Abstract
Appropriate treatment of non-healing diabetic foot ulcers is challenging as the whole skin repair process is complicated. Current therapeutic strategies have to be evolved for better efficacy. Staphylococcus epidermidis (S. epidermidis) is a commensal having probiotic properties. The wound healing potential of its lysate was explored using skin biopsies. Full-thickness skin, obtained from individuals with and without diabetes, was wounded using an excisional punch. Skin explants were cultured using a serum-free medium in the presence and absence of S. epidermidis lysate. The ability of the lysate to stimulate re-epithelialisation was assessed in an ex-vivo human skin model of wounding from patients with and without diabetes. Epithelial tongue length was significantly reduced in diabetic wounds in comparison to non-diabetic samples, even after 12 days post-wounding. Re-epithelialisation was augmented considerably in S. epidermidis lysate-treated wounds ex-vivo. In non-diabetic samples, the increase was twofold greater but in diabetic samples, only a slight enhancement in tongue length was observed in comparison to the control (untreated sample). The epidermal thickness also increased 7.7-fold in treated wounds in comparison to 4.4-fold in untreated samples. The cell number per unit area showed a significant increase in comparison to untreated control and the number of Ki-67 positive cells was slighter greater indicating increased expression of the proliferation marker in the newly formed epithelial tongue area in lysate-treated samples (both diabetic and non-diabetic). S. epidermidis lysate promotes re-epithelialisation in both diabetic and non-diabetic skin biopsies and may be used to accelerate healing in chronic diabetic ulcer wounds. Its therapeutic potential needs to be explored further with in vivo and molecular studies.
1. Introduction
Diabetes is a chronic metabolic disorder resulting from defects in insulin secretion and/or activity [Citation1]. Uncontrolled diabetes can cause several complications leading to conditions, such as neuropathy, nephropathy, retinopathy and radiculopathy, as consistently high sugar (hyperglycaemia) can damage blood vessels and nerves with time [Citation2]. Diabetic foot is one such complication where one foot of the patient is ulcerated as a result of associated neuropathy and/or peripheral arterial disease of the lower limb [Citation3]. Diabetic foot ulcers are prevalent in 4–10% of the patient population and the risk increases with age. It is estimated that the chance of developing a diabetic foot in a person’s lifetime is ∼15%. It is devastating, as at least 1% ultimately leads to amputations (usually neuroischaemic ulcers); having a traumatic social, emotional and psychological impact on the person [Citation4]. Hence, it is of utmost importance to get diabetic foot ulcers treated at the earliest. The slow process of re-epithelialisation in diabetic wounds can be enhanced with the help of wound healing therapies [Citation5]. Although diabetic foot ulceration is a major health problem, its prevention and management still need to be studied thoroughly so that the foot at risk can be identified within time and amputation may be avoided.
Effective and timely wound closure would require enhanced re-epithelialisation wherein keratinocytes, the key epidermal cells, proliferate and migrate towards the injury site. Re-epithelialisation is impaired in all types of chronic wounds, including diabetic ulcers, and hence the healing process gets delayed. Thus, novel therapeutic approaches need to be explored that can augment the closure of such chronic wounds. There are some studies that investigated that the topical application of Cinnamomum hydroethanolic extract improves wound healing by enhancing re-epithelialisation and keratin biosynthesis in streptozotocin-induced diabetic; Quercus infectoria gall extract aids wound healing in a streptozocin-induced diabetic mouse model and hydroethanolic extract of Trifolium pratense (red clover) accelerates wound healing by reducing apoptosis and improving re-epithelialisation [Citation6–8], but all these studies were done on a mouse model.
The human skin surface is colonised by several diverse bacterial species that are beneficial for maintaining a healthy environment. These commensal microbes strengthen the host immune system and affect the skin microenvironment during wound healing [Citation9]. S. epidermidis is one such commensal that colonises the host skin and does not cause any serious infection but rather protects from the more pathogenic bacterial species [Citation10]. These Gram-positive bacteria can induce the accumulation of T cells (CD8+) at the wound site and hence may have a role to play in cell protection and tissue repair. It can enhance the process of re-epithelisation of skin wounds and hence accelerate wound closure [Citation11]. S. epidermidis produces several biomolecules that have antitumor and wound healing properties [Citation11]. Some have been identified but a lot more research has to be done to unravel the cause for its efficacy. It produces a compound 6-N-hydroxyaminopurine that has anti-tumour potential without causing any systemic toxicity in vivo. Another compound reported to be secreted by this bacteria, N-formyl methionine peptide, enhances CD8+ T lymphocyte activity [Citation12]. S. epidermidis upregulates the expression of antimicrobial protein perforin-2 in human skin ex vivo [Citation13]. Hence it plays a significant role in modulating the host’s innate immune responses and protects from skin infections.
Antibiotics, antiseptics and other conventional antimicrobials display a broad spectrum of anti-infective activity on wounds but show undesirable side effects including host toxicity and multidrug resistance. Their topical applications can have an adverse effect on the host epidermal cells and other tissues [Citation14]. Wound healing is a multifaceted and actively progressive phenomenon that involves several stages of re-epithelialisation. A synchronised equilibrium is established between specific cells and molecules, besides extracellular matrix components and the cutaneous microflora. Normal skin repair is found to be inappropriately accomplished in chronic diabetic wounds and ulcers [Citation15]. Hence, novel strategies are required that promote skin repair in all types of wounds.
Since human and mouse skin differ in thickness and cell number (human skin is 100 μm thicker), the process of wound healing in both differ to a large extent. Hence, ex vivo studies using human skin explants would give a better understanding of wound healing instead of murine skin repair models [Citation11]. It has been observed that S. epidermidis and its extracts (lysate and spent culture fluid) show protective and wound healing properties in vitro (unpublished data). The aim of the present study was to explore re-epithelialisation in the human skin explants of diabetic and non-diabetic patients post-wounding. The length of the new epithelial tongue was estimated in the absence and presence of S. epidermidis lysate in both conditions. Quantification was done on the basis of Haematoxylin and Eosin (H&E)-stained sections of human skin wounds monitored for 12 days. Re-epithelialisation rate was also estimated in terms of the thickness of the epidermal layer and the number of cells per unit area. Cell proliferation was studied by estimating the expression of nuclear marker protein Ki-67 during epidermal repair in diabetic and non-diabetic wound healing ex vivo [Citation16].
2. Methods and materials
2.1. Preparation of bacterial lysate
S. epidermidis cultures (Isolated from our participants in the previous study [Citation17]) (100 ml) were centrifuged at 12,000g for10 min. The harvested cells were washed with phosphate-buffered saline (0.01 M PBS, pH = 7.4), and finally re-suspended in 100 ml of PBS. This solution was boiled and cooled immediately on ice for 5 min [Citation18]. The lysates were passed through 0.22 µm Millex-GV syringe filters from Millipore (Bedford, MA, USA). An aliquot of 100 μl was plated on agar to confirm the complete removal of all viable bacteria.
2.2. Re-epithelialisation in human skin model ex-vivo
Human skin was obtained from three diabetic participants with foot ulcers (two females aged 48 and 46 years) who attended the Prince Abdul-Aziz Ben Maged Ben Abdul-Aziz diabetic center in Al Madinah, KSA. Healthy skin controls were obtained from two other participants who had undergone elective cosmetic surgery (two females aged 45 and 47 years) (Table ). The “punch-in-a-punch” model was used wherein 6 mm incisions were made in the skin using a biopsy punch and then smaller (2 mm) biopsy punches were used to create skin wounds (Figure ) [Citation19]. These were positioned individually, with the epidermis facing upwards, in six-well plates and cultured in 500 μl serum-free William’s E medium at 37°C in a 5% CO2 incubator. The skin was cultured in the absence (treated with 500 μl PBS) or presence of 500 μl S. epidermidis lysate. The sample medium was replaced after every 48 h. Biopsies were collected at a specified time period (day 0, 3, 7, and 12) and then mixed in 10% formalin for 24 h followed by 70% ethanol at room temperature before the staining. This study was authorised by the Medical Ethical Committee of the Faculty of Applied Medical Sciences at Taibah University (CLS 201801). All the participants signed the written informed consent.
Figure 1. Wound-healing assay design Full-thickness wounds were created using a 2-mm in 6-mm biopsy punch. Photographs of “punch-in-a-punch” biopsy injury on human skin.
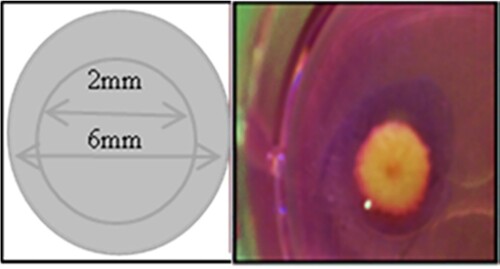
Table 1. Demographic data of participants and sources of skin explants.
2.3. Histological studies – slide preparation and staining
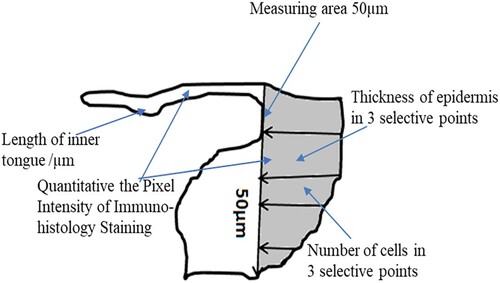
The fixed samples were stained with Mayer’s Haematoxylin (Merck, Darmstadt, Germany) and 0.1% Eosin E (Sigma-Aldrich, St Louis, MO, USA), as described previously in the literature review. Images were captured at 20× magnification using a Zeiss Microscope (ZEISS Group, Germany) and evaluated using the ImageJ 64 software program (obtained from http://imagej.nih.gov). This determines the wound area and quantification of re-epithelialisation by establishing the whole length of the newly formed inner epithelial tongue. In another set of experiments, the stained sections were used to determine the number of keratinocytes and the thickness of the epidermis in 50 μm areas (Thickness of epidermis at three selected points by measuring from the top of the stratum corneum to the basement membrane). Images were captured at 40× magnification, and the bars indicate 100 μm for three randomly selected fields, as depicted in the schematic diagram (Figure ). The experiment was done three times with triplicate samples within each individual experiment.
2.4. Immunostaining of ex-vivo wounded skin using the Ki-67 proliferation marker
Human snih kin sections were housed on slides prepared as described previously. Anti Ki-67 primary antibodies [SP6] (ab16667, Abcam, Cambridge, UK) were used at 1:100 dilution to stain the Ki-67 proliferation marker, further identified with goat anti-mouse-IgG-FITC/-Rhodamine (JIR, Westgrove, PA; USA; 1:200). Images were taken using a Zeiss Microscope (ZEISS Group, Germany) and evaluated with the ImageJ 64 software program (obtained from http://imagej.nih.gov). The proliferating cells were thus defined in the wound area and the new epithelial tongue was determined by counting the number of coloured cells. Images were captured at 60× magnification; bars = 100 μm and the measurement scale is demonstrated in Figure .
2.5. Statistical analysis
All assays were conducted thrice with triplicate samples for each individual experiment. For experiments assessing two or more treatments, a two-way ANOVA with a post hoc Tukey test was applied to analyse the main effects. The results were considered significant if P ≤ .05, and all analyses were done using the GraphPad program.
3. Results
3.1. Re-epithelialisation rate of human skin wound ex-vivo in diabetic and non-diabetic patients
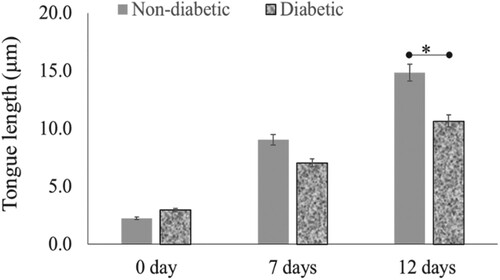
To assess the feasibility of the current model, the wound healing process was analysed in both diabetic and non-diabetic human skin wounds cultured in a serum-free medium. Re-epithelialisation rate was detected on the basis of epithelial tongue length measurements, as illustrated in Figure . Re-epithelialisation in the diabetic patient wound was significantly less as can be seen in the form of reduced epithelial tongue length than non-diabetic patients, even after 12 days’ post-wounding (Figure ). In seven days, the tongue length was 9 and 7 µm in non-diabetic and diabetic patient wounds, respectively. After 12 days’ post-wounding, the tongue length was 14.8 μm and 10.6 μm, in respective patient wounds (P = .04, n = 3).
Figure 3. Re-epithelialisation of human skin in the diabetic and non-diabetic wound. The length of the new epithelial tongue in the non-diabetic section was significantly longer (14.8 μm) than that of the diabetic section (10.6 μm) after 12-day post-wounding (P = .04, n = 3). Data are illustrative of three experiments, *P < .05.
3.2. S. epidermidis lysate augments re-epithelialisation in healthy human skin ex-vivo
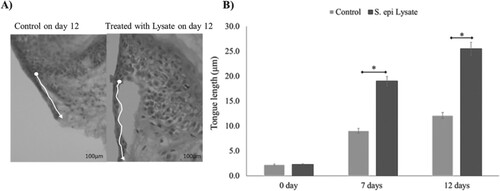
After treatment with 500 μl S. epidermidis lysate, the skin biopsies were harvested on days 0, 7 and 12 post-wounding. Histological staining of the sections with H&E showed that the lysate significantly enhanced the re-epithelialisation of treated wounds in comparison to that of untreated wounds (P = .002, n = 3) (Figure (A)). On day 7, the epithelial tongue length was 9 and 19 µm in untreated and treated wounds, respectively. After 12 days, the tongue length was 12.1 and 25.5 µm, respectively, while the values on day zero were only 2.2 and 2.3, respectively (Figure (B)).
Figure 4. Re-epithelialisation of human skin wound in the absence and presence of S. epidermidis lysate. (A) H&E-stained sections of human skin wounds on day 12 (20× magnification; scale bar represents 100 μm). The new epithelial tongue is depicted by white lines. (B) Length of new epithelial tongue measured in µm at specified time periods. Significance relative to control data is denoted by *P < .05.
3.3. S. epidermidis lysate augments re-epithelialisation in diabetic human skin ex-vivo
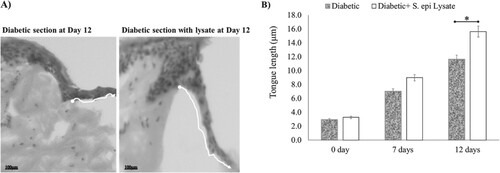
Histological studies performed on diabetic skin explants reveal that wound healing was slightly faster in the presence of S. epidermidis lysate. Figure shows that lysate exposure of 12 days post-wounding, increased the epithelial tongue length to 15.6 µm while in the case of untreated diabetic wounds the length increased to 11.6 µm (P = .03, n = 3). Hence, it was observed that S. epidermidis lysate enhances the re-epithelialisation process even in diabetic wounds (Figure (A,B)).
Figure 5. Effect of S. epidermidis lysate on wound re-epithelialisation rate in diabetic skin explant. (A) H&E-stained images of diabetic wound sections after treatment with or without S. epi lysate on day 12 post-wounding (white arrows indicate the tongue length at 20× magnification, scale bar represents 100 μm). (B) Quantification of wound re-epithelialisation by measuring the tongue length in diabetic patients with and without S. epi lysate after day 7 and 12 post-wounding (P = .03, n = 3). Data are illustrative of three experiments, *P < .05.
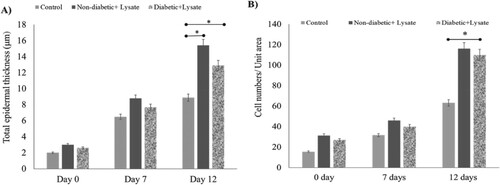
3.4. S. epidermidis lysate increases the thickness of the epidermis in wounded skin
Figure shows the analysis for H&E-stained sections of S. epidermidis lysate-treated human skin punch wounds on day 12 along with untreated control. Figure (A) records the effect of lysate on the thickness of the epidermis in human skin explant. The total thickness of the epidermal layer in the wound area was evaluated based on the H&E-stained human skin sections treated with S. epidermidis lysate (P = .03, n = 3). The present study shows that there was a significant enhancement in the total thickness of the epidermis in wounds treated with lysate in comparison to the untreated control. On day 12, the total epidermal layer thickness was 15.4 µm in treated wounds (non-diabetic) and 12 µm in treated diabetic wounds while in the case of untreated wounds the thickness was 8.9 μm (P = .03, n = 3). Figure (B) gives the number of cells per unit area in the epidermal layer of the wound area as evaluated from the H&E-stained human skin sections after treatment. Significance relative to control data was indicated by *P < .05. To evaluate the cause of this augmented thickness, the number of cells in the epidermal layers was determined in three different areas of the sections in the wound area. On day 12, the cell number per unit area increased to 116.5 ± 0.65, 108 ± 0.45 (31, 29 on day 0, non-diabetic and diabetic respectively) in treated wounds, while untreated control showed a number of 63 ± 0.39 (16 on day 0) cells per unit area. It was observed that treatment with the bacterial lysate led to a noteworthy increase in the cell numbers (P = .04, n = 3) with a significant increase in the total epidermal thickness. Approximately, the same results were noticed in diabetic samples (Figure (A,B), P = .04, n = 3).
Figure 6. Effect of S. epidermidis lysate on epidermis thickness and keratinocyte numbers in human skin explant. (A) The total thickness of epidermal layers in the wound area as evaluated from H&E-stained human skin sections treated with S. epi lysate for diabetic and Non-diabetic samples (P = .03, P = .04, respectively, n = 3). (B) Cell number per unit area in the epidermis of wound area as evaluated from H&E-stained human skin sections after treatment. Significance was relative to control data indicated by *P < .05.
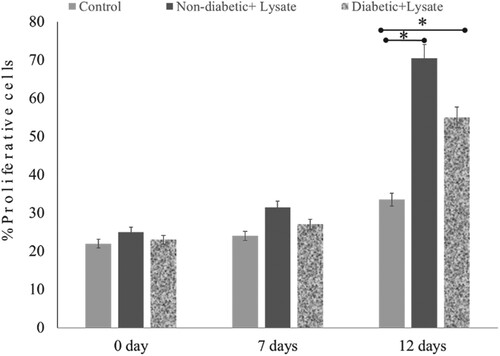
3.5. S. epidermidis lysate stimulates cell proliferation in skin wounds
The repair of skin wounds involves cell proliferation which plays an important role in the mechanism of re-epithelialisation. The extent of Ki-67 expression in treated and untreated cells was used to estimate the percentage of proliferation in skin wounds. The Ki-67 antigen was detected in the nucleus of proliferating cells as a red stain in the images and the number of cells was calculated. Figure shows the effect of S. epidermidis lysate on cell proliferation in both diabetic and non-diabetic human skin explants measured post-wounding. Ki-67 immunoreactivity was quantified in newly formed epithelial tongues and the number of Ki-67 positive cells was detected in the treated wounds on days 7 and 12. Increased proliferation was seen in wounds of both healthy and diabetic skin explants. On day 12, the number of Ki-67 positive cells was significantly high in both cases. In the case of non-diabetic wounds, the percentage of positive cells increased from 25% on day 0 to 71% on day 12 upon lysate treatment. On the other hand, untreated control wounds showed an increase in positive cells from 22% on day 0 to 34% on day 12 (Figure ). Furthermore, interestingly re-epithelialisation in diabetic skin wounds was enhanced significantly. It was observed that on day 12, the number of Ki-67 positive cells in treated diabetic skin wounds was 55.15%, while in untreated wounds it was 34% (P = .04, n = 3) (Figure ) indicating further enhancement of skin repair in diabetic wounds also.
Figure 7. Effect of S. epidermidis lysate on cell proliferation in non-diabetic and diabetic human skin explants measured post-wounding. Quantification of Ki-67 immunoreactivity was done in newly formed epithelial tongues 50μm length behind the new tongue (schematic diagram 1). The number of Ki-67 positive cells was detected in the treated wound on days 7 and 12, and compared with the control (P = .02, n = 3, * P < .05).
4. Discussion
Diabetic foot ulcers that do not heal are a major cause of concern for chronic diabetic patients. If left untreated these can be infested with bacterial and fungal secondary infections and can lead to amputations, which can be avoided with required management and treatment. Wound healing has become a slow process, especially in immunocompromised patients. Moreover, multidrug resistance has made several conventional antibiotics unusable, further slowing down the healing process [Citation20]. Hence, exploring new options for wound healing has become imperative today. Probiotics have shown great potential in both the treatment of secondary infections and wound healing [Citation21,Citation22]. Previous studies have shown that cell lysates, spent culture fluids, supernatants and metabolites extracted from probiotic bacteria have shown immense potential and are equally beneficial [Citation23,Citation24]. Based on several studies it has been hypothesised that extracts of bacteria, especially lysates, prove to be safer alternatives to live bacteria in wound healing as their chance of causing any potential infection is minimal [Citation25,Citation26]. Furthermore, the lysates of S. epidermidis and other probiotic bacteria have shown efficacy against wounds by enhancing keratinocyte proliferation and migration to the wound site. Since re-epithelialisation is vital to wound healing, the present study demonstrates the efficacy of S. epidermidis lysate in promoting re-epithelialisation of wounds in diabetic and non-diabetic ex vivo human skin explants.
The re-epithelialisation phase of tissue formation plays a major role in the restoration of the damaged epidermis at the wound site. The migratory activity of epithelial cells is noticed within a few hours post-wounding. Successfully complete re-epithelialisation depends on several factors, such as patient age and location of the wound. The size, depth and cleanness of the wound also have an effect on the repair phenomenon [Citation27]. In the present study we used the “punch-in-a-punch” model for creating wounds in skin biopsies [Citation18]. These were then used to study re-epithelialisation process in wounds created in skin explants taken from both diabetic and non-diabetic patients. The length of epithelial tongue formation was monitored for 12 days. It was observed that tongue length increased 6.7-fold in non-diabetic wounds, while it increased only 3.6-fold in diabetic wounds. Hence re-epithelialisation process was almost two-fold slower in diabetics than healthy controls (Figure ).
For histological studies, the biopsy skin samples were fixed and stained. Hematoxylin marks the nuclear material blue-black in the images, while eosin stains the cytoplasmic content along with the connective tissue. The images were evaluated to quantify re-epithelialisation by estimating the length of the newly formed inner epithelial tongue, the number of keratinocytes and the thickness of the epidermis. As shown in Figure , the epithelial tongue length increased several folds in the presence of S. epidermidis lysate. Within seven days, there was an eight-fold increase in the tongue length while control showed a four-fold increase. After 12 days of exposure post-wounding, an increase of 11-fold was observed while untreated cells showed an increase of only 5.5-fold. In the case of diabetic skin biopsies, the epidermal tongue length increased 4.7-fold and 4-fold, respectively in treated and untreated samples on day 12 (Figure ). The present study further shows that there was a significant augmentation in the total wound epidermal thickness in lysate-treated non-diabetic samples in comparison to the untreated control. On day 12, the total epidermal thickness increased 7.7-fold in treated wounds while in the case of untreated samples the increase was only 4.4-fold on day 12. Although an amplified cell number per unit area was observed, we noted a very significant increase in comparison to untreated control. In the case of both non-diabetic and diabetic samples, approximately a four-fold increase was observed (Figure ).
Ki-67 expression was detected in the new epithelial tongue area in treated and untreated samples. Ki-67 is a nuclear protein that expresses more in newly dividing cells. This proliferation marker is expressed by all the cells of the outer layer of human skin for at least five days post-wounding [Citation28], both in young and elderly skin. The Ki-67 antigen can be detected in the nucleus of proliferating cells specifically in the interphase. It is expressed in all the phases of the cell cycle except the resting phase [Citation29]. The percentage of Ki-67 positive was quantified with the help of a staining technique using anti-Ki-67 primary antibodies and goat anti-mouse-IgG-FITC/-Rhodamine. The greater the number of positive cells, the greater the frequency of cell division and formation of new cells. The proportion of cells expressing Ki-67 antigen increased in the presence of S. epidermidis lysate in both diabetic and non-diabetic healthy skin wounds (Figure ). On day 12, a significant increase was observed in the number of Ki-67 positive cells. In general, most antiseptics are toxic to the main cellular components (keratinocytes, fibroblasts, epithelial cells, endothelial cells) involved and ultimately cause a delay in wound healing. Bacterial extracts have shown potential in having both antimicrobial [Citation17] and wound healing properties. Since S. epidermidis lysate is nontoxic to host cells and promotes re-epithelialisation in both diabetic and non-diabetic samples, it has immense potential to be used in wound healing.
However, further investigation is required, such as the lysate effects on the immune system could be investigated since the ex-vivo skin model has its own resident immune cells. Combination models might also be studied where the ability of the lysate to heal a wound in the presence of S. aureus could be tested.
5. Conclusion
The need to develop novel therapeutic strategies for the management of diabetic foot ulcers is vital. S. epidermidis lysate can be used to fasten the wound healing process as it promotes re-epithelialisation in both diabetic and non-diabetic human skin wounds alike. These lysates can lower health care costs as they are easily available, protect epidermal keratinocytes and increase total epidermal thickness and cell number per unit area. The wound healing ability of S. epidermidis lysates needs to be explored further by performing in vivo studies and other studies at the molecular level. These alternative approaches can be used along with the conventional treatments to synergistically enhance the treatment of chronic diabetic foot ulcers and other wounds. The host microflora has immense potential as a novel therapeutic candidate for skin wound repair.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2009;32:S62–S67. DOI:10.2337/dc09-S062
- Deshpande AD, Harris-Hayes M, Schootman M. Epidemiology of diabetes and diabetes-related complications. Phys Ther. 2008;88:1254–1264.
- Alexiadou K, Doupis J. Management of diabetic foot ulcers. Diabetes Ther: Res Treat Educ Diabetes Relat Disord. 2012;3:4.
- Oliver TI, Mutluoglu M. Diabetic foot ulcer. [Updated 2020 Aug 10]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537328/
- Jeffcoate WJ, Price P, Harding KG. International working group on wound healing and treatments for people with diabetic foot ulcers. Wound healing and treatments for people with diabetic foot ulcers. Diabetes Metab Res Rev. 2004;20:S78–S89.
- Daemi A, Lotfi M, Farahpour MR, et al. Topical application of Cinnamomum hydroethanolic extract improves wound healing by enhancing re-epithelialization and keratin biosynthesis in streptozotocin-induced diabetic mice. Pharm Biol. 2019;57(1):799–806.
- Bonab FS, Farahpour MR. Topical co-administration of Pistacia atlantica hull and Quercus infectoria gall hydroethanolic extract improves wound-healing process. Comp Clin Pathol. 2017;26:885–892.
- Farahpour MR, Vahid M, Oryan A. Effectiveness of topical application of ostrich oil on the healing of Staphylococcus aureus and Pseudomonas aeruginosa-infected wounds. Connect Tissue Res. 2018;59(2018):212–222.
- Baldwin HE, Bhatia NC, Friedman A, et al. The role of cutaneous microbiota harmony in maintaining a functional skin barrier. J Drugs Dermatol. 2017;16:12–18.
- Brown MM, Horswill AR. Staphylococcus epidermidis – skin friend or foe? PLoS Pathog. 2020;16:e1009026.
- Leonel C, Sena I, Silva WN, et al. Staphylococcus epidermidis role in the skin microenvironment. J Cell Mol Med. 2019;23:5949–5955.
- Linehan JL, Harrison OJ, Han SJ, et al. Non-classical immunity controls microbiota impact on skin immunity and tissue repair. Cell. 2018;172:784–796.e18.
- Pastar I, O'Neill K, Padula L, et al. Staphylococcus epidermidis boosts innate immune response by activation of gamma delta T cells and induction of perforin-2 in human skin. Front Immunol. 2020;16:550946.
- Punjataewakupt A, Napavichayanun S, Aramwit P. The downside of antimicrobial agents for wound healing. Eur J Clin Microbiol Infect Dis. 2019;38:39–54.
- Pastar I, Stojadinovic O, Yin NC, et al. Epithelialization in wound healing: a comprehensive review. Adv Wound Care. 2014;3:445–464.
- Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311–322.
- Mohammedsaeed W. Identification of skin microbiota in Saudi female community and their effects on keratinocytes viability (in-vitro). J Taibah Univ Sci. 2022;16:24–30.
- Fujiwara K, Doi N. Biochemical preparation of cell extract for cell-free protein synthesis without physical disruption. PLoS One. 2016;11:e0154614.
- Moll I, Houdek P, Schmidt H, et al. Characterization of epidermal wound healing in a human skin organ culture model: acceleration by transplanted keratinocytes. J Invest Dermatol. 1998;111:251–258.
- Trivedi U, Parameswaran S, Armstrong A, et al. Prevalence of multiple antibiotic resistant infections in diabetic versus nondiabetic wounds. J Pathog. 2014;2014:173053.
- Lukic J, Chen V, Strahinic I, et al. Probiotics or pro-healers: the role of beneficial bacteria in tissue repair. Wound Repair Regen Off Publ Wound Heal Soc [and] Eur Tissue Repair Soc. 2017;25:912–922.
- Sinha A, Sagar S, Jabez Osborne W. Probiotic bacteria in wound healing: an in-vivo study. Iran J Biotechnol. 2019;17:e2188.
- Mohammedsaeed W, McBain AJ, Cruickshank SM, et al. Lactobacillus rhamnosus GG inhibits the toxic effects of Staphylococcus aureus on epidermal keratinocytes. Appl Environ Microbiol. 2014;80:5773–5781.
- Ramos AN, Cabral ME, Noseda D, et al. Antipathogenic properties of Lactobacillus plantarum on Pseudomonas aeruginosa: the potential use of its supernatants in the treatment of infected chronic wounds. Wound Repair Regen. 2012;20:552–562.
- Mohammedsaeed W, McBain AJ, Cruickshank SM, et al. Lactobacillus rhamnosus GG lysate increases re-epithelialization of keratinocyte scratch assays by promoting migration. Sci Rep. 2015;5:16147.
- Brandi J, Cheri S, Manfredi M, et al. Exploring the wound healing, anti-inflammatory, anti-pathogenic and proteomic effects of lactic acid bacteria on keratinocytes. Sci Rep. 2020;10:11572.
- Rittié L. Cellular mechanisms of skin repair in humans and other mammals. J Cell Commun Signal. 2016;10:103–120.
- Sun X, Kaufman PD. Ki-67: more than a proliferation marker. Chromosoma. 2018;127:175–186.
- Landén NX, Li D, Ståhle M. Transition from inflammation to proliferation: a critical step during wound healing. Cell Mol Life Sci. 2016;73:3861–3885.