Abstract
As a prescribed antioxidant, N-Acetylcysteine is used in treating disease and supportive care. However, N-acetylcysteine’s role in cancer therapy is controversial and needs to be investigated thoroughly. Presently, renal cell carcinoma is one of the most common forms of malignancy and needs new anti-cancer drugs with high efficacy and low side effect. Herein, we studied the effect of N-acetylcysteine on cell proliferation, oxidant stress, and apoptosis on human renal cell carcinoma cell line A498 and tried to understand the mechanisms driving these effects. The results show that N-acetylcysteine could enhance antioxidative activities against oxidative damage and suppress the expression of the directly apoptotic protein to inhibit apoptosis when used at low concentration (<10 mM), thereby protecting A498 cells. On the other hand, N-acetylcysteine induced oxidative stress, leading to mitochondria damage and directly activating apoptotic protein to induce apoptosis at high concentrations (>10 mM), thereby killing A498 cells. Therefore, N-acetylcysteine has two contradictory activities towards renal cell carcinoma cells.
1. Introduction
A precursor of reduced glutathione (GSH), N-Acetylcysteine (NAC), is a commonly prescribed antioxidant and reductant. The excellent reducing property of NAC is due to the thiol functional group. The sulphhydryl groups can effectively scavenge free radicals. Moreover, cysteine can promote the synthesis of reduced GSH during metabolism. Therefore, NAC is a considered effective antioxidant [Citation1–3]. Due to its antioxidant activity, NAC is used in treating diseases, such as Alzheimer’s disease [Citation4], diabetic neuropathy [Citation5], age-related hearing loss and memory impairment [Citation6], influenza [Citation7], asthma [Citation8], insulin resistance [Citation9], intrauterine growth retardation [Citation10], heavy metal toxicity [Citation11] and sports injury prevention [Citation12]. These effects are primarily due to the radical scavenging activity of NAC to protect cells against oxidative damage. However, NAC’s role in cancer therapy is controversial. The reduction of reactive oxygen species (ROS) and oxidative stress by antioxidants could lead to the survival of cancer cells and the progression of tumour [Citation13]. In the JunD-deficient mice, NAC treatment decreased lung oxidative damage from lung emphysema but concomitantly induced the development of lung adenocarcinoma [Citation14]. Besides, the combined use of NAC and vitamin E increased tumour progression by disrupting the ROS-p53 axis [Citation15]. NAC has been widely implicated in the prevention and therapy of several cancesr. NAC decreased the malignant characteristics of glioblastoma cells by inhibiting Notch2 signalling [Citation16]. Furthermore, NAC could enhance the cytotoxicity of some anti-cancer drugs by inducting ROS-independent apoptosis in cancer cells [Citation17,Citation18]. Thus, NAC’s role in cancer therapy seems dichotomous and needs to be thoroughly investigated.
Renal cell carcinoma (RCC) is still one of the most common malignant tumours in humans, the most lethal urothelial tract cancer known so far [Citation19]. Unfortunately, the presently available treatments (surgical intervention, chemotherapy, radiotherapy, and hormonal therapy) for RCC do not provide satisfactory curative results. Currently, there is no clinically proved therapy to cure RCC [Citation20] completely. Although drug therapy as an important tumour treatment method can kill cancer cells quickly and rapidly control the disease, five-year survival rates of RCC with advanced disease profiles remain unacceptably low. The immunity drugs and chemotherapeutics still do not provide desired curative solutions [Citation21]. Keeping this in mind, it is imminent to find new anti-cancermedicines with natural properties, low side effects, and high efficacy.
Although NAC may be a potential chemotherapeutic agent, no study is available in the literature focusing on RCC treatment using NAC. NAC’s role in RCC treatment remains unclear, and any bioactivity of NAC other than antioxidation is largely unknown. In this study, we evaluated the cytotoxic activity of NAC on RCC cells (A498) and investigated the therapeutic or protective effects of NAC on RCC. We also studied the ROS-independent apoptosis in A498 cells induced by NAC to elucidate its toxic mechanism. The present study provides a new idea for applying NAC in the drug therapy of RCC.
2. Materials and methods
2.1. Materials
Human RCC cell lines A498 were purchased from ATCC (USA). Dulbecco's modified eagle medium (high glucose) (DMEM), pancreatin, penicillin and streptomycin (PS), and fetal bovine serum (FBS) were obtained from Gibco (USA). N-Acetylcysteine was purchased from Sigma (USA). Cell counting kit-8 (CCK-8) was obtained from Dojindo (Japan). Reactive oxygen species (ROS) assay kit (DCFH-DA method), total superoxide dismutase assay kit (SOD, WST-8 method), catalase assay kit (CAT), cellular glutathione peroxidase assay kit (GSH-PX, NADPH method), lipid peroxidation assay kit (MDA), total antioxidant capacity assay kit (T-AOC, rapid ABTS method), mitochondrial membrane potential assay kit (JC-1 method) were purchased from Beyotime (China). Antibodies against Nrf2 (bs-1074R), HO-1 (bs-23397R), NQO1 (bs-2184R), NFĸB p65 (bs-0465R), caspase-3 (bs-33277M), caspase-9 (bs-0049R), PARP (bs-2138R), β-actin (bs-0061R) and rabbit anti-rat IgG antibody (bs-0293R) were bought from Bioss (China). Anti-rabbit antibodies against cleaved PARP (380374) and tubulin α (341001) were obtained from ZEN BIO (China). Anti-rabbit antibodies against cleaved caspase-9 (GTX132331) and cleaved caspase-3 (GTX86952) were purchased from GeneTex (USA).
2.2. Cell culture
Human RCC cell lines A498 were maintained in a DMEM medium supplemented with 10% FBS and 1% PS. A498 were cultured at 37°C in a humidified incubator with 5% CO2. All cells in the exponential growth phase were used for experiments.
2.3. CCK-8 assay
A498 cells were seeded into 96-well plates with 5000 cells per well. After 12 h, NAC solution in different concentrations (2.5, 5, 10, 20, 25 mM) was added to the medium. Cellular viability was measured using the CCK-8 assay after successive cultivation for 24 h under humidified atmosphere. A498 cells without any treatment were used as the positive control to fix the cellular viability to 100%. The above data were used to calculate the 50% inhibitory concentration (IC50) of NAC. The cellular viability of cell exposure time to NAC of 48 and 72 h was also investigated.
2.4. Measurement of intracellular ROS
The treatment method for A498 cells was the same as Section 2.3. The initial culture medium was taken out, and a fresh medium, containing 10 µM/L of DCFH-DA, was added to submerge the cells. A498 cells were cultured for another 20 min. Then cells were washed thrice with DMEM. Their fluorescence absorbances were detected using a microplate reader at the excitation and emission wavelengths of 488 and 525 nm, respectively. A498 cells without any treatment were set as the blank control, and cells treated with Rosup were set as the positive control.
2.5. Detection of mitochondrial membrane potential
A498 cells were treated the same as Section 2.3. The cells were incubated for 20 min after treating with 100 µL of JC-1 working solution. The cells were then washed twice with cool JC-1 staining buff, followed by adding 100 µL of DMEM. The fluorescence absorbances of JC-1 polymerides were detected using a microplate reader at the excitation and emission wavelengths of 525 and 590 nm, respectively. And the fluorescence absorbances of JC-1 monomers were detected using a microplate reader at the excitation and emission wavelengths of 490 and 530 nm, respectively. Meanwhile, a fluorescence microscope was also used to observe their fluorescence intensities. A498 cells treated with CCCP were set as the positive control.
2.6. Determination of antioxidant system
A498 cells were seeded into 96-well plates with 5000 cells per well. After allowing the A498 cells to adhere to the plates overnight, NAC solution in different concentrations was added to five experimental groups. After 24 h, the cellular activities of SOD, CAT, GSH-PX, MDA and T-AOC were determined using corresponding assay kits according to their explanatory protocol.
2.7. Western blot analysis
According to their explanatory protocol, cells were washed with ice-cold PBS and collected. A498 cells were blotted with primary antibodies against Nrf2, HO-1, NQO1, NFĸB p65, caspase-3, caspase-9, PARP, cleaved caspase-9, cleaved PARP, cleaved caspase-3, or β-actin and tubulin α (as an internal loading control, respectively), and with a secondary anti-IgG antibody. The immunoblots were visualized by the super signal west pico chemiluminescence substrate and imaged by the VersaDoc imaging system.
2.8. Statistical analysis
All the experiments were repeated six times. A one-way or two-way ANOVA test determined the statistical difference. Statistical significance was accepted at P < 0.05.
3. Results
3.1. Cytotoxicity
A498 was treated with different concentrations of NAC for 24 h. The cellular viability was slightly increased at the NAC concentration of 2.5 mM. And the cellular viability was sharply decreased when the concentration of NAC was higher than 5 mM (Figure S1). As derived from the standard curve, IC50 of NAC was 18.62 mM.
The cytotoxic effect of NAC on A498 cells for a longer exposure time (48 & 72 h) at different concentrations was also investigated. Similarly, there were non-apoptotic effects of the NAC at lower levels but apparent cytotoxicity at higher levels (Figures S2 & S3).
3.2. Oxidative stress and mitochondrial injury
The intracellular ROS levels in A498 cells varied according to the concentration of NAC. The green fluorescence intensity of NAC-treated cells intensified with increased NAC concentration (Figure (A)). The yield of ROS increased significantly upon using NAC above 20 mM concentration compared to the blank control (Figure (B)). But, in the groups treated with low doses of NAC, the level of cellular ROS was close to that of the blank control group. The results indicated that NAC could trigger increased oxidative stress in A498 cells correlated with the treatment concentration. This suggests that NAC can induce oxidative stress-mediated injury in A498 cells.
Figure 1. The green fluorescence intensity (A) and productivity (B) of ROS, and changes in JC-1 level (C) and quantitative analysis (D) in A498 induced by NAC. *P < 0.05 compared to the blank control.
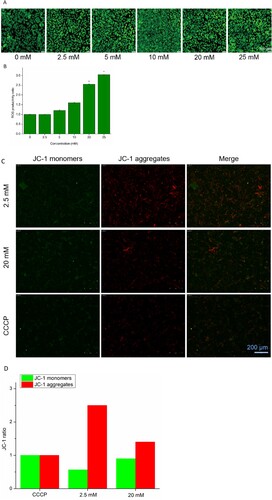
A mitochondrial membrane potential detection kit was used to detect the change in mitochondrial membrane potential. Figure (C) shows the changes in values of A498 mitochondrial membrane potential being treated with NAC. The green fluorescence intensity was enhanced, while the red fluorescence intensity was reduced in the groups treated with high doses of NAC. The results obtained were similar to the status of the CCCP-treated group. The results showed that , the mitochondrial membrane potential can also be depolarized by NAC, similar to CCCP. In contrast, in the groups treated with low doses of NAC, the red fluorescence intensity was stronger, indicating the stable mitochondrial membrane potential in A498 cells. Their quantitative analysis is shown in Figure (D), which held the same tendency that a low dose of NAC enhanced red fluorescence intensity, while a high dose of NAC enhanced green fluorescence intensity. NAC can change the mitochondrial membrane potential of A498 cells at higher concentrations, which eventually damages mitochondria, while stabilized mitochondrial membrane potential at low concentrations helps protect cells.
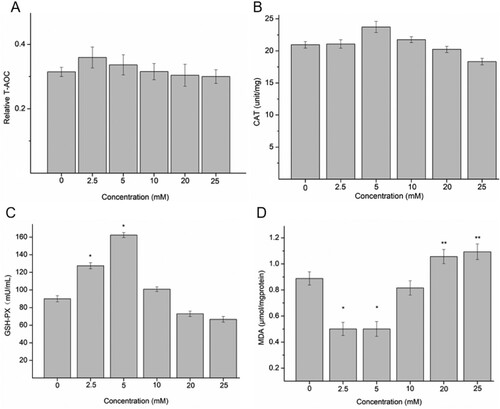
3.3. Effects of NAC on antioxidant activities
The effects of NAC on the activities of T-AOC, CAT, GSH-PX, SOD and on the MDA content of A498 cells are presented in Figure . NAC could increase T-AOC and CAT of A498 cells at a concentration below 10 mM. However, upon increasing the concentration of NAC above 10 mM, the T-AOC and CAT contents of A498 cells were decreased (Figure (A,B)). The GSH-PX activity of 2.5 and 5 mM NAC-treated groups was significantly higher than those of other groups. And the GSH-PX activity of 20 and 25 mM NAC-treated groups was lower than that of the blank control group (Figure (C)). In addition, there was a significant decrease of MDA in 2.5 and 5 mM NAC-treated groups and a significant increase of MDA in 20 and 25 mM NAC-treated groups (Figure (D)). These results revealed that NAC had enhanced antioxidant capacity against oxidative damage at low concentrations (<10 mM), whereas antioxidant capacity is reduced to cause oxidative damage to A198 cells at high concentrations of NAC (>20 mM).
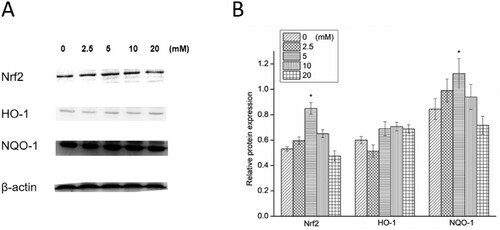
3.4. Influence of NAC on Nrf2-HO-1/NQO-1 signalling pathway
Generally, the expression of Nrf2, HO-1 and NQO-1 was increased in the low-dose-treated NAC groups and decreased in the high-dose-treated NAC groups (Figure ). NAC significantly increased the expression of Nrf2 at 5 mM, which enhanced the expression of downstream HO-1 and NQO-1. On the contrary, a high dose of NAC suppressed the expression of Nrf2 and NQO-1. This result indicated that at low doses, NAC could initiate the Nrf2-HO-1/NQO-1 signalling pathway to increase the expression of peroxiredoxins to keep A498 cells safe from oxidative stress. Nevertheless, at high doses, NAC could suppress the Nrf2-HO-1/NQO-1 signalling pathway to decrease the expression of peroxiredoxins and eventually aggravate oxidative stress.
3.5. Effects of NAC on A498 apoptosis
The expressions of apoptosis-related proteins in A498 cells exposed to NAC are shown in Figures and . An increase was found in pro-apoptotic protein (caspase-9) level, especially for high-dose NAC treatment. The expression of apoptotic protein (PARP) level was down-regulated in the groups treated with a low dose of NAC and up-regulated in the groups treated with a high dose of NAC. The expression cleaved caspase-9 was up-regulated, and PARP was cleaved by a high dose of NAC treatment. Then caspase-3 and cleaved caspase-3 were activated.
Figure 4. Western blotting (A) and relative protein expression of caspase-9, PARP and NF-κB (B). *P < 0.05 compared to the blank control.
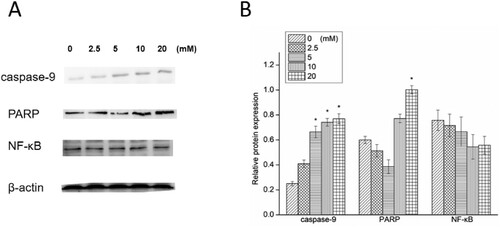
Figure 5. Western blotting (A) and relative protein expression of cleaved PARP, cleaved caspase-9, caspase-3 and cleaved caspase-3 (B). *P < 0.05 compared to the blank control.
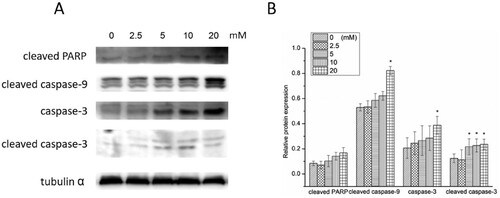
Anti-apoptotic protein expression in A498 cells exposed to NAC was also investigated. NF-κB expression was down-regulated, and that was to a greater extent in high-dose NAC treated groups.
These results showed that NAC could induce apoptosis in A498 cells by stimulating caspase-9 to activate PARP and suppress the expression of NF-κB at its high concentration. However, NAC did not activate PARP to induce A498 cellular apoptosis in low concentrations.
4. Discussion
The NAC’s role on RCC cell lines A498 was investigated in this study. We measured the intracellular ROS level and mitochondrial membrane potential related to the mitochondrial injury. We explored the effect and mechanism of NAC as an antioxidant in A498 cells. Besides, apoptosis-related proteins in A498 were also determined. We found that NAC could enhance cellular activities against oxidative damage and suppress the expression of the directly apoptotic protein to inhibit apoptosis at its low concentration (<10 mM), thereby protecting A498 cells. On the other hand, NAC could induce oxidative stress to damage mitochondria and activate directly apoptotic protein to induce apoptosis at its high concentrations (>10 mM), thereby killing A498 cells.
Although antioxidant compounds are currently explored as many prophylactics against many ailments (such as malignant tumours) [Citation22,Citation23], recent studies have reported that antioxidant compounds may even be conducive to cancer initiation and progression [Citation24,Citation25]. There is an evident two-faced nature of antioxidants towards cancer [Citation13]. NAC is the synthetic precursor of intracellular cysteine and GSH, so it is considered an important antioxidant [Citation26]. In this study, we also have found two faces of NAC towards RCC.
ROS are the key molecules in oxidative stress. When cellular activities fail in scavenging excess ROS, mitochondrial dysfunction may occur. As a result, an imbalance in antioxidant defense happens, certainly causing cell death [Citation27]. ROS can also damage DNA and regulate related antioxidation activities [Citation28,Citation29]. As a clinical antioxidant, NAC has been frequently used to scavenge ROS to protect cells against oxidative stress [Citation30–32]. Yan et al. [Citation33] noted that NAC raises GSH levels while reducing MDA levels. Similarly, in our study, NAC in low doses did not raise the ROS level in A498 cells. Consequently, the changes in mitochondrial membrane potential have not been observed. An increase in GSH-PX level and a decrease in MDA level were also observed. As one of the most important cells defence mechanisms, the Nrf2 signalling pathway can regulate the protective gene expression influenced by environmental stress factors. When cells are attacked by ROS, Nrf2 moves into the nucleus and activates the transcription of antioxidant-related proteins (such as HO-1, NQO-1) to protect the cells from oxidative stress [Citation34]. The findings of this study testify that the expression of Nrf2, HO-1, and NQO1 increased when A498 cells were treated with a low dose of NAC. The results show that NAC can protect against A498 oxidative stress-induced cell damage by increasing the expression of Nrf2-regulated antioxidant enzymes, including HO-1 and NQO1 [Citation35]. These results confirm that NAC can protect A498 cells against oxidative damage by scavenging ROS by activating the Nrf2 signalling pathway.
Many studies report that NAC could reduce apoptosis by suppressing the pro-apoptotic protein (caspase-9) expression [Citation36,Citation37]. In this way, the downstream proteins’ (such as PARP) expression is down-regulated [Citation38], and the cell apoptosis is inhibited by NAC. The current study found that low-dose NAC did not up-regulate the caspase-9 expression and significantly suppressed the apoptotic protein (PARP) expression. These results are consistent with the protective effect of NAC on other cancer cells [Citation39–41].
The interesting finding in this study is that when A498 cells are treated with high-dose NAC, the results turned out to be almost the opposite of results obtained after treatment with a low dose. High doses of NAC induced oxidative stress resulting in mitochondrial damage and up-regulated upstream caspase-9 to activate caspase-3 to initiate the caspase cascade and cleaved PARP, causing induction of irreversible apoptosis. Keeping this in mind, NAC can be developed as a chemotherapeutic drug for RCC treatment. Cao et al. [Citation16] have reported that NAC inhibited glioblastoma cell viability effectively when the concentration was higher than 10 mM. Lee et al. [Citation42] found that the NAC suppressed prostate cancer cell growth at a concentration of 20 mM. NAC could effectively inhibit invasive oral cancer cells at a 30 mM concentration [Citation43]. In this study, the growth of A498 cells was well inhibited by NAC at 20 mM and oxidative stress and apoptosis of A498 were induced by NAC when its concentration was higher than 10 mM. Therefore, the therapeutic dose of NAC can be adjusted according to the types of cancer. Moreover, the dose of NAC at 10 mM is attainable in vivo [Citation44]. Accordingly, it is important to determine the dose of NAC to be used as a chemotherapeutic drug. Only a high dose of NAC can exhibit cancer inhibition activity.
Unfortunately, the results of the present study cannot explain why NAC plays two completely different roles when used in different concentrations. However, these results are very important for the application of NAC in cancer therapy, and we plan to work on it in the future.
In summary, NAC has two contradictory activity profiles towards RCC cells. It protects RCC cells against oxidative stress and apoptosis at low concentrations and destroys them by inducing oxidative stress and apoptosis at high concentrations. These results may implicate a novel application of NAC as an anti-cancer drug after thorough research and consideration.
Supplemental Material
Download MS Word (7.1 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Faghfouri AH, Zarezadeh M, Tavakoli-Rouzbehani OM, et al. The effects of N-acetylcysteine on inflammatory and oxidative stress biomarkers: a systematic review and meta-analysis of controlled clinical trials. Eur J Pharmacol 2020;884:173368.
- Shih WL, Chang CD, Chen HT, et al. Antioxidant activity and leukemia initiation prevention in vitro and in vivo by N-acetyl-L-cysteine. Oncol Lett 2018;16:2046–2052.
- Aldini G, Altomare A, Baron G, et al. N-Acetylcysteine as an antioxidant and disulphide breaking agent: the reasons why. Free Radical Res 2018;52:751–762.
- Skvarc DR, Dean OM, Byrne LK, et al. The effect of N-acetylcysteine (NAC) on human cognition – a systematic review. Neurosci Biobehav R. 2017;78:44–56.
- Kamboj SS, Vasishta RK, Sandhir R. N-acetylcysteine inhibits hyperglycemia-induced oxidative stress and apoptosis markers in diabetic neuropathy. J Neurochem 2010;112:77–91.
- Marie A, Meunier J, Brun E, et al. N-acetylcysteine treatment reduces age-related hearing loss and memory impairment in the senescence-accelerated prone 8 (SAMP8) mouse model. Aging Dis. 2018;9:664–673.
- Lai KY, Ng WY, Chan PKO, et al. High-dose N-acetylcysteine therapy for novel H1N1 influenza pneumonia. Ann Intern Med 2010;152:687–688.
- Lee PH, Hong J, Jang AS. N-acetylcysteine decreases airway inflammation and responsiveness in asthma by modulating claudin 18 expression. Korean J Intern Med 2020;35:1229–1237.
- Shen FC, Weng SW, Tsao CF, et al. Early intervention of N-acetylcysteine better improves insulin resistance in diet-induced obesity mice. Free Radical Res 2018;52:1296–1310.
- Zhang H, Li Y, Chen Y, et al. N-Acetylcysteine protects against intrauterine growth retardation-induced intestinal injury via restoring redox status and mitochondrial function in neonatal piglets. Eur J Nutr 2019;58:3335–3347.
- Yeh IJ, Wang TY, Lin JC, et al. Optimal regimen of N-acetylcysteine on chromium-induced renal cell damage. Metabolites. 2019;9:172.
- Rhodes K, Braakhuis A. Performance and side effects of supplementation with N-acetylcysteine: a systematic review and meta-analysis. Sports Med 2017;47:1619–1636.
- Dastmalchi N, Baradaran B, Latifi-Navid S, et al. Antioxidants with two faces toward cancer. Life Sci 2020;258:118186.
- Breau M, Houssaini A, Lipskaia L, et al. The antioxidant N-acetylcysteine protects from lung emphysema but induces lung adenocarcinoma in mice. JCI Insight. 2019;4:e127647.
- Sayin VI, Ibrahim MX, Larsson E, et al. Antioxidants accelerate lung cancer progression in mice. Sci Transl Med 2014;6:221ra15.
- Deng J, Liu AD, Hou GQ, et al. N-acetylcysteine decreases malignant characteristics of glioblastoma cells by inhibiting Notch2 signaling. J Exp Clin Canc Rese. 2019;38:2.
- Rakshit S, Bagchi J, Mandal L, et al. N-acetyl cysteine enhances imatinib-induced apoptosis of Bcr-Abl+ cells by endothelial nitric oxide synthase-mediated production of nitric oxide. Apoptosis. 2009;14:298–308.
- Wu MS, Lien GS, Shen SC, et al. N-Acetyl-L-cysteine enhances fisetin-induced cytotoxicity via induction of ROS-independent apoptosis in human colonic cancer cells. Mol Carcinogen. 2014;53:E119–E129.
- Song X, Tian Y, Li H, et al. Research progress on advanced renal cell carcinoma. J Int Med Res 2020;48:UNSP 0300060520924265.
- Neha, Das P, Verma SP. Dual role of G-quadruplex in translocation renal cell carcinoma: exploring plausible cancer therapeutic innovation. BBActa-Gen Subjects. 2020;1864:129719.
- Parmar A, Sander B, Bjarnason GA, et al. Systemic therapy in metastatic renal cell carcinoma: emerging challenges in therapeutic choice. Crit Rev Oncol Hemat. 2020;152:102971.
- Glasauer A, Chandel NS. Targeting antioxidants for cancer therapy. Biochem Pharmacol 2014;92:90–101.
- Yasueda A, Urushima H, Ito T. Efficacy and interaction of antioxidant supplements as adjuvant therapy in cancer treatment: a systematic review. Integ Cancer Ther. 2016;15:17–39.
- Kennedy L, Sandhu JK, Harper ME, et al. Role of glutathione in cancer: from mechanisms to therapies. Biomolecules. 2020;10:1429.
- Jaune-Pons E, Vasseur S. Role of amino acids in regulation of ROS balance in cancer. Arch Biochem Biophys 2020;689:108438.
- Mishra S, Divakar A, Srivastava S, et al. N-acetyl-cysteine in combination with celecoxib inhibits deoxynivalenol induced skin tumor initiation via induction of autophagic pathways in Swiss mice. Free Radical Bio Med. 2020;156:70–82.
- Zheng Q, Li Q, Zhao G, et al. Alkannin induces cytotoxic autophagy and apoptosis by promoting ROS-mediated mitochondrial dysfunction and activation of JNK pathway. Biochem Pharmacol 2020;180:114167.
- Walter L, Canup B, Pujada A, et al. Matrix metalloproteinase 9 (MMP9) limits reactive oxygen species (ROS) accumulation and DNA damage in colitis-associated cancer. Cell Death Dis 2020;11:767.
- Zhou J, Geng S, Ye W, et al. ROS-boosted photodynamic therapy against metastatic melanoma by inhibiting the activity of antioxidase and oxygen-producing nano-dopants. Pharmacol Res 2020;158:104885.
- Zhou W, Wang L, Yu H, et al. N-acetylcysteine alleviates PCB52-induced hepatotoxicity by repressing oxidative stress and inflammatory responses. PeerJ 2020;8:e9720.
- Mercantepe T, Topcu A, Rakici S, et al. The radioprotective effect of N-acetylcysteine against x-radiation-induced renal injury in rats. Environ Sci Pollut R. 2019;26:29086–29094.
- Liu X, Wang L, Cai J, et al. N-acetylcysteine alleviates H2O2-induced damage via regulating the redox status of intracellular antioxidants in H9c2 cells. Int J Mol Med 2019;43:199–208.
- Gao W, Liang J, Ma C, et al. The protective effect of N-acetylcysteine on ionizing radiation induced ovarian failure and loss of ovarian reserve in female mouse. BioMed Res Int 2017;2017:4176170.
- Zhu X, Oseghale AR, Nicole LH, et al. Mechanisms of NRF2 activation to mediate fetal hemoglobin induction and protection against oxidative stress in sickle cell disease. Exp Biol Med 2019;244:171–182.
- Al-Saeedi FJ. Mangiferin protect oxidative stress against deoxynivalenol induced damages through Nrf2 signalling pathways in endothelial cells. Clin Exp Pharmacol P. 2021;48(3):389–400.
- Fan H, Le J, Zhu J. Protective effect of N-acetylcysteine pretreatment on acute kidney injury in septic rats. J Surg Res 2020;254:125–134.
- Shieh P, Jan CR, Liang WZ. The protective effects of the antioxidant N-acetylcysteine (NAC) against oxidative stress-associated apoptosis evoked by the organophosphorus insecticide malathion in normal human astrocytes. Toxicology. 2019;417:1–14.
- Liu X, Wang Y, Yan Y, et al. Investigation of cadmium-induced apoptosis and the protective effect of N-acetylcysteine in BRL 3A cells. Mol Med Rep 2016;14:373–379.
- Li Y, Wang P, Zhao P, et al. Apoptosis induced by sonodynamic treatment by protoporphyrin IX on MDA-MB-231 cells. Ultrasonics. 2012;52:490–496.
- Li Q, Zhao X, Sun J, et al. Anti-proliferative and apoptosis-inducing activities of juglone in LS-174T cells. Bangl J Pharmacol. 2013;8:65–72.
- Lee CS. Effect of depletion and oxidation of cellular GSH on cytotoxicity of mitomycin c in small cell lung cancer cells. Biomol Ther. 2004;12:92–100.
- Lee YJ, Lee DM, Lee CH, et al. Suppression of human prostate cancer PC-3 cell growth by N-acetylcysteine involves over-expression of Cyr61. Toxicol in Vitro. 2011;25:199–205.
- Lee MF, Chan CY, Hung HC, et al. N-acetylcysteine (NAC) inhibits cell growth by mediating the EGFR/Akt/HMG box-containing protein 1 (HBP1) signaling pathway in invasive oral cancer. Oral Oncol 2013;49:129–135.
- Moazzen H, Lu X, Ma NL, et al. N-Acetylcysteine prevents congenital heart defects induced by pregestational diabetes. Cardiovasc Diabetol 2014;13:46.