?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
This manuscript describes the preparation and characterization of silica-nickel oxide (SiO2-NiO) xerogel nanocomposite and its catalytic application in the hydrogenation of p-nitrophenol. Hydrochloric acid and ammonium hydroxide were used as the acid and base catalyst, respectively during the synthesis of SiO2-NiO. The Fourier Transformation Infra-Red spectroscopic results supported the formation of the silica xerogel skeleton structure with corresponding characteristic peaks of siloxane linkage. The surface of SiO2-NiO xerogel nanocomposite was observed to be porous along with some irregular cracks. The X-Ray diffraction analysis showed that the SiO2-NiO xerogel nanocomposite was amorphous in nature. The synthesized xerogel nanocomposite was employed as a catalyst for the hydrogenation of p-nitrophenol with sodium borohydride in water. The rate of hydrogenation of p-nitrophenol was observed to be increased with the increased amount of catalyst as well as the temperature. The maximum reduction rate of p-nitrophenol was found as high as 0.26 min-1.
1. Introduction
The emission of organic pollutants from various industries in wastewater is extremely affecting the people and the environment. In industries, there are many processes such as the preparation of pesticides, herbicides, explosives, textiles, and paper where nitroaromatic compounds are used. These compounds are released in industrial effluents and are considered as water pollutants. Among the nitroaromatic compounds, para-nitrophenol (p-NP) and its corresponding derivatives are the most common and toxic organic pollutants which are accompanied by industrial and agricultural effluents. These pollutants are generally liberated from the process of formation of synthetic dyes, insecticides, herbicides, plastics, leather, and paints [Citation1,Citation2]. Due to the wide range of applications of p-NP, it is liberated from many industries and contaminated in water resources including ground water and surface water. This contamination affects public health and aquatic life at a large scale [Citation3]. According to the reports of the Agency for Toxic Substances and Disease Registry of the United States, p-NP causes inflammation and irritation of the skin, eyes, and respiratory tract. It can remain contacted with blood for long times and this delayed interaction results in the formation of methaemoglobin which is responsible for many diseases including methemoglobinemia, confusion, potentially causing cyanosis and unconsciousness. Upon ingestion, p-NP causes vomiting and abdominal pain. Long time contact of p-NP with skin may also cause an allergic response. Due to these toxic effects, the Environmental Protection Agency (EPA) of the United States has listed p-NP among the top 126 priority pollutants list. Due to the high toxicity and widespread of p-NP, there is a need to degrade this pollutant to protect public health and aquatic life from its harmful effects. Many methods have been reported to degrade p-NP [Citation4–9]. As compared to other methods, hydrogenation of p-NP into p-aminophenol (p-AP) with the assistance of a catalyst has been reported as a very useful one. One of the main factors which make this reaction important is the obtained product i.e. p-AP, which is used in the preparation of some antipyretic and analgesic medicines. In dye industries, the p-AP is also employed as a wood stain and dyeing agent for fur and feathers [Citation10]. So, it is important to convert p-NP into p-AP to take advantage of getting rid of a harmful pollutant and obtaining a useful product from the pollutant. It is very easy to change p-NP into p-AP in water with sodium borohydride (NaBH4) which acts as a reducing agent under mild conditions provided that the reaction mixture contains a suitable catalyst. In recent years, metal nanoparticles have received much attention as catalysts. These catalysts have been found to possess excellent catalytic properties due to high surface-to-volume ratios, and exceptional electronic and surface properties [Citation11,Citation12]. However, the usage of metal nanoparticles or nanomaterials as catalysts is hindered due to their high inclination towards agglomeration. Various methods have been investigated by researchers to stabilize metal-based nanoparticles. For example, oleylamine was found as a suitable stabilizing agent for copper nanoparticles by Masoud et el. [Citation13]. Fatima et al., synthesized CoTiO3/CoFe2O4 nanocomposites via sol–gel auto-combustion technique using glucose, maltose, and starch as stabilizing agents [Citation14]. The chemical precipitation method was used by Maryam et al., to prepare Zn2GeO4/graphene nanocomposite using acacen as a capping agent for Zn2GeO4 nanostructure [Citation15]. The combustion method was used by Ali et al., for the synthesis of binary metal oxides (Dy3Fe5O12), and fine crystalline nanoparticles were obtained [Citation16]. Similarly, Sahar et al., reported that Nd2Zr2O7-Nd2O3 nanocomposites can be synthesized with tunable morphology and can be used as effective photocatalysts [Citation17]. Nanomaterials have also been synthesized by using green chemistry. For example, Farnosh et al. synthesized graphene nanosheets using pomegranate juice as a reductant as well as a capping agent [Citation18].
Among the various available methods, dispersion of the nanoparticles in a matrix is a widely studied method to overcome the agglomeration of nanoparticles. The nanoparticles of noble metals such as Au, Ag, Pt, and Pd have been stabilized in polymeric matrices and used as efficient catalysts [Citation19–22]. However, these catalysts are very expensive, therefore, they have found limited practical applications. On the other hand, metal oxide nanoparticles are being considered as promising cost effective catalysts owing to their higher reactivity, chemical and thermal stability, easy recovery, and reusability [Citation23]. Metal oxide nanoparticles such as TiO2 and LaFeO3 have been reported as competent catalysts for the hydrogenation of p-NP into p-AP. Despite the above-mentioned advantages, there are very limited reports on the catalytic usage of nanoparticles of metal oxides in the hydrogenation of p-NP. So, there is a need to explore the catalytic properties of metal oxide nanoparticles to design promising cost-effective catalysts. In this context, we have synthesized a novel SiO2-NiO nanostructured xerogel composite with sol–gel method. The aim of the present study was to explore the catalytic behaviour of NiO nanoparticles stabilized by SiO2 xerogel matrix. SiO2-NiO nanostructure xerogel composite was synthesized and employed as the catalyst for the first time in the degradation of a common and harmful industrial pollutant p-nitrophenol.
2. Experimental
2.1. Materials
The chemicals used in this work are given in Table along with the chemical formula, purity, and supplier name. Deionized water (DIW) was prepared in the laboratory and used all over the experimental work.
Table 1. Chemicals used along with chemical formula, purity, and supplier name.
2.2. Synthesis of SiO2-NiO xerogel nanocomposite
First, 100 ml, 8% aqueous solution of sodium silicate was prepared in a 100 ml volumetric flask using deionized water as a solvent. The pH of this solution was measured and found to be ∼13. The ion exchanger (Amberlite® IR-120) was packed in a 25 in. long column with a diameter of 2 in.. A 20 ml of 8% aqueous solution of sodium silicate was slowly passed through the column containing the ion exchanger. After passing through the ion exchange column, sodium silicate was converted to silicic acid sol (H2SiO3) that was collected in a polypropylene beaker. The pH of the collected H2SiO3 was measured and found to be ∼3. Afterward, as an acid catalyst, 0.05 M HCl was slowly added to the silicic acid sol with continuous stirring till the pH of the sol was changed to 2.60. The sol was kept on stirring for another half hour to homogenize. On the other hand, 1 mg nickel nitrate hexahydrate was dissolved in 10 ml DIW and mixed with 20 ml silicic acid sol with continuous stirring. To this mixture, 0.05 molar NH4OH was added slowly as a basic catalyst till the pH of the sol reached to 5. This mixture of reactants was further stirred for half hour and then kept at room temperature for 72 h for gelation and ageing. Afterward, the solvent exchange was carried out by placing the gel in DIW for four days and replacing DIW after every 24 h. In the end, the SiO2-NiO xerogel nanocomposite was dried in an oven at 65°C for 4 h, 80°C for 2 h, and finally at 120°C for 2 h.
2.3. Characterization
Fourier Transformation Infrared (FT-IR) Spectroscopy was used to confirm the formation of the xerogel composite as well as to analyze the functional groups present in the xerogel composite. The FT-IR spectrum was taken from Nicole’s FTIR Nexus 470 spectrometer. The amorphous behaviour of the composite material was confirmed by X-Ray Diffraction technique. XRD pattern was obtained by Using X’PRO PANalytical XRD spectrophotometer. SEM image was recorded by SEM JEOL model, 5910 LV. The accelerating voltage of 20 kV and high vacuum mode was applied to record secondary electron image from SEM to study the surface morphology of SiO2-NiO xerogel nanocomposite. UV-Visible spectrophotometer (Shimadzu 1601) was used to monitor the catalytic hydrogenation.
2.4. Catalytic reduction of p-nitrophenol
The reaction involving the reduction of p-NP with NaBH4 in DIW was selected as a model reaction to evaluate the catalytic activity. The prepared SiO2-NiO xerogel nanocomposite was used as a catalyst. In a representative reaction, 1 ml aqueous solution of each of the p-NP (0.01 mM) and NaBH4 (1.5 mM) was taken with a cuvette of UV-Visible spectrophotometer. A certain amount (0.2, 0.4 or 0.6 mg) of SiO2-NiO xerogel nanocomposite was added to the reaction mixture as a catalyst. The reaction’s progress was tracked with a UV-Visible spectrophotometer coupled with a thermostat. The temperature of the reaction mixture was maintained with a thermostat. The extent of reaction was observed from the decrease in the absorbance at 400 nm with the passage of time.
3. Results and discussion
SiO2-NiO xerogel nanocomposite was synthesized by a two-step sol gel method. In this method, when sodium silicate passes through the ion exchanger the positively charged Na+ ions are replaced by the positively charged H+ ions which are much smaller in size and more mobile than Na+ ions. This reaction results in the production of silicic acid. This silicic acid acts as a silica precursor which can condense with the passage of time to form the silica gel, but this process is quite time consuming. To speed up the gelation process, a hydrochloric acid solution was used as a catalyst. On the other hand, ammonium hydroxide solution was used to catalyze the condensation reaction of silicic acid (H2SiO3) to silica. The siloxane linkage (Si-O-Si) in silica gel propagates and strengthens up the prepared gel network during the process of condensation and gelation.
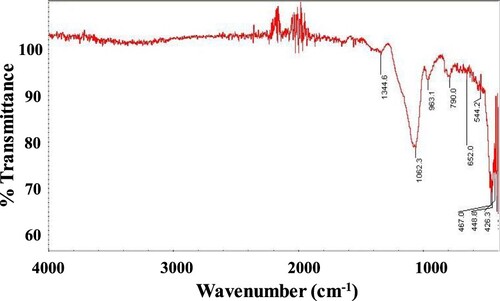
The formation of the SiO2-NiO xerogel nanocomposite skeleton structure was confirmed by FT-IR spectroscopy. Xerogel’s FT-IR spectrum is shown in Figure . The presence of characteristic peaks for Si-O-Si at 1062 and 790 cm−1 indicated the formation of a silica network [Citation24]. The absorption peak at 960 cm−1 appeared due to the Si – O stretch of silanol (Si–OH) groups [Citation25]. The absorption bands in the range of 420–790 cm−1 correspond to Ni-O stretching vibration mode [Citation26–28]. So, the FTIR spectrum indicates the presence of both the SiO2 and NiO in the sample and hence approves the formation of SiO2-NiO xerogel nanocomposite.
Morphology of the SiO2-NiO xerogel nanocomposite was studied by SEM. The SEM image of the SiO2-NiO xerogel nanocomposite is shown in Figure . This SEM image depicts that the surface of xerogel nanocomposite is not smooth but has an irregular pattern with some cracks. The SEM image also depicts some porosity on the surface of xerogel nanocomposite. The cracks and porosity are produced due to the evaporation of water from the structure of xerogel. The SiO2-NiO xerogel nanocomposite was dried by heating at temperatures from 65 to 120°C. This process is accompanied by the removal of water trapped in the microstructure of SiO2-NiO xerogel nanocomposite. Since the ageing was carried out before the evaporation of water, the spaces created in the structure of SiO2-NiO xerogel nanocomposite were left unoccupied and led to the appearance of cracks and porosity. Czarnobaj has also observed similar results when prepared silica xerogel by sol–gel process for their application as drug carriers [Citation29]. The surface roughness in xerogels owing to the evaporation of water was also observed by Yamasaki at al. [Citation30].
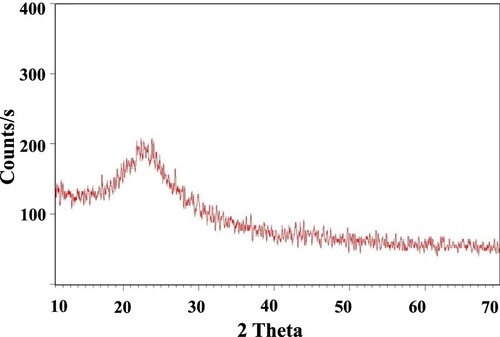
XRD spectrum of the synthesized SiO2-NiO xerogel nanocomposite is shown in Figure . Around 2θ angle of 22° a broad peak was observed. This broad peak indicated that our nanocomposite was having amorphous nature. It is well known fact that the presence of sharp peaks in the XRD pattern indicates the crystallinity of the subject material and the XRD pattern of our prepared SiO2-NiO xerogel nanocomposite was free from any sharp peaks. So, the results of the XRD study indicate that the prepared nanocomposite has an amorphous structure. The amorphous nature further demonstrates that SiO2 and NiO were arranged in SiO2-NiO xerogel nanocomposite in an irregular pattern leading to an anisotropic structure.
3.1. Catalytic activity
The potential of the synthesized composite to catalyze the hydrogenation of p-NP to p-AP was studied in an aqueous medium by employing its small amounts as a catalyst. The selection of this reaction was done to ease of tracking of its progress. The consumption of p-NP is associated with a suppression in its absorption at 400 nm. While the formation of the major product p-AP is associated with the domination of absorbance at 300 nm. So, both the consumption of reactant and formation of product can be observed with a single scan of the UV-visible spectrophotometer. Additionally, nitrophenols are very toxic pollutants and these toxins are released in industrial wastewater [Citation31]. p-NP and similar toxic pollutant molecules are released from many industrial processes [Citation1]. Such a widespread existence of p-NP motivated to select this reaction for analysis. Although both the p-NP and p-AP are considered as poisonous but the toxicity of p-AP is far less than p-NP and there is a great demand of p-AP as a reagent for the synthesis of various products in many industries [Citation32]. Owing to the above-mentioned features, this reaction is of prime importance both academically as well as technologically.
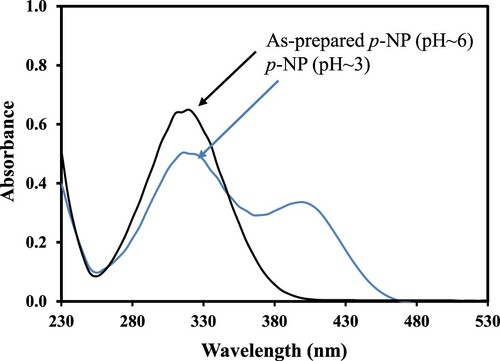
The aqueous solution of p-AP exhibited an absorption maximum at 317 nm as shown by the UV-Visible spectrum in Figure (a). The addition of NaBH4 in the aqueous solution of p-AP leads to the formation of phenolate and a shift in the absorption maximum from 317 to 400 nm as shown by the UV-Visible spectrum in Figure (a). It is also important to note that the formation of phenolate ion was accompanied by an increase in the intensity of the colour of the solution as well as its absorbance. This observation is due to the higher molar absorption coefficient of p-nitrophenolate ion as compared to its parent specie i.e. p-NP. Also, only a small decrease in the absorbance at 400 nm was observed when NaBH4 was used to reduce p-AP without the aid of any catalyst, and it showed that the reduction of p-AP with NaBH4 was deadly slow. Similar results have also been observed by many researchers [Citation33–35]. The thermodynamics of the reduction of p-NP into p-AP by NaBH4 suggests that this reaction can take place. However, the electron donor and acceptor of this reaction have a big potential gap among them which creates a kinetic barrier large enough to suppress the hydrogenation process in this reaction. This large kinetic barrier hinders the reaction to proceed over time no matter how large an amount of the NaBH4 is added as a reducing agent [Citation33,Citation36]. However, the hydrogenation of p-NP at an appreciable rate can be made feasible with the aid of a small amount of a catalyst. A substantial loss in the height of the absorption band of p-NP at 400 nm and concomitant rising of a new peak at ∼300 nm indicates the reduction of p-NP to p-AP [Citation37]. In the present study, to the reaction mixture of aqueous solutions of p-NP and NaBH4 a small amount of newly prepared xerogel nanocomposite catalyst was added. The reduction process was tracked by time dependent UV-Vis spectra as shown in Figure (b). The altitude of absorbance peak of p-NP was decreased gradually as the time moved on and a new peek appeared at ∼300 nm. The decrease in height of the absorbance band of p-NP with the passage of time was observed due to the decolorization of the reaction mixture from bright yellow [Citation38]. This observation of catalytic reaction is in accordance with results reported earlier [Citation39]. The isosbestic points at 330 and 270 nm in the UV-Visible spectra of the samples taken from the reaction mixture during the reduction of p-NP also demonstrated the reduction of p-NP to p-AP. These results are in good agreement with those observed by Anas et al., in the reduction of p-NP catalyzed by palladium nanoparticles [Citation40]. It is important to mention that the aqueous solution of p-NP becomes colourless in an acidic medium (below pH ∼5). So, only decolorization cannot be considered as a sufficient reason for the reduction of p-NP to p-AP. Therefore, we also recorded the UV-Visible spectra of the as-prepared aqueous solution of p-NP and after adjusting the pH to 3 as shown in Figure . It is demonstrated from Figure that both the as-prepared aqueous solution of p-NP and that having pH∼3 exhibit absorption maxima at 317 nm which is different from that of p-AP (∼300 nm) observed during its reduction as can be in Figure (b). From the difference in absorption maxima of p-AP and the aqueous solution of p-NP having pH∼3 it can be concluded that in our present work p-NP was converted into p-AP. In this catalytic reaction, NiO nanoparticles facilitated electron transportation from electron donor BH4¯ to acceptor p-NP [Citation41–43]. So, during this reduction process, the reaction was catalyzed by NiO nanoparticles present in the xerogel to overcome the kinetic barrier.
Figure 4. (a) Plots of absorbance of an aqueous solution of p-NP as a function of wavelength in the absence and presence of a reducing agent. (b) Plots of absorbance of an aqueous solution of p-NP as a function of wavelength at different intervals of time during catalytic reduction at 45 °C.
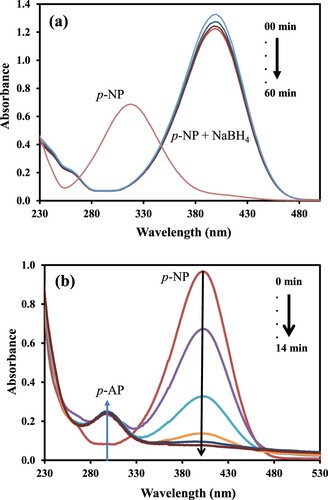
Figure 5. UV-Visible spectra of the as-prepared aqueous solution of p-NP and after adjusting the pH to 3.
In the reduction of p-NP, the concentration of NaBH4 was 150 times than that of p-NP, so we supposed that the rate of this reaction was independent of NaBH4 concentration. Therefore, this reduction reaction can be assumed as a pseudo-first order reaction. Therefore, we applied pseudo-first-order kinetics with respect to the concentration of p-NP for the calculation of the rate constant. The straight-line form of this equation can be given as follows
(1)
(1) At = concentration of p-NP at different times t; Ao = concentration of p-NP at time 0; kapp = apparent rate constant.
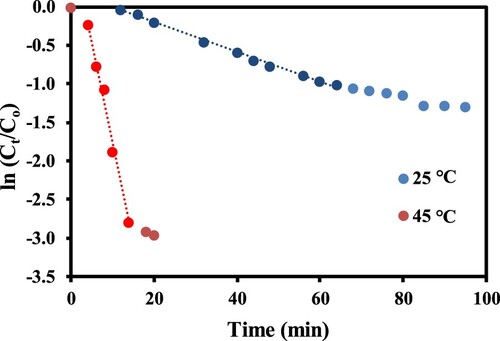
Figure represents the plot of ln(At/Ao) against time. The slope of this plot gives the value of kapp.
The effect of the catalyst’s dose was analyzed by applying different amounts of xerogel composite in the hydrogenation reaction. By increasing the amount of catalyst, more catalytic sites become available for the reactants, and hence the frequency of formation of substrate–catalyst complex is increased which leads to rapid reduction of p-NP as depicted by increasing slopes of pseudo first order plots in Figure . The apparent rate constant was increased from 0.013 to 0.019 min−1 and from 0.122 to 0.260 min−1 by increasing the amount of catalyst from 0.2 to 0.6 mg at 25 and 45°C, respectively. So, the variation in the amount of catalyst can be used as a useful tool to control the speed of reduction of p-NP.
Figure 6. Pseudo first order plots for catalytic hydrogenation of p-NP with three different amounts of catalysts.
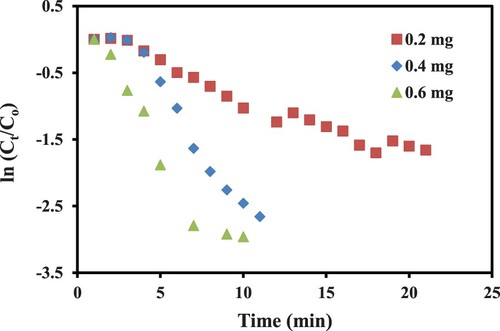
Temperature is another important parameter that affects the rate of reactions and helps to calculate the activation energy as well. So, we also carried out the catalytic reactions at two different temperatures. The temperature’s effect on reaction rate is shown in Figure in terms of pseudo first order plots. With the rise in temperature from 25 to 45°C the reduction rate was increased from 0.019 to 0.260 min−1. Such an increase in the rate of hydrogenation reaction is attributed due to the fact that at higher temperature conditions kinetic energy of the reacting substances is increased which enhances the diffusion rate of reactants towards the NiO catalyst adsorbed on xerogels surface. The increase in the rate of diffusion of reactants increases the rate of effective collisions as well as the rate of formation of the activated complex which in turn causes an increase in reaction rate. The effect of temperature was studied with three different amounts of catalyst and the apparent rate constants were calculated from the slopes of linear regions of pseudo first order plots and are given in Table .
Table 2. Values of rate constants for the reduction of p-nitrophenol catalyzed by SiO2-NiO nanocomposite catalyst at different temperatures and with different amounts of catalyst.
The increase in the rate of reaction associated to an increase in temperature was observed irrespective of the amount of catalyst. The following form of the Arrhenius equation Equation(2)(2)
(2) was considered for the calculation of activation energy (Ea) and its value was found to be 88 kJmol−1.
(2)
(2) A time delay was observed in all cases of catalytic reactions. However, this time delay was decreased when the reaction was carried out at a higher temperature. Also, the reaction occurred with a higher reduction rate at higher temperatures and was completed in a shorter time as shown in Figure . Such a time delay has also been observed in the previous studies of catalytic reactions by metal nanoparticles. This time delay appears due to the presence of oxygen in the reaction medium. NaBH4 is more reactive towards oxygen and less reactive towards p-nitrophenol. Thus, NaBH4 start to reduce p-NP only when all O2 is consumed [Citation44]. In addition, in a catalytic reaction, the catalyst is first activated by the removal of oxides or any other impurities from the active sites and then begins its action. This process of activation of the catalyst may take time resulting in a time delay in this catalytic hydrogenation reaction [Citation45]. Furthermore, p-NP will have to diffuse into the pores of xerogel and adsorb on the surface of NiO nanoparticles to form a substrate–catalyst complex, and then the collision of these complexes will result in the reduction of p-NP. Due to this process at the initial stage of the reaction, a time delay may also appear.
4. Conclusions
This work includes the applications of porous structured xerogels, showing that the xerogels and metal oxide nanoparticles can be combined to produce a venerable material that can be directly employed as a catalyst. In this study, we have successfully synthesized a novel SiO2-NiO nanostructured xerogel composite using sol–gel method. The prepared xerogel composite showed catalytic potential with appreciable efficiency for the reduction of p-NP which is considered as toxic organic compound. The FT-IR spectroscopic analysis has confirmed the formation of xerogel nanocomposite. The XRD analysis has revealed that the prepared material was amorphous in nature while the porous nature was evidenced by SEM analysis. From the application point of view, this SiO2-NiO nanostructured xerogel composite was found as an active catalyst in the reduction of p-NP. The rate of catalytic reaction was enhanced by raising the temperature of the reaction mixture and amount of catalyst suggesting that the catalytic activity of this system can be tuned easily. The apparent rate constant for the reduction of p-nitrophenol was increased from 0.013 to 0.019 min−1 and from 0.122 to 0.260 min−1 by increasing the amount of catalyst from 0.2to 0.6 mg at 25 and 45°C, respectively. The activation energy for the catalytic reduction of p-NP was found to be 88 kJmol−1. A time delay of 16 min appeared for each catalytic reaction which was decreased at higher temperature conditions.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Zhang JT, Wei G, Keller TF, et al. Responsive hybrid polymeric/metallic nanoparticles for catalytic applications. Macromol Mater Eng. 2010;295:1049.
- Shaoxiang T, Guoping S, Xianwei L, et al. Progress in Chemistry. 2009;21:534.
- Shi H, Xu X, Xu X, et al. Journal of Environmental Sciences (China). 2005;17:926.
- Agarry S, Durojaiye A, Solomon B. Microbial degradation of phenols: a review. Int J Environ Pollut. 2008;32:12.
- Ahmed S, Rasul M, Martens WN, et al. Advances in heterogeneous photocatalytic degradation of phenols and dyes in wastewater: A review. Water Air Soil Pollut. 2011;215:3.
- Bo L, Zhang Y, Quan X, et al. Microwave assisted catalytic oxidation of p-nitrophenol in aqueous solution using carbon-supported copper catalyst. J Hazard Mater. 2008;153:1201.
- Babuponnusami A, Muthukumar K. Removal of phenol by heterogenous photo electro Fenton-like process using nano-zero valent iron. Sep Purif Technol. 2012;98:130.
- Bazrafshan E, Biglari H, Mahvi AH. Fresenius Environ Bull. 2012;21:364.
- Pimentel M, Oturan N, Dezotti M, et al. Phenol degradation by advanced electrochemical oxidation process electro-Fenton using a carbon felt cathode. Appl Catal, B. 2008;83:140.
- Jiang Y, Chen R, Xing W. Chemical Industry and Engineering Progress. 2011;2.
- Ajmal M, Farooqi ZH, Siddiq M. Silver nanoparticles containing hybrid polymer microgels with tunable surface plasmon resonance and catalytic activity. Korean J Chem Eng. 2013;30:2030.
- Begum R, Naseem K, Farooqi ZH. A review of responsive hybrid microgels fabricated with silver nanoparticles: synthesis, classification, characterization and applications. J Sol-Gel Sci Technol. 2016;77:497.
- Salavati-Niasari M, Fereshteh Z, Davar F. Synthesis of oleylamine capped copper nanocrystals via thermal reduction of a new precursor. Polyhedron. 2009;28:126.
- Ansari F, Sobhani A, Salavati-Niasari M. Simple sol-gel synthesis and characterization of new CoTiO3/CoFe2O4 nanocomposite by using liquid glucose, maltose and starch as fuel, capping and reducing agents. J. Colloid Interface Sci. 2018;514:723.
- Masjedi-Arani M, Salavati-Niasari M. Novel synthesis of Zn2GeO4/graphene nanocomposite for enhanced electrochemical hydrogen storage performance. Int J Hydrogen Energy. 2017;42:17184.
- Salehabadi A, Salavati-Niasari M, Ghiyasiyan-Arani M. Self-assembly of hydrogen storage materials based multi-walled carbon nanotubes (MWCNTs) and Dy3Fe5O12 (DFO) nanoparticles. J. Alloys Compd. 2018;745:789.
- Zinatloo-Ajabshir S, Salavati-Niasari, M, Zinatloo-Ajabshir Z. Nd2Zr2O7-Nd2O3 nanocomposites: New facile synthesis, characterization and investigation of photocatalytic behaviour. Mater. Lett. 2016;180:27.
- Tavakoli F, Salavati-Niasari M, Mohandes F. Green synthesis and characterization of graphene nanosheets. Mater. Res. Bull. 2015;63:51.
- Zhai Z, Wu Q, Li J, et al. Enhanced catalysis of gold nanoparticles in microgels upon on site altering the gold–polymer interface interaction. J Catal. 2019;369:462.
- Farooqi ZH, Khalid R, Begum R, et al. Facile synthesis of silver nanoparticles in a crosslinked polymeric system by in situ reduction method for catalytic reduction of 4-nitroaniline. Environ Technol. 2019;40:2027.
- Naseem K, Begum R, Farooqi ZH. Platinum nanoparticles fabricated multiresponsive microgel composites: synthesis, characterization, and applications. Polym Compos. 2018;39:2167.
- Chen T, Fang Q, Zhou L, et al. Comparative study of cross-linked and linear thermo-responsive carriers supported palladium nanoparticles in the reduction of 4-nitrophenol: structure, catalytic activity and responsive catalysis property. React Funct Polym. 2019;142:104.
- Mandlimath TR, Gopal B. Catalytic activity of first row transition metal oxides in the conversion of p-nitrophenol to p-aminophenol. J Mol Catal A: Chem. 2011;350:9.
- Vasiliu I, Gartner M, Anastasescu M, et al. Sio x -P2O5 films—promising components in photonic structure. Opt Quantum Electron. 2007;39:511.
- Li H, Kunitake T. Efficient proton conduction of nanometer-thick film of porous silica as prepared by oxygen plasma treatment. Microporous Mesoporous Mater. 2006;97:42.
- Sharma AK, Desnavi S, Dixit C, et al. Extraction of nickel nanoparticles from electroplating waste and their application in production of bio-diesel from biowaste. International Journal of Chemical Engineering and Applications. 2015;6:156.
- Srihasam S, Thyagarajan K, Korivi M, et al. Phytogenic generation of NiO nanoparticles using stevia leaf Extract and evaluation of their In-vitro antioxidant and antimicrobial properties. Biomolecules. 2020;10:89.
- Mohaideen HM, Fareed SS, Natarajan B. Role of calcination temperatures on the structural and optical properties of NiO nanoparticles. Surf. Rev. Lett. 2019;26:1950043.
- Czarnobaj K. Preparation and characterization of silica xerogels as carriers for drugs. Drug Deliv. 2008;15:485.
- Yamasaki S, Sakuma W, Yasui H, et al. Nanocellulose xerogels with high porosities and large specific surface areas. Front Chem. 2019;7:316.
- Begum R, Rehan R, Farooqi ZH, et al. Physical chemistry of catalytic reduction of nitroarenes using various nanocatalytic systems: past, present, and future. J Nanopart Res. 2016;18:231.
- Vaidya MJ, Kulkarni SM, Chaudhari RV. Synthesis ofp-aminophenol by catalytic hydrogenation ofp-nitrophenol. Org Process Res Dev. 2003;7:202.
- Begum R, Farooqi ZH, Ahmed E, et al. Catalytic reduction of 4-nitrophenol using silver nanoparticles-engineered poly(N-isopropylacrylamide-co-acrylamide) hybrid microgels. Appl Organomet Chem. 2017;31:e3563.
- Begum R, Farooqi ZH, Butt Z, et al. Engineering of responsive polymer based nano-reactors for facile mass transport and enhanced catalytic degradation of 4-nitrophenol. J Environ Sci. 2018;72:43.
- Serrà A, Artal R, Pozo M, et al. Simple environmentally-friendly reduction of 4-nitrophenol. Catalysts. 2020;10:458.
- Ajmal M, Siddiq M, Al-Lohedan H, et al. Highly versatile p(MAc)–M (M: Cu, Co, Ni) microgel composite catalyst for individual and simultaneous catalytic reduction of nitro compounds and dyes. RSC Adv. 2014;4:59562.
- Ajmal M, Demirci S, Siddiq M, et al. Betaine microgel preparation from 2-(methacryloyloxy) ethyl] dimethyl (3-sulfopropyl) ammonium hydroxide and its use as a catalyst system. Colloids Surf, A. 2015;486:29.
- Naeem H, Ajmal M, Qureshi RB, et al. Facile synthesis of graphene oxide–silver nanocomposite for decontamination of water from multiple pollutants by adsorption, catalysis and antibacterial activity. J Environ Manag. 2019;230:199.
- Naeem H, Ajmal M, Muntha S, et al. Synthesis and characterization of graphene oxide sheets integrated with gold nanoparticles and their applications to adsorptive removal and catalytic reduction of water contaminants. RSC Adv. 2018;8:3599.
- Iben Ayad A, Luart D, Ould Dris A, et al. Kinetic analysis of 4-nitrophenol reduction by “water-soluble” palladium nanoparticles. Nanomaterials. 2020;10:1169.
- Gangula A, Podila R, Karanam L, et al. Catalytic reduction of 4-nitrophenol using biogenic gold and silver nanoparticles derived fromBreynia rhamnoides. Langmuir. 2011;27:15268.
- Jana S, Ghosh SK, Nath S, et al. Synthesis of silver nanoshell-coated cationic polystyrene beads: a solid phase catalyst for the reduction of 4-nitrophenol. Appl Catal, A. 2006;313:41.
- X. Du, J. He, J. Zhu, L. Sun, S. An. Ag-deposited silica-coated Fe3O4 magnetic nanoparticles catalyzed reduction of p-nitrophenol, Appl Surf Sci 258,2717 (2012)
- Mei Y, Lu Y, Polzer F, et al. Catalytic activity of palladium nanoparticles encapsulated in Spherical Polyelectrolyte Brushes and core−shell microgels. Chem Mater. 2007;19:1062.
- Mei Y, Sharma G, Lu Y, et al. High catalytic activity of platinum nanoparticles immobilized on spherical polyelectrolyte brushes. Langmuir. 2005;21:12229.