?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
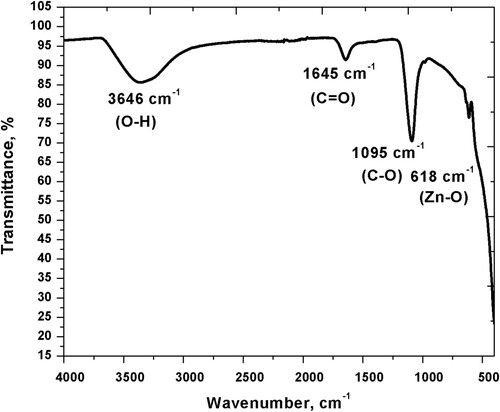
Parabens are frequently used in cosmetics and their high quantities in wastewater are detrimental to the ecosystem. The effect of sonication, light aid and a combination of both on nano-sized zinc oxide-mediated degradation of methylparaben (MP) was investigated using electronic spectroscopy and differential conductometry. The time-dependent absorption at λmax of MP (i.e. 254 nm) was used to monitor the system. The degradation process that followed pseudo-first-order kinetics was augmented by the presence of ZnO. Light exposure gave better results than sonication; however, a negative synergy was observed under the combined catalysis. This limitation was overcome using constant sonication time, a high amount of catalyst and variable light exposure duration. Conductivity measurements revealed an excess of free radical or neutral species. X-ray diffraction analysis (XRD) indicated a wurtzite hexagonal structure and crystallite size of 39 nm for ZnO NPs. In Fourier transform infrared spectrum, the Zn-O band was observed at 618 cm−1.
1. Introduction
Parabens are C1 – C4 alkyl esters of 4-hydroxybenzoic acid [Citation1,Citation2]. They are frequently utilized in food preservation, cosmetics and pharmaceuticals owing to their antimicrobial properties [Citation3–5]. Paraben products are daily used by humans and hence they are regularly released into water bodies [Citation6]. The long time use of cosmetic products containing paraben affects reproductive organs [Citation7], causes skin cancer, DNA damage and leads to metabolic disorders [Citation8]. In addition, it produces thyroid hormone irregularities responsible for disability in offspring and premature birth [Citation9]. Hence, it is imperative to degrade parabens.
Nanoparticles are used for the degradation of organic compounds [Citation10]. Their high catalytic activity is due to a high surface-to-volume ratio and apparently large surface area. In the field of medicine, targeted delivery of drugs to the site of action is achieved through nanoparticle carriers. It is one of the most important aspects of drug delivery systems. These nanoparticle carriers protect the drug entity in the systemic circulation, restrict the access of the drug to the chosen sites and deliver the drug at a controlled and sustained rate. Different types of polymers have been used in the preparation of nanoparticles for drug delivery research. It increases the therapeutic benefit and minimizes the side effects. Nanoparticles are used for cancer therapy. These are used for the destruction of tumour cells through photothermal therapy. Metal nanoparticles make several chemical reactions take place at an elevated rate [Citation11,Citation12]. They are important because they are generally environment-friendly, cheap, biocompatible and less toxic, but due to the small size of nanoparticles, their inhalation causes serious health issues [Citation13–15]. Among semiconductor nanoparticles, ZnO NPs are most used for degradation. ZnO NPs are inorganic metal oxides, used as water disinfectants, antimicrobials and preservative agents. ZnO NPs can show photo reactivity in visible light [Citation16,Citation17]. They have high chemical stability and oxidation capacity, low cost and non-toxic nature [Citation18].
Nanoparticles with ultrasound waves work in a synergistic way that increases the degradation rate of methylparaben significantly [Citation19]. An ultrasonic transducer converts the electrical energy into countless high-frequency sound waves around 20,000 Hz. Sound waves propagate because of rarefaction and compression. In the rarefaction cycle, water molecules move apart resulting in the formation of nucleation sites, which trap the gases. The compression cycle generates small vacuum bubbles in solution, bubbles absorb the energy. When they can no longer obtain the energy, they start to burst and collapse. This causes the release of energy into the solution and produces molecular degradation. This sensation is called acoustic cavitation, as shown in Figure (A). Under sonication, the degradation of organic pollutants occurs in three regions: cavitation, interfacial and bulk. The cavitation region has high temperature and pressure than the interfacial region, while the bulk region has ambient temperature [Citation17].
Figure 1. Degradation of methylparaben by (A) sono-catalytic reaction mechanism and (B) photo-catalytic reaction mechanism [Citation24].
![Figure 1. Degradation of methylparaben by (A) sono-catalytic reaction mechanism and (B) photo-catalytic reaction mechanism [Citation24].](/cms/asset/d3d9438f-c045-4d8f-b07b-250b11c60245/tusc_a_2131992_f0001_oc.jpg)
Photoreactions occur in the presence of light and catalysts [Citation20]. The photocatalytic reaction is environmental-friendly in nature and has a 99% degradation rate of pollutants [Citation21,Citation22]. Tungsten filaments are the source of visible light, which converts electrical energy into heat and light. From the total energy, 90% energy is converted into heat and 10% into light. When a certain wavelength of light falls on the semiconductor, and the energy of incident light is equal to the band gap energy of the semiconductor, an excitation of electron (e-) from the valence band to the conduction band takes place. This generates the hole (h+) in the valence band [Citation23]. ZnO NPs exhibit the power to degrade organic waste by absorbing light in the UV/visible region. Hydroxyl radical and other reactive species are generated during sono-catalytic and photo-catalytic processes (Figure ), which degrade organic contaminants [Citation24,Citation25].
Sonophoto-catalysis is the combined effect of sonolysis and photolysis in the presence of a catalyst. During the degradation process, different charged or radical species are formed [Citation26]. The extent of their formation in an aqueous solution can be determined by spectrophotometry [Citation27] and conductometry [Citation28]. The present work is based on a comparative study of the degradation of methylparaben by ZnO nanoparticles [Citation23,Citation29]. The effect of light, sonication and the combined effect of both on the degradation of MP have been investigated [Citation30,Citation31]. In the dilute solution of MP, different quantities of ZnO NPs were added to the MP solution and subjected to sonication and light exposure separately and simultaneously at various intervals of time [Citation32]. The degradation was monitored by measuring absorbance at 254 nm (i.e. λmax of MP) [Citation33]. The intensity of the lamp was 1100 μW·cm−2.
2. Experimental
Zinc sulphate heptahydrate (ZnSO4.7H2O), sodium hydroxide (NaOH), methanol (CH3OH) and methylparaben (C8H8O3) were obtained from Sigma Aldrich. They were of high purity and used as received. Ultrapure water (conductivity < 0.055 μS·cm−1 at 25°C) was used in all aqueous preparation.
2.1. Synthesis of zinc oxide nanoparticles
ZnO NPs were prepared by the co-precipitation method. The aqueous solution of ZnSO4.7H2O (0.1 M) was added gradually to aqueous NaOH (0.2 M) with continuous stirring. The resulting turbid mixture was sonicated for 1 h, followed by centrifugation at 5000 rpm for 15–20 min. The solid thus obtained was washed with methanol and water to remove water-soluble impurities comprising neutral salts. Afterwards, the zinc oxide nanoparticles were dried in an oven for 1 h at 55 °C. The following chemical reaction occurs during co-precipitation [Citation34,Citation35].
2.2. Preparation of methylparaben solution for degradation studies
0.1 M stock solution of methylparaben was prepared by dissolving 0.76 g methylparaben in distilled water. The sample solutions, each containing 0.05 M MP and the desired quantity of ZnO NPs (either 0.01 g·L−1 or 0.04 g·L−1) were prepared through appropriate dilution of the stock solution [Citation32].
2.3. Degradation experiment
To examine the effect of sonication or light on the degradation of MP, MP solutions containing ZnO NPs were subjected to sonication or exposed to the light of intensity 1100 μW·cm−2 for different time intervals up to 1 h. Photosonocatalysis was also performed where the combined effect of light and sonication was observed. In addition, an experiment was performed with the quantity of catalyst (0.08 g·L−1) and sonication time (30 min) kept constant and the system was exposed to light for different time durations. The process was followed by recording time-dependent electronic spectra in the wavelength range 230–320 nm and observing the change in absorbance at λmax of MP [Citation28]. Simultaneously, the change in conductivity of each solution was noted as a function of time. Conductometer was calibrated using 0.01 M KCl solution as the conductivity standard (conductivity 1413 μS·cm−1 at 25°C) before measurement of the conductivity of sample solutions.
According to Beer-Lambert Law (Equation 1), absorbance is directly proportional to the concentration and path length of the sample solution. As a result of degradation, the concentration of MP decreases over time causing a gradual reduction in the magnitude of absorbance at 254 nm.
(1)
(1) The initial absorbance Ao and absorbance recorded at time t (At) were converted to corresponding concentration (Co and C) using molar absorptivity of MP i.e. 1.4 × 104 L·mol−1·cm−1.
3. Results and discussion
3.1. Characterization of zinc oxide nanoparticles
3.1.1. Fourier transform infrared spectroscopy
Figure illustrates the FT-IR spectrum of synthesized ZnO NPs. The absorption band of metal oxide is observed in the fingerprint region below 1000 cm−1 [Citation36]. The characteristic absorption of Zn-O appears at 618 cm−1 due to the stretching vibration, confirming the formation of ZnO NPs [Citation37,Citation38]. Water adsorption takes place on the metal surface causing the stretching and deformation of -OH at 3646 cm−1, C = O peak is present at 1645 cm−1 due to the symmetric stretching vibration. A similar peak may originate from the chemical or physical adsorption of water. The peak at 1095 cm−1 shows the presence of CO stretching vibration. Residual absorptions are caused by atmospheric CO2 and moisture individually. Functionalization and calcination also affect absorptions in the infrared region [Citation36–40].
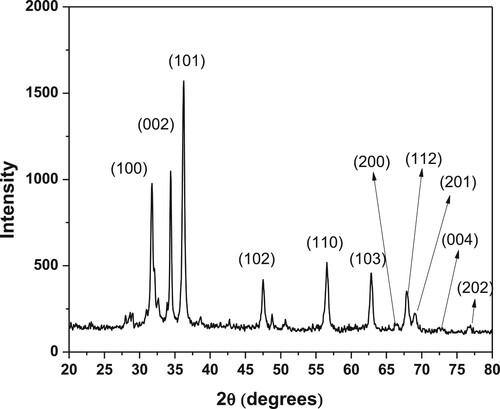
3.1.2. X-Rays diffraction analysis
Figure shows the XRD spectrum of ZnO NPs. The hexagonal wurtzite structure of ZnO NPs was confirmed by the XRD analysis. Braggs equation was used to calculate the distance between the adjoining crystal planes (d)
(2)
(2) For the plane (100), spacing (d) is 0.281 nm, while for the plane (101), spacing (d) is 0.247 nm. The crystal geometry equation for XRD was used to calculate the volume of the unit cell and the angle between the plane and lattice parameter (a, b and c).
(3)
(3)
(4)
(4) where interplanar spacing (d), lattice parameter (a and c), Miller indices (h, k, l) Two planes (100 and 002) were used to calculate the lattice parameter of the prepared ZnO NPs that is a = 3.249 Å, b = 3.249 Å and c = 5.205 Å (α = β = 90o; γ = 120o), volume of unit cell 47.58 Å. The crystal size of ZnO NPs was 39 nm, as calculated by the Scherrer equation [Citation40,Citation41].
3.2. Degradation studies
3.2.1. Sono-catalytic degradation of methylparaben
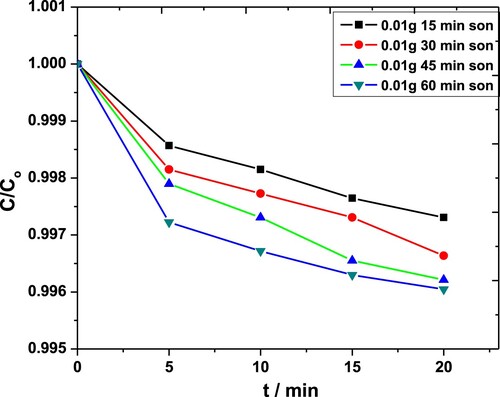
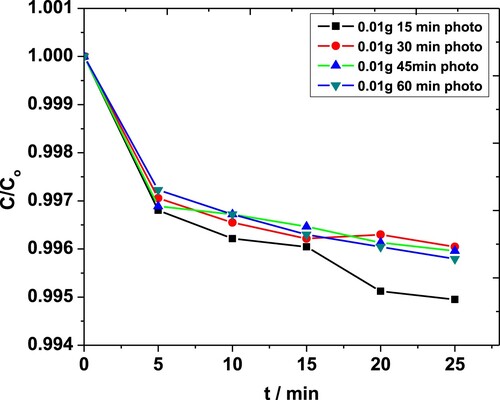
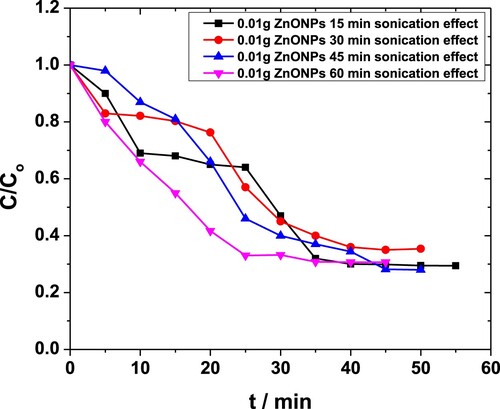
Experiments were performed using different quantities of ZnO NPs i.e. 0.01 and 0.04 g·L−1 in 0.05 M solutions of MP. Each of these solutions was subjected to sonication for 15-, 30-, 45- and 60-min. For 0.01 g·L−1 catalyst, the time dependence absorbance was recorded, and corresponding C/Co values were plotted as a function of time (Figure ). For less than 45 min, the degradation occurred in steps, probably due to the low amount of energy available in the system. For t > 30 min, the conversion took place smoothly. The maximum degradation observed in this case was almost 71% [Citation42,Citation43].
Figure 4. Effect of sonication time on degradation of MP in the presence of 0.01 g·L−1 ZnO NPs obtained through the absorbance at 254 nm.
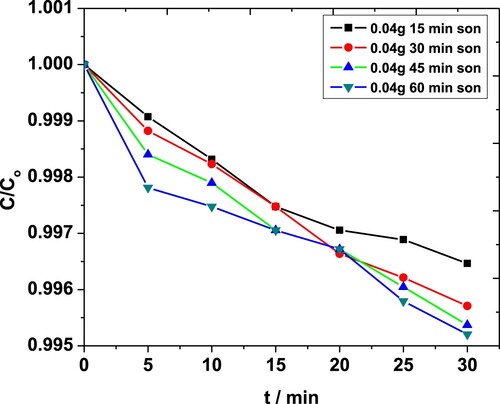
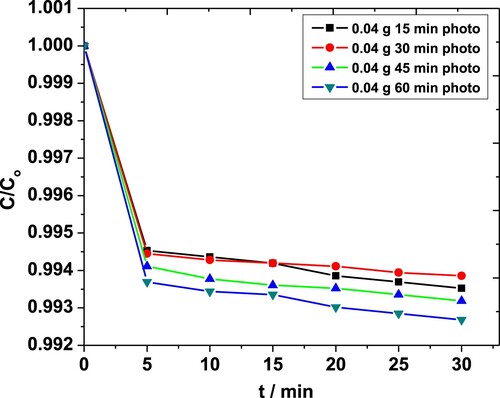
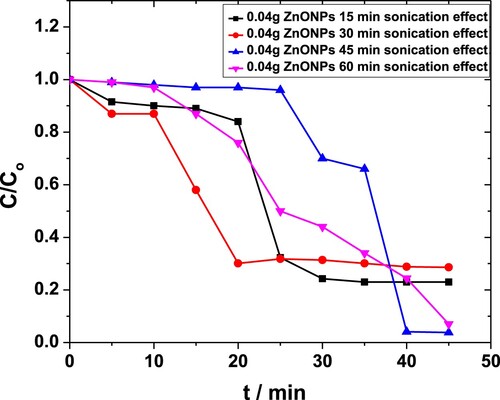
Effect of sonication on MP degradation in the presence of 0.04 g·L−1 ZnO NPs is shown in Figure . Above 99% degradation was observed for sonication times exceeding 30 min compared to the maximum 71% seen with 0.01 g·L−1 of catalyst earlier [Citation44]. The results obtained in the case of 0.08 g·L−1 of NPs were comparable for all sonication times (not shown).
Figure 5. Effect of sonication time on degradation of MP in the presence of 0.04 g·L−1 ZnO NPs as obtained from the absorbance at 254 nm.
The rate constant for each system was obtained from the slope of the linear plot of [1 + log(OD)] vs. t. The linearity of these plots revealed that the degradation followed pseudo-first-order kinetics [Citation45]. The variation in rate constants reflects a trade-off of energy released into the system and energy transferred to MP via catalyst, as shock waves produced as a result of cavitation cause desorption of MP from the catalyst surface [Citation46]. For 15 min sonication time the rate constant decreases with an increase in the amount of catalyst. However, for 0.01 g·L−1 of catalyst the rate constant increases with increasing sonication time. A random variation in rate constant was seen with higher quantities of NPs. The results are given in Table .
Table 1. The effect of sonication times on the rate constant of methylparaben degradation occurring with different quantities of zinc oxide nanoparticles.
The conductivity of the solution increased over time because of the ionization of MP or its degradation products. However, C/Co values computed using differential conductance between MP and ionic products were reduced only to a small extent with time (Figure ), apparently due to the presence of the small amount of charged species in the solution [Citation47]. With 0.01 g·L−1 of ZnO, the maximum effect was seen with 60 min of sonication. Similar behaviour was seen with a 0.04 g·L−1 catalyst-containing system (Figure ).
3.2.2. Photocatalytic degradation of MP
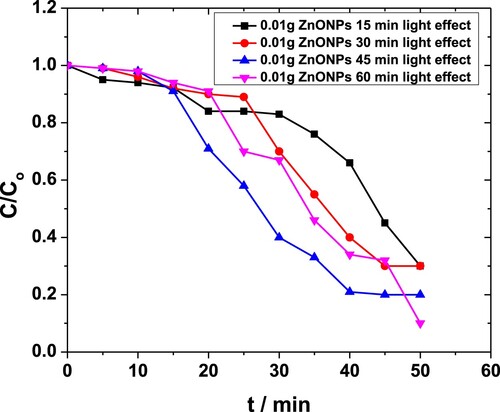
Figure shows the effect of light exposure time on degradation of MP by 0.01 g·L−1 ZnO NPs. This quantity of catalyst requires longer exposure times for a greater percentage of degradation. In comparison to sonication where merely 71% conversion was seen, 90% conversion was seen when the system was exposed to light for 1 h [Citation48,Citation49]. Another important feature also unravelled by the plot is insignificant degradation occurring during the initial 15 min that was almost independent of the exposure times.
Figure 8. Effect of light exposure on the degradation of MP in the presence of 0.01 g·L−1 ZnO NPs as obtained from the absorbance at 254 nm.
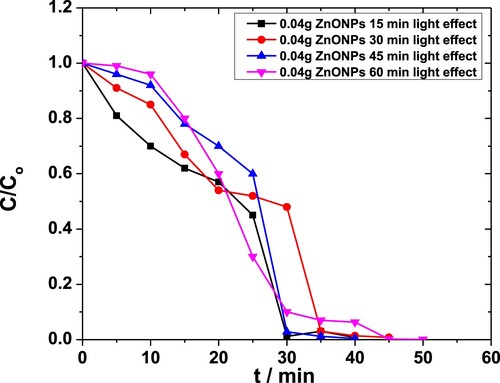
When the amount of catalyst was raised to 0.04 g·L−1, 100% degradation was achieved as C/Co values fell to null. The trends are shown in Figure [Citation50]. Again, photocatalysis yielded better results than sonocatalysis.
Figure 9. Effect of light exposure on the degradation of MP in the presence of 0.04 g·L−1 ZnO NPs as obtained from the absorbance at 254 nm.
Photocatalytic degradation of methylparaben followed pseudo-first-order kinetics as revealed from the plots between [1 + log(OD)] and t. The rate constants for 45 min of photocatalytic degradation increased with an increase in the amount of catalyst (Table ) [Citation51,Citation52]. However, for a fixed amount of catalyst, the rate showed small dependence on ZnO quantity.
Table 2. The effect of light exposure times on rate constant of methylparaben degradation occurring with different quantities of zinc oxide nanoparticles.
The effect of light exposure on C/Co values obtained from differential conductivity in 0.01 g·L−1 and 0.04 g·L−1 of the catalyst containing MP solutions is shown in Figures and , respectively [Citation53]. Under photocatalysis by 0.04 g·L−1 ZnO, the values dropped a little further than the similar system under sonocatalysis, indicating the presence of a comparatively greater concentration of ionic species. However, the overall change is negligibly small and leads to the inference that the system is rich in free radicals or neutral compounds.
3.2.3. Photosono-catalytic degradation of methylparaben
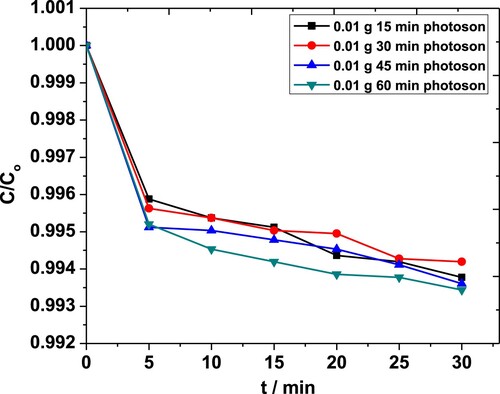
The combined effect of light exposure and sonication time on degradation of MP by 0.01 g·L−1 of catalyst is shown in Figure . The plot indicates that degradation is markedly compromised, and C/Co values are not altered too much to match either of those obtained under individual sono – or photo-catalysis. Although the production of hydroxyl radicals, increased catalytic activity for sono luminescence below 375 nm and ultrasound de-aggregation of ZnO NPs promote the degradation [Citation54,Citation55]; however, the desorption of MP from catalysts surface by shock waves produced by cavitation bubbles diminishes the utility of photochemically produced hydroxyl radicals because they mostly degrade MP adsorbed on the surface of the catalyst. As a result, degradation is reduced [Citation46]. The maximum MP degradation observed under combined catalysis was 46% only. This was endorsed by the decrease in rate constant seen with 0.01 g·L−1 of ZnO NPs and exposure times ranging from 30 to 60 min (Table ). A random trend was seen when the amount of catalyst was raised by eight folds making it arduous to draw any conclusion from the data.
Figure 12. The combined effect of light exposure and sonication on the degradation of MP in the presence of 0.01 g·L−1 ZnO NPs as obtained from the absorbance at 254 nm.
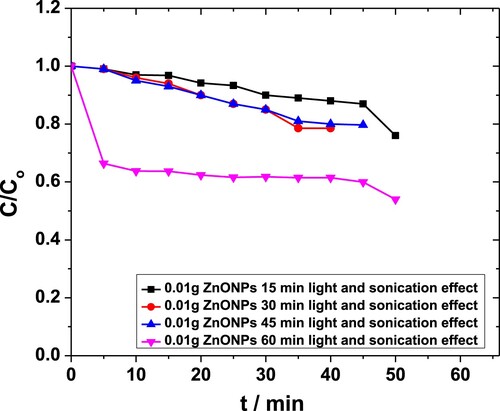
Table 3. The combined effect of sonication and light exposure on the rate constant of methylparaben degradation occurring with different quantities of zinc oxide nanoparticles.
The effect of light exposure on C/Co values obtained from differential conductivity in 0.01 g·L−1 of the catalyst containing MP solutions is shown in Figure . Under combined catalysis, the decline in values is somewhat similar to that observed with either of the photo – or sono-catalysis alone. Based on this it is inferred that the system is rich only in free radicals or neutral compounds [Citation56,Citation57].
3.2.4. Degradation of methylparaben under constant sonication time and variable duration of light exposure
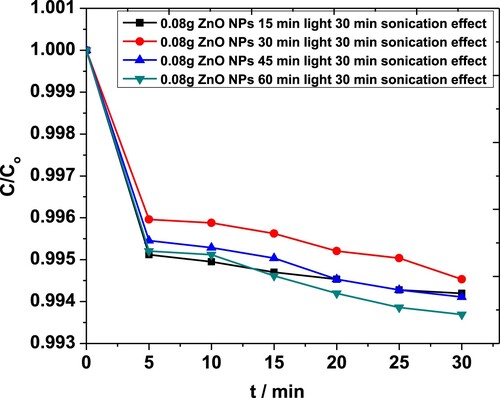
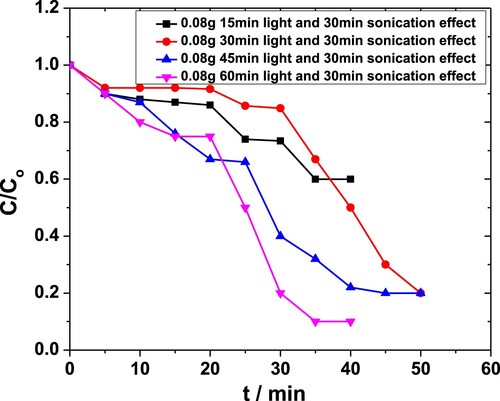
To further investigate the system, the degradation of MP was studied with 0.08 g·L−1 of the catalyst under constant sonication time, while the system was exposed to light for a different duration. The corresponding plots between C/Co vs. t are shown in Figure . With reduced sonication time and higher duration of light exposure, above 90% of degradation was achieved at the maximum, unravelling the possibility of overcoming the negative synergy between sonolysis and photolysis observed earlier.
Figure 14. The effect of light exposure time on the degradation of MP in the presence of 0.08 g·L−1 ZnO NPs as obtained from the absorbance at 254 nm. The system was sonicated for 30 min duration in each case.
The effect of light exposure under fixed sonication of 30 min on C/Co values obtained from differential conductivity in 0.08 g·L−1 of the catalyst containing MP solutions is shown in Figure . Under combined catalysis the decline in values is somewhat like that observed with other systems, showing the excess of free radicals or neutral compounds.
4. Conclusions
The degradation of methylparaben by synthesized ZnO NPs of size 30 nm was studied. The effect of sonication or light and a combination of both was investigated using different quantities of catalyst (between 0.01 and 0.08 g·L−1). The results proved that photocatalysis was more yielding than sonocatalysis under similar conditions. With 0.01 g·L−1 of catalyst, the maximum degradation recorded under mere sonication was 71% compared to 90% obtained under photocatalysis. The difference was eliminated when the quantity of catalyst was raised to 0.04 g·L−1. A negative effect on degradation was seen under combined photosonocatalysis. The extent of degradation declined to 46% maximum, showing the negative synergy between two catalysis aids viz. light and sonication. It was possible to overcome this problem using a fixed sonication time of 30 min and variable exposure to light when the amount of catalyst used was 0.08 g·L−1. The differential conductivity measurements revealed the excess of free radicals and neutral species. Based on the findings of this study, it is concluded that ZnO NPs can effectively be harnessed for the removal or degradation of parabens from wastewater.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Terasaki M, Makino M, Tatarazako N. Acute toxicity of parabens and their chlorinated by-products with Daphnia magna and Vibrio fischeri bioassays. J Appl Toxicol. 2009;29(3):242–247.
- Asgari E, Esrafili A, Rostami R, et al. O3, O3/UV and O3/UV/ZnO for abatement of parabens in aqueous solutions: effect of operational parameters and mineralization/biodegradability improvement. Process Saf Environ Prot. 2019;125:238–250.
- Mincea MM, Lupsa IR, Cinghita DF, et al. Determination of methylparaben from cosmetic products by ultra performance liquid chromatography. J Serb Chem Soc. 2009;74(6):669–676.
- Lee J-W, Lee H-K, Moon H-B. Contamination and spatial distribution of parabens, their metabolites and antimicrobials in sediment from Korean coastal waters. Ecotoxicol Environ Saf. 2019;180:185–191.
- Zúñiga-Benítez H, Peñuela GA. Methylparaben removal using heterogeneous photocatalysis: effect of operational parameters and mineralization/biodegradability studies. Environ Sci Pollut Res. 2017;24(7):6022–6030.
- Shirasangi R, Kohli HP, Gupta S, et al. Separation of methylparaben by emulsion liquid membrane: optimization, characterization, stability and multiple cycles studies. Colloids Surf, A. 2020;597:Article 124761.
- Gupta P, Pushkala K. Parabens: The love-hate molecule. Obstet Gynecol. 2020;3:037–038.
- Haider, K., Rehman K, Sabir A, et al., Parabens as endocrine disrupting chemicals and their association with metabolic disorders. In: Akash MSH, Rehman K, Hashmi MZ, editors. Endocrine disrupting chemicals-induced metabolic disorders and treatment strategies. Cham: Springer; 2020. p. 367–379.
- Baker BH, Wu H, Laue HE, et al. Methylparaben in meconium and risk of maternal thyroid dysfunction, adverse birth outcomes, and attention-deficit hyperactivity disorder (ADHD). Environ Int. 2020;139:Article 105716.
- Ngigi EM. Photocatalytic degradation of parabens in aqueous solutions with modified tungsten oxide nanoparticles. Johannesburg: University of Johannesburg; 2018.
- Khalafi T, Buazar F, Ghanemi K. Phycosynthesis and enhanced photocatalytic activity of zinc oxide nanoparticles toward organosulfur pollutants. Sci Rep. 2019;9(1):1–10.
- Ijaz I, Gilani E, Nazir A, et al. Detail review on chemical, physical and green synthesis, classification, characterizations and applications of nanoparticles. Green Chem Lett Rev. 2020;13(3):223–245.
- Al-Hakkani MF. Biogenic copper nanoparticles and their applications: A review. SN Appl Sci. 2020;2(3):1–20.
- Buazar F. Impact of biocompatible nanosilica on green stabilization of subgrade soil. Sci Rep. 2019;9(1):1–9.
- Moavi J, Buazar F, Sayahi MH. Algal magnetic nickel oxide nanocatalyst in accelerated synthesis of pyridopyrimidine derivatives. Sci Rep. 2021;11(1):1–14.
- Saleh TA. Nanomaterials: classification, properties, and environmental toxicities. Environ Technol Innov. 2020;20:Article 101067.
- Mano G, Harinee S, Sridhar S, et al. Microwave assisted synthesis of ZnO-PbS heterojuction for degradation of organic pollutants under visible light. Sci Rep. 2020;10(1):1–14.
- Gao F. An overview of surface-functionalized magnetic nanoparticles: preparation and application for wastewater treatment. ChemistrySelect. 2019;4(22):6805–6811.
- Zanias A, Frontistis E, Vakros J, et al. Degradation of methylparaben by sonocatalysis using a Co–Fe magnetic carbon xerogel. Ultrason Sonochem. 2020;64.
- Yashni G, Al-Gheethi A, Mohamed R, et al. Photocatalysis of xenobiotic organic compounds in greywater using zinc oxide nanoparticles: a critical review. Water Environ J. 2020;35(1):190–217.
- Safat S, Buazar F, Albukhaty S, et al. Enhanced sunlight photocatalytic activity and biosafety of marine-driven synthesized cerium oxide nanoparticles. Sci Rep. 2021;11(1):1–11.
- Asgari E, Hashemzadeh B, Hassani G, et al. Enhancement the BuP photo-catalytic degradability by UVC/ZnO through adding exogenous oxidant: mechanism, kinetic, energy consumption. J Environ Chem Eng. 2020;8(1):Article 103576.
- Sheikhmohammadi A, Asgari E, Manshouri M. Enhancement the phenylmethyl ester photo degradability in the presence of O3 and H2O2. Optik (Stuttg). 2021;228:Article 166204.
- Yap H, Pang YL, Lim S, et al. A comprehensive review on state-of-the-art photo-, sono-, and sonophotocatalytic treatments to degrade emerging contaminants. Int J Environ Sci Technol. 2019;16(1):601–628.
- Moradi M, Elahinia A, Vasseghian Y, et al. A review on pollutants removal by sono-photo-Fenton processes. J EnvironChem Eng. 2020;8(5):Article 104330.
- Arslan E, Hekimoglu BS, Cinar SA, et al. Hydroxyl radical-mediated degradation of salicylic acid and methyl paraben: an experimental and computational approach to assess the reaction mechanisms. Environ Sci Pollut Res. 2019;26(32):33125–33134.
- Edison TJI, Sethuraman M. Instant green synthesis of silver nanoparticles using Terminalia chebula fruit extract and evaluation of their catalytic activity on reduction of methylene blue. Process Biochem. 2012;47(9):1351–1357.
- Alves MM, Araujo JMM, Martins IC, et al. Insights into the interaction of bovine serum albumin with surface-active ionic liquids in aqueous solution. J Mol Liq. 2020;322:Article 114537.
- Tritt TM. Thermal conductivity: theory, properties, and applications. New York: Springer; 2004.
- Castro I, Teixeira JA, Salengke S, et al. Ohmic heating of strawberry products: electrical conductivity measurements and ascorbic acid degradation kinetics. Innov Food Sci Emerg Technol. 2004;5(1):27–36.
- Calloway D. Beer-Lambert law. J Chem Educ. 1997;74(7):744.
- Dhahir SA, Hussein H. Spectrophotometric determination of methyl paraben in pure and pharmaceutical oral solution. Adv Nat Sci. 2013;6:69–74.
- Sheikhmohammadi A, Asgari E, Hashemzadeh B, et al. The application of co-oxidant in order to enhancement the parabens photo-catalytic degradability. Optik (Stuttg). 2020;224:Article 165667.
- Ullah I, Ali F, Ali Z, et al. Glycol stabilized magnetic nanoparticles for photocatalytic degradation of xylenol orange. Mater Res Express. 2018;5(5):Article 055509.
- Becheri A, Dürr M, Nostro PL, et al. Synthesis and characterization of zinc oxide nanoparticles: application to textiles as UV-absorbers. J Nanopart Res. 2007;10(4):679–689.
- Buazar F, Alipouryan S, Kroushawi F, et al. Photodegradation of odorous 2-mercaptobenzoxazole through zinc oxide/hydroxyapatite nanocomposite. Appl Nanosci. 2015;5(6):719–729.
- Buazar F, Bavi M, Kroushawi F, et al. Potato extract as reducing agent and stabiliser in a facile green one-step synthesis of ZnO nanoparticles. J Exp Nanosci. 2016;11(3):175–184.
- Du Y, Wang R, Yue H, et al. Dose response and stability of silicone-based deformable radiochromic dosimeters (FlexyDos3D) using spectrophotometer and flatbed scanner. Radiat Phys Chem. 2020;168:Article 108574.
- Jayarambabu N, Kumari BS, Rao KV, et al. Beneficial role of zinc oxide nanoparticles on green crop production. Int J Multidiscip Adv Res Trends. 2015;10:273–282.
- Zak AK, Razali R, Abu Majid WHB, et al. Synthesis and characterization of a narrow size distribution of zinc oxide nanoparticles. Int J Nanomed. 2011;6:1399–1403.
- Kasi G, Seo J. Influence of Mg doping on the structural, morphological, optical, thermal, and visible-light responsive antibacterial properties of ZnO nanoparticles synthesized via co-precipitation. Mater Sci Eng C. 2019;98:717–725.
- Wang X, Sun T, Zhu H, et al. Roles of pH, cation valence, and ionic strength in the stability and aggregation behavior of zinc oxide nanoparticles. J Environ Manage. 2020;267:Article 110656.
- Bampos G, Frontistis Z. Sonocatalytic degradation of butylparaben in aqueous phase over Pd/C nanoparticles. Environ Sci Pollut Res. 2019;26(12):11905–11919.
- Larrarte F, Francois P. Attenuation of an ultrasonic beam by suspended particles and range of acoustic flow meters in sewer networks. Water Sci Technol. 2012;65(3):478–483.
- Yaqoob A, Noor N, Umar K, et al. Graphene oxide-ZnO nanocomposite: an efficient visible light photocatalyst for degradation of rhodamine B. Appl Nanosci. 2021;11(4):1291–1302.
- Chakma S, Moholkar VS. Investigation in mechanistic issues of sonocatalysis and sonophotocatalysis using pure and doped photocatalysts. Ultrason Sonochem. 2015;22:287–299.
- Kroflič, A., A. Apelblat, and M. Bešter-Rogač. Dissociation constants of parabens and limiting conductances of their ions in water. J Phys Chem B. 2012;116(4):1385–1392.
- Berberidou C, Poulios I, Xekoukoulotakis NP, et al. Sonolytic, photocatalytic and sonophotocatalytic degradation of malachite green in aqueous solutions. Appl Catal, B. 2007;74(1-2):63–72.
- Sasi S, Rayaroth MP, Devadasan D, et al. Influence of inorganic ions and selected emerging contaminants on the degradation of methylparaben: a sonochemical approach. J Hazard Mater. 2015;300:202–209.
- Chatterjee D, Dasgupta S. Visible light induced photocatalytic degradation of organic pollutants. J Photochem Photobiol C. 2005;6(2-3):186–205.
- Ameta R, Benjamin R, Ameta A, et al. Photocatalytic degradation of organic pollutants: A review. Mater Sci Forum. 2012;734:247–272.
- Fang H, Gao Y, Li G, et al. Advanced oxidation kinetics and mechanism of preservative propylparaben degradation in aqueous suspension of TiO2 and risk assessment of its degradation products. Environ Sci Technol. 2013;47(6):2704–2712.
- Suliman MA, Gondal MA, Dastageer MA, et al. Method for visible light-induced photocatalytic degradation of methylparaben in water using nanostructured Ag/AgBr@ m-WO3. Photochem Photobiol. 2019;95(6):1485–1494.
- Joseph CG, Puma GL, Bono A, et al. Sonophotocatalysis in advanced oxidation process: a short review. Ultrason Sonochem. 2009;16(5):583–589.
- Karim AV, Shriwastav A. Degradation of ciprofloxacin using photo, sono, and sonophotocatalytic oxidation with visible light and low-frequency ultrasound: degradation kinetics and pathways. Chem Eng J. 2020;392:Article 124853.
- Kalpana V, Devi Rajeswari V. A review on green synthesis, biomedical applications, and toxicity studies of ZnO NPs. Bioinorg Chem Appl. 2018;2018:Article 3569758.
- Chantipmanee N, Sonsa-ard T, Fukana N, et al. Contactless conductivity detector from printed circuit board for paper-based analytical systems. Talanta. 2020;206:Article 120227.