo dyes tartrazine and carmoisine on biochemical parameters related to renal, hepatic function and oxidative stress biomarkers in young male rats. Food Chem Toxicol. 2010;48(10):2994–2999.
- Nisa A, Zahra N, Butt YN. Sudan dyes and their potential health effects. Pak J Biochem Mol Biol. 2016;49(1):29–35.
- Prokurat S. Palm oil–strategic source of renewable energy in Indonesia and Malaysia. Journal of Modern Science. 2013;3:425–443.
- Oguntibeju OO, Esterhuyse AJ, Truter EJ. Red palm oil: nutritional, physiological and therapeutic roles in improving human wellbeing and quality of life. Br J Biomed Sci. 2009;66(4):216–222.
- Genualdi S, MacMahon S, Robbins K, et al. Method development and survey of Sudan I–IV in palm oil and chilli spices in the Washington, DC, area. Food Additives & Contaminants: Part A. 2016;33(4):583–591.
- Andoh SS, Nuutinen T, Mingle C, et al. Qualitative analysis of Sudan IV in edible palm oil. Journal of the European Optical Society-Rapid Publications. 2019;15(1):1–5.
- Zhang Y, An Y, Jiang L, et al. The role of oxidative stress in Sudan IV-induced DNA damage in human liver-derived HepG2 cells. Environ Toxicol. 2011;26(3):292–299.
- WHO (World Health Organization). (2019). Healthy diet (No. WHO-EM/NUT/282/E). [accessed 2022 Feb 24]. Regional Office for the Eastern Mediterranean. https://apps.who.int/iris/bitstream/handle/10665/325828/EMROPUB_2019_en_23536.pdf.
- Schneider CA, Rasband WS, Eliceiri KW. NIH image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–675.
- Engwa GA, Ferdinand PU, Nwalo FN, et al. In: O Karcioglu, B Arslan, editor. Poisoning in the modern world - New tricks for an Old Dog? London: IntechOpen; 2019. 10.5772/intechopen.82511.
- Sharma A, Goyal RP, Chakravarty G, et al. Haemotoxic effects of chocolate brown, a commonly used blend of permitted food colour on Swiss albino mice. Asian J Exp Sci. 2005;19(2):93–103.
- Sharma G, Gautam D, Goyal RP. Tartrazine induced haematological and serological changes in female Swiss albino mice, Mus musculus. Pharmacologyonline. 2009;3:774–788.
- Elbanna K, Sarhan OM, Khider M, et al. Microbiological, histological, and biochemical evidence for the adverse effects of food azo dyes on rats. J Food Drug Anal. 2017;25(3):667–680.
- Parmar AI, Shah AI. Haematological parameters and histopathological alterations in the gills of fish, catla catla exposed to Azo Dye acid Red -97. Advances in Zoology and Botany. 2020;8(4):342–350.
- An Y, Jiang L, Cao J, et al. Sudan i induces genotoxic effects and oxidative DNA damage in HepG2 cells. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 2007;627(2):164–170.
- Cox JA, White PA. The mutagenic activity of select azo compounds in MutaMouse target tissues in vivo and primary hepatocytes in vitro. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 2019;844:25–34.
- Monteiro ACB, Iano Y, França RP, et al. (2020, December). Hematology: A review of the main methodologies of clinical analyses. In Proceedings of the 5th Brazilian Technology Symposium: Emerging Trends, Issues, and Challenges in the Brazilian Technology, Volume 2 (Vol. 202, p. 49). Springer Nature.
- Camiolo M, Gauthier M, Cohn L, et al. Biology of lymphocytes. middleton's allergy E-book: principles and practice. elsevier health sciences. Philadelphia: Saunders; 2019. 203–214.
- Zhang C, Yang M, Ericsson AC. Function of macrophages in disease: current understanding on molecular mechanisms. Front Immunol. 2021;12:635.
- Aleksandrova K, Koelman L, Rodrigues CE. Dietary patterns and biomarkers of oxidative stress and inflammation: A systematic review of observational and intervention studies. Redox Biol. 2021;42:101869.
- Peng B, Kong G, Yang C, et al. Erythropoietin and its derivatives: from tissue protection to immune regulation. Cell Death Dis. 2020;11(2):1–12.
- Kuhrt D, Wojchowski DM. Emerging EPO and EPO receptor regulators and signal transducers. Blood, The Journal of the American Society of Hematology. 2015;125(23):3536–3541.
- Grzegorzewska AE, Mostowska A. Monocyte Chemotactic Protein-1 (Cytokine, Receptors, and Gene Polymorphisms) in Hepatitis. In: Patel V, Preedy V, eds. Biomarkers in liver disease. Biomarkers in disease: methods, discoveries and applications. Switzerland (AG): Springer Nature; 2017. p. 927–955.
- Haller H, Bertram A, Nadrowitz F, et al. Monocyte chemoattractant protein-1 and the kidney. Curr Opin Nephrol Hypertens. 2016;25(1):42–49.