?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The present study aimed to investigate the hepatoprotective activity of the hydroethanolic (HSE) and methanolic extracts (MSE) of Pericopsis laxiflora stem bark on carbon tetrachloride-induced hepatotoxicity in rats. The stem bark of P. laxiflora was extracted with 50% ethanol and methanol. Hepatoprotective activities were assessed using the CCl4 model and tested against extracts at 100, 250, and 500 mg/kg bwt. Biochemical, antioxidant enzymes and proinflammatory cytokines in liver homogenate were assayed. Treatment with HSE and MSE resulted in a significant increase in SOD, CAT, and GSH levels and a significant decrease in MDA and MPO levels in livers of CCl4 treated rats. There was also restoration of liver biomarkers and pro-inflammatory cytokines to near-normal levels. The present investigation suggests P. laxiflora extracts possess good hepatoprotective properties. This could be attributed in part to the inhibitory effect of the extracts on oxidative stress and the suppression of proinflammatory cytokines.
1. Introduction
The liver is an organ that is commonly known for the production of reactive oxygen species that results in tissue damage through lipid peroxidation or inflammation. These generated free radicals can also be considered as important mediators of pathogens leading to many diseases such as diabetes, cancer, heart infection, and gout [Citation1]. The presence of antioxidant enzymes provides a defence mechanism by scavenging the reactive oxygen species into a less toxic substance [Citation1]. Diseases of the hepatocytes are regarded as a tremendous global health concern, especially in Africa, however, in allopathic medicine, few effective drugs for liver protection against serious disorders have been discovered [Citation2]. Various herbal plants have been recorded to perform a crucial function in the healing and management of different types of liver diseases.
Pericopsis laxiflora is a plant that is regularly used by diabetes patients. In Cameroon, Bum [Citation3] reported P. laxiflora as a medicinal plant that possess anti-diabetic effect when compared to Combretum molle. P. laxiflora continue to remain as one of the most popular and well-known plants with a long history of use among the Ghanaian population for the treatment of jaundice and body weakness [Citation4].
In Ghana, numerous herbal plants have been investigated for their hepatoprotective effects and actions [Citation5,Citation6], however, no research has been done to determine the hepatoprotective effects of Pericopsis laxiflora [Citation7]. Therefore, the purpose of this investigation was to examine the hepatoprotective potential of Pericopsis laxiflora stem bark extract, to help improve the outcomes of patients with liver disorders. Pericopsis laxiflora is a savannah, perennial, deciduous shrub, or tree that belongs to the Leguminosae family. It is mostly planted in the communities and villages due to its fond shade capabilities. It is commonly called Satinwood [Citation7]. Carbon tetrachloride (CCl4) and certain analgesics, such as acetaminophen, are prominent liver damage models used to test for hepatoprotective drugs. In this investigation, CCl4 was chosen as the toxicant because of its high reactive metabolite produced. The ability of P. laxiflora stem bark extract to reverse damage caused by CCl4 to near normal will therefore prove its hepatoprotective role.
Silymarin a flavonolignan molecule has been considered a standard drug for treating liver diseases in many experiments due to its hepatoprotective properties [Citation8]. Silymarin comprises structural components such as silibinin, silydianine, and silychristine and was extracted from the Silybum marianum plant [Citation9]. According to the current study, hepatoprotective effects of silymarin in acute hepatitis poisoning by Amanita phalloides, ethanol, carbon tetrachloride, and thioacetamide were reported [Citation10], however, the hepatoprotective properties of the hydroethanolic (HS) and methanolic (MSE) stem extract of P. laxiflora have not yet been reported. Thus, the present study was carried out to investigate the hepatoprotective properties and mechanism of action 50% hydroethanolic and methanolic extracts of Pericopsis laxiflora on carbon tetrachloride-induced liver damage in rats.
2. Material and methods
2.1 Chemicals and reagents
Ethanol 99.9% was procured from Changshu Hongsheng Fine Chemicals Co. Ltd., China. Ethylenediaminetetraacetic acid (EDTA) was procured from Thermo Fisher Scientific India Pvt. Ltd., (Mumbai, India). All other chemicals and reagents used in the study were of analytical grade and obtained from standard suppliers.
2.2 Collection and authentication of plant materials
P. laxiflora fresh stem bark was obtained in February 2019 from healthy, fully developed plants in the Ejura Savannah forest reserve in Ghana's Ashanti region. (Latitude location – N 070°, 21°, 03.4; Longitude location – W 001°, 22°, 28.9). Mr. Jonathan Dabo, a botanist from the Centre for Scientific and Industrial Research, Kumasi, authenticated the plant samples. A voucher identification number (*******) was assigned, and the voucher specimen was deposited at the herbarium of the Herbal Medicine Department, ********. The phytochemical components is published elsewhere.
2.3 Preparation of extracts
The plant, Pericopsis laxiflora, was separated into the leaves, flowers, and stem barks components. The stem bark was washed, dried in the shade, and milled into a powder. Fifty percent hydroethanolic extract of the stem bark (HSE) was prepared using cold maceration procedure by suspending 100 grams of the powder in 500 ml of 50 percent ethanol (50:50, ethanol: water, v / v). Similarly, methanolic extract of the stem bark (MSE) was prepared by suspending 100 grams of the powder stem bark material in 500 ml of methanol. The extraction was done at room temperature on a shaker for 24 h. Subsequently, the extracts were filtered through cotton wool and concentrated under pressure using a rotary evaporator. The extracts were frozen at – 20oC freeze-dried and stored until use.
2.4 Animals
HSE and MSE were evaluated for their hepatoprotective effects in the adult male of Sprague–Dawley rats (body weight, 150–200 g) in good health. Animals were purchased from the University of Ghana Medical School in Legon, Accra, and were kept in polypropylene cages coated with wood shavings. They were acclimatized for one week before testing in the Department of Biochemistry and Biotechnology's animal holding facility at ******, where they were kept under normal circumstances (temperature 252°C, relative humidity 65 percent, light/dark cycle 12/12 h). The rats were fed with a normal pellet diet (Agricare, Kumasi), and provided with drinking water ad libitum. For easy identification, they were only painted on their tails using permanent markers. All animal research was carried out by the Committee for the Monitoring and Control of Animal Experimentation's guidelines. Protocol for all animal experiments were verified and approved by a veterinarian on the research team.
2.5 Experimental design
Seventy-five (75) male rats weighing 150–200 g were divided into 15 groups and treated for 7 days (n = 5). Table displays the groups and treatments. All animals were starved for 12 h before the first oral dose was administered, and they had unlimited access to food and freshly distilled water throughout the experiment. After an overnight fast under light ether anesthesia, all rats were slaughtered on the eighth day. Blood samples were collected from the animals’ necks in gel-activated tubes for biochemical analysis and EDTA tubes for haematological examination. In liver homogenates, antioxidant enzymes, oxidative stress markers, and pro-inflammatory cytokines were all assessed.
Table 1. Animal groups and treatments.
2.6 Effect of extracts on body weight of rats
On the 1st (D1) and 7th days (D7) of treatment, the body weights of all animals were measured using the formula below [Citation11].
where Weight n is the weight on day 7 and Weight o is the weight on day 1.
2.7 Effect of extracts on organ weights of rats
The rats’ livers and kidneys were collected, cleaned in a buffered saline solution, dried on tissue paper, visually evaluated, and weighed to estimate the absolute organ weight (AOW).
The Relative Organ Weight (ROW) of each organ was calculated using the formula below [Citation11].
2.8 Effect of the treatments on haematological parameters of rats
An automated haematological analyser was used to examine the haematological profile of the animals (Sysmex XS-1000i). The following parameters were measured: haemoglobin, white blood cell count (WBC), red blood cell count (RBC), haematocrit (HCT), platelets (PLT), mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH), mean corpuscular haemoglobin concentration (MCHC), and differential leucocyte count (neutrophils, lymphocytes, eosinophils, monocytes).
2.9 Effect of treatments on serum biochemistry of rats
To extract serum, blood samples in activated gel tubes were allowed to coagulate before being centrifuged at 1500 × g for 15 min. The following biochemical parameters were measured: glucose, creatinine, urea, sodium, potassium, chloride, total protein, albumin, globulin, total bilirubin (TBil), direct bilirubin (DBil), alanine transaminase (ALT), aspartate transaminase (AST), triglyceride (TG), and cholesterol (total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol) using an automated biochemistry analyzer (ADVIA 2400, Siemens Healthcare) and analyzer specific reagents Aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), gamma-glutamyl transferase (GGT), and total bilirubin were used to compute percentage protection (TBIL). The following formula was used to compute percentage protection for liver functional indices [Citation12]:
2.10 Antioxidant and oxidative stress biomarkers
After homogenizing the liver (1.0 g) in 10 mL phosphate buffer (0.1 M, pH 7.4) and centrifuging at 10,000 rpm for 20 min at 4°C, the post-mitochondrial fraction was extracted (PMF). The supernatant was collected and utilized for antioxidant assays such as reduced glutathione (GSH), catalase (CAT), superoxide dismutase (SOD), malondialdehyde (MDA), and myeloperoxidase (MPO) using methods described by Beutler et al. [Citation13], Jollow et al. [Citation14] and Oyagbemi et al. [Citation15], with modifications as described by Genfi et al. [Citation16].
2.11 Inflammatory cytokine assay
Using homogenized liver samples, measurement of anti-inflammatory proteins of specific cytokines, namely Tumour Necrosis Factor-alpha (TNF-α), Transforming Growth Factor-Beta1 (TGF-β1), Nuclear Factor Kappa B (NFkβ), Cyclo-oxygenase 2 (COX-2), Interleukin – 17 (IL-17) and Interleukin – 23 (IL-23) were tested using appropriate ELISA kits. TNF-α, NFkβ, and COX-2 ELISA kits were obtained from RayBio – tech, Inc. (Norcross, GA, USA) and TGF-β1, IL-17 and IL-23 kits were from Aviscera Bioscience, Inc. (Santa Clara, CA, USA).
2.12 Histopathological studies
Each slice of liver tissue was immersed in a 10% buffered formaldehyde solution to guarantee adequate 24 h fixing. Following that, the samples were rinsed with double distilled water and dehydrated using methanol and ethanol dilutions. Specimens were purified in xylene before being embedded in paraffin for 24 h at 56oC in an oven. Using a sled microtome, paraffin beeswax tissue blocks with a thickness of 4 m were produced for sectioning. For analysis, the tissue slices were mounted on glass slides, deparaffinized, and counterstained with haematoxylin and eosin. The stained tissues were viewed under a microscope at X40 (BX-51) magnification and shot using a charge-couple device (CCD) camera.
2.13 Statistical analyses
All statistical data were analysed using GraphPad Prism for Windows version 9.0 (GraphPad Software, San Diego, CA, USA) with a one-way analysis of variance test, and presented as means ± SEM. The Tukey Multiple Comparison Test was employed and, significant differences between groups were considered as p < 0.05.
3. Results
3.1 Effect of the treatments on rat body weight
Table shows the impact of HSE and MSE on the body weight of normal and CCl4 – induced rats. On the 7th day of the experiment, there was a considerable loss in body weight in the CCl4 group when compared to the control group. Co-treated of HSE or MSE with CCl4 resulted in weight increase when compared with CCl4 only treatment group.
Table 2. Effect of treatment on body weight of rats.
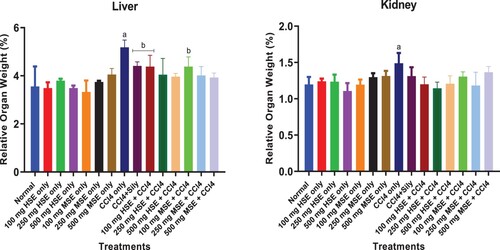
3.2 Effect of the treatments on relative organ weight
Figure shows the effect of HSE and MSE on the relative weight of liver and kidney samples of CCl4 treated rats. Administration of CCl4 resulted in a significant increase in the relative liver (p < 0.05–0.001) and kidney (p < 0.5–0.001) weight of the CCl4 treated group when compared to the normal (untreated or control) group. CCl4 and HSE or MSE co-treatment groups did not show any significant changes compared to the normal group, at all doses for both liver and kidney samples, indicating the hepatoprotective effects of HSE and MSE against the CCl4 insult.
3.3 Effect of treatment on haematological parameters
The effect of HSE and MSE treatment on hematological indices of CCl4 – intoxicated rats is shown in Table . The hematological indices were measured to assess the effect of the CCl4, HSE, and MSE on the rats. Generally, the treatments did not cause significant changes to the haematological indices of the rats.
Table 3. Effect of treatment on haematological parameters.
3.4 Effect of treatment on biochemical profile
Table shows the effect of treatment on the biochemical profile of rats after CCl4 treatment. Administration of CCl4 resulted in a significant increase in most of the biochemical parameters tested when compared to the normal group. Treatment with 250 mg HSE and MSE separately, resulted in significant decreases in most of the parameters.
Table 4. Effect of treatment on biochemical parameters.
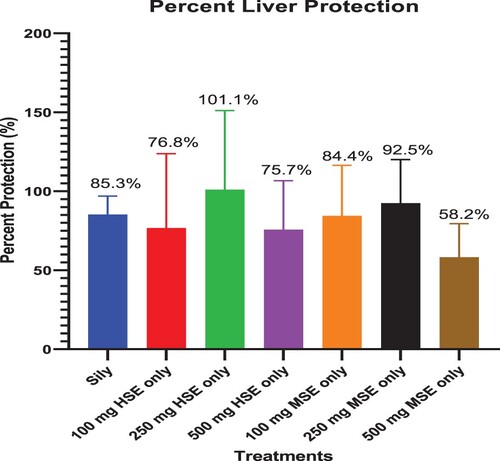
3.5 Percentage hepatoprotection
Figure shows the percent protection of silymarin, HSE, and MSE against CCl4. HSE treatment at 250 mg/kg b.wt recorded the highest liver protection against the CCl4 – induced hepatotoxicity. Principal indicators used in the calculation included ALT, AST, GGT, and TBIL.
3.6 Effect of treatment on electrolytes
The effects of HSE and MSE treatment on electrolytes are shown in Table . CCl4 administration resulted in a non-significant difference in all electrolytes indices.
Table 5. Effect of HSE on electrolytes.
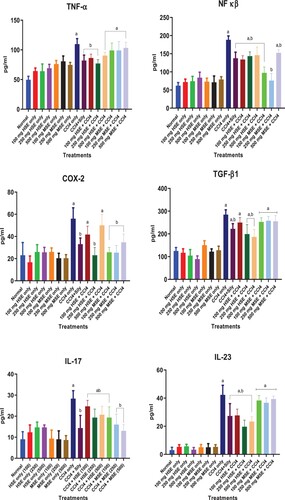
3.7 Effect of treatments on inflammation
The effect of treatment on inflammatory cytokine is shown in Figure . In the CCl4 treatment group, TNF-α, TGF-β1, NF kβ1, COX-2, IL-17, and IL-23 levels were significantly higher (p < 0.05–0.001) than the Normal control. Groups treated with extracts only showed no significant difference in TNF-α, TGF-β1, NF kβ, COX-2, IL-17, and IL-23 levels when compared with the normal group.
3.8 Effect of treatments on hepatic antioxidants and oxidative stress markers
Table shows the effects of HSE and MSE on antioxidants and hepatic oxidative stress. Administration of CCl4 resulted in a significant increase in hepatic oxidative stress biomarkers of the CCl4 treated group when compared to the Normal group. However, groups co-treated with CCl4 and HSE or MSE at all doses recorded significant changes (p < 0.05) in antioxidants and hepatic oxidative stress biomarkers when compared to the CCl4 group.
Table 6. Effect of treatments on antioxidants and hepatic oxidative stress.
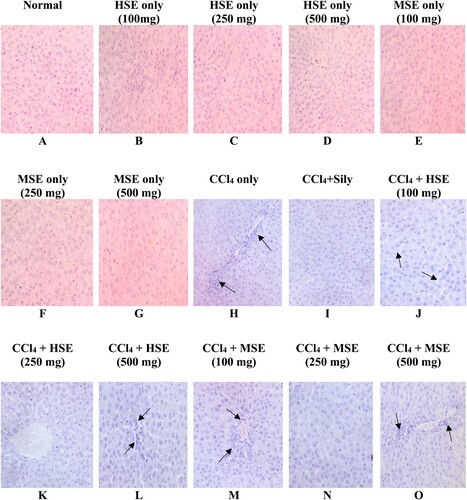
3.9 Effect of treatment on liver histology
Figure depicts liver micrographs from both normal and treated rats. While there were no unfavourable morphological changes in the Normal group, the CCl4 group had significant hepatocellular abnormalities. In animals treated with 250 mg/kg bwt of either HSE or MSE and Silymarin, these changes were reverted to near to normal architecture.
Figure 4. Photomicrographs of liver from male rats administered orally for 7 days Photomicrograph (A) – (G) shows normal hepatocytes with no observable lesion (F0); (H) showed severe destruction of the hepatic architecture with portal fibrous strands. (black arrows, F3); (I) shows no observable hepatic degeneration (F0); (J) shows mild degeneration with sinusoidal dilation (black arrows, F1); (K) recorded no observable lesion (F0); (L) shows mild degeneration with sinusoidal dilation (F1); (M) shows mild necrosis with centrilobular atrophy of hepatocytes (black arrows, F2); (N) shows normal hepatocytes with no observable lesion (F0); (O) shows degeneration of hepatocytes with moderate necrosis (black arrows, F2). (H&E x 400). From Metavir system; level of fibrosis from F0 to F3.
4 Discussion
Liver diseases are a global health concern that causes a high rate of mortality and morbidity in more than two million people yearly [Citation17]. Regardless of the recent improvement in orthodox medicine, effective medicine with hepatoprotective properties and little effects is not easily available. Exposing toxins or chemicals to the liver cells is one good approach hepatoprotective drugs can be developed [Citation18]. In this work, the hepatoprotective property of HSE and MSE were investigated against liver toxicants carbon tetrachloride. Silymarin, a common chemical known to possess a hepatoprotective role [Citation19] was used as a standard drug in the study.
Extensive research has not yet been carried out on P. laxiflora. Hence information on the plant is scanty, The hepatoprotective ability of hydroethanolic (HSE) and methanolic (MSE) extracts of P. laxiflora at different doses (100, 250, and 500 mg/kg doses) on CCl4 treated rats, was comparable to silymarin. The difference in organ weight is mostly considered to be a benchmark to investigate the toxic effect of toxins substances was used in the experiment [Citation20]. HSE and MSE at all doses resulted in increased body weight gain following acute liver toxicity, however, CCl4 treated group recorded a significant reduction in body weight on termination of the experiment as expected. Loss of appetite has a direct effect on body weight loss because it causes a disturbance in the carbohydrate, protein, and fat metabolism of an individual [Citation17]. The significant decrease in body weight was attributed to the toxic effect of CCl4 while the reported increases following administration of HSE and MSE were probably due to the averting effect of CCl4 by the extracts. Increases in weight of rat livers and kidneys were possibly a result of enlargement occurring from the toxicity of CCl4, which resulted in the infiltration of chemicals such as fatty acids and glycerol into the hepatic and nephron cells [Citation21]. Earlier reports have indicated that treatment with CCl4 caused an increase in the weight of organs such as the liver and kidney [Citation4–6,Citation18].
The concentration of biomarkers such as AST, ALT, ALP, and GGT in blood serum is usually used to determine the normal functioning and cellular integrity of the liver [Citation4–6].
In earlier studies, a group of animals treated with CCl4 only showed a significant increase in AST, ALT, ALP, and GGT (at P < 0.05–0.0001) when compared to the normal or control group. Thus, the presence of CCl4 was suggested to destroy the liver cells resulting in the leaking of liver enzymes into the blood serum [Citation4]. Co-treatment with standard drug, silymarin and HSE or MSE was able to significantly decrease the levels of AST, ALT, GGT, and ALP, thus bringing them to near normal range. In this work, the treatment with CCl4 resulted in increased activity of these enzymes. This may lead to the destruction of the hepatocytes and adverse effects on membrane permeability. These findings lend support to a previously reported study [Citation5]. Co-treatment with the extracts and silymarin resulted in a reduction of these enzymes concentration to near-normal levels, implying that the extracts have a beneficial role in ameliorating liver damage. The current study also revealed a significant increase in lactate dehydrogenase (LDH) (P < 0.01) and creatine kinase (P < 0.001) in the CCl4 treated group when compared to the normal group. Previous studies by Kotoh and colleagues [Citation20] indicated that free radicals generated by CCl4 caused oxidative damage to the liver cell membrane resulting in increased serum concentration of LDH and CK. CCl4 toxicity resulted in a similar outcome in the brain of treated rats. Pre-treatment with HSE or MSE and silymarin were however able to significantly reduce the level of LDH and CK to near normal at 250 mg/kg b.wt. dose of HSE, showing the greatest significant reduction when compared to the normal levels. This suggests the protective effects of this extract. CCl4 treatment also resulted in a significant increase in blood creatinine and urea levels, thus suggesting possible kidney damage. The combined reduction in albumin, creatinine, and urea level occurring in rats treated with the extracts suggested the protective role of HSE and MSE. Cholesterol plays a key role in the cell membrane and hormone formation. The level of total cholesterol mostly depends on the balance between the body’s rate of production and the rate of absorption from the diet. Hence liver damage may arise when there is a high level of cholesterol accumulation. In this study, significant increases in total cholesterol and triglyceride levels by the CCl4 insults were reversed to normal by pre-treatment with either HSE or MSE. These results suggest that the extracts are promising hepatoprotective agents against CCl4 toxicity, with 250 mg/kg b.wt. of HSE treatment dose having the highest activity.
The CCl4 treatment could also cause the generation of free radicals which ultimately results in rapid haemolysis of red blood cells. Excessive breakdown of RBC can also lead to hyper-bilirubin or a high accumulation of bilirubin in the liver beyond the capacity of the liver cells.
Breakdown of haem results in the formation of a yellowish pigment called bilirubin. Hyper-bilirubin mostly occurs when there is a deficiency in erythropoiesis [Citation22].
Hyper-bilirubin is a build-up of bilirubin due to the endangered bile duct or damaged liver cells, thus resulting in the liver’s inability to excrete a normal amount of bilirubin through the bile duct. A significant increase (P < 0.5–0.0001) in both total bilirubin and direct bilirubin was observed after CCl4 treatment. On the other hand, a significant reduction was reported after co-treatment with silymarin and HSE or MSE at all doses.
At all doses, the ability of HSE and MSE to prevent an increase in organ weight, body weight, and liver enzymes in blood plasma caused by CCl4 was an important marker of the hepatoprotective potential of these extracts. Further investigation on the protection by 250 mg/kg b.wt. HSE and MSE are warranted. Free radicals generated from the treatment with CCl4 result in lipid peroxidation which eventually damages the liver cells [Citation4]. Therefore, hepatoprotective drugs must possess antioxidant properties to facilitate the scavenging of free radicals produced by hepatotoxicants. The HSE and MSE contain known antioxidants such as flavonoids, alkaloids, and Phenols [Citation7].
In the current study, significant changes in levels of CAT, GSH, SOD, MPO, and MDA after CCl4 treatment, thus suggesting a potential liver injury caused by oxidative stress. Superoxide dismutase plays a key role in the antioxidant defence system. A significant increase in SOD activity in the hepatocytes was caused by P. aculeate, thus preventing oxidative damage to the liver which is usually caused by the high production of free radicals [Citation23]. In the present study, the activity of SOD was significantly reduced in the liver of the CCl4 treated group compared with the control group. Co-treatment of CCl4 with HSE or MSE at all doses normalized the activity of the SOD enzyme, thus resulting in a significant difference when compared to the CCl4 group and a non-significant difference when compared to the normal group. Treatment with CCl4 also led to a significant reduction in CAT protein concentration than the normal group. Subsequent, treatment of the rats with HSE or MSE at all doses resulted in a significant increase (P < 0.05–0.001) in CAT level compared to the CCl4 treated group. Elevated levels of MPO and MDA (which are considered indices of hepatic infiltration) were observed in the CCl4 treatment group, which was also associated with lipid peroxidation enhancement. Pre-treatment with HSE and MSE showed a significant reduction in a hepatic infiltration index (MPO and MDA) when compared with the CCl4 treatment group. The reduction of MPO and MDA by HSE and MSE could partially be due to the antioxidant property of the extracts.
Histological examination of liver microstructure corroborated the protective effect of HSE and MSE extracts at 250 mg/kg bwt. Histology of CCl4-treated liver showed massive necrosis, also the micrographs of the HSE and MSE – treated groups at 100 and 500 mg/kg b.wt. corroborated the low percentage of protection, however HSE and MSE co-treated groups at 250 mg/kg b.wt. respectively showed that they prevented cellular organelle degeneration by reversing the damage. In accordance according to the biochemistry results, MSE treatment at 500 mg/kg b.wt also provided the least protection for hepatocytes and hepatic enzymes as shown in the liver histology.
There is also proof that free radical-mediated oxidative stress is accountable for CCl4 toxicity [Citation7,Citation24]. We proposed that the pathway of defence by HSE and MSE may be due to the inhibition of reactive oxygen species (ROS) through the suppression of cytochrome P450 from activating CCl4 to its reactive form (trichloromethyl free radical) and highly reactive metabolites such trichloromethyl peroxyl radical.
The capacity of HSE and MSE to mitigate CCl4-induced hepatic damage may be partly attributed to its ability to decreased phosphorylation of Erk1/2 expression while subjected to oxidative stress [Citation25]. The toxicity of CCl4 can also relate to pro-inflammatory cytokine generation in an experimental model using rats [Citation26]. This was concomitant with the high increased expression of TNF-α, NF kβ, COX-2, TGF-β, IL-17, and IL-23 after CCl4 administration. These pro-inflammatory cytokines play important roles in the formation of liver injury, especially in the activation of hepatic stellate cells (HSCs), which is an essential initiator for liver cell damage [Citation27]. This means that NF kβ signalling is involved in CCl4-induced hepatotoxicity. Oxidative stress is one of the activators of NF kβ which is released from cytosolic inhibitors, transported to the nucleus, and eventually to NF kβ target genes for expression [Citation28]. Cyclooxygenase-2 (COX-2) is an isoenzyme that is regulated by growth factors and many inflammatory cytokines such as IL-6 and TNF; hence, it can be upregulated during inflammation [Citation29].
The cytokines, IL-17 and IL-23, which are involved in inflammation, immune response, muscle injury, and haematopoiesis control, were highly expressed in response to CCl4 [Citation30]. Treatment with HSE or MSE and silymarin blocked the NF kβ inflammatory cascade (slightly due to the brevity of the experiment), resulting in suppression of the pro-inflammatory cytokines COX-2, IL-17, and IL-23.
Thus, in this study, co-treatment of CCl4 with HSE or MSE at all doses resulted in a significant reduction in the levels of pro-inflammatory cytokines. The hepatoprotective effect of P. laxiflora was partly due to the presence of phytochemicals such as alkaloids, flavonoids, cyanogenic glycosides, triterpenes, saponins, polyphenols, tannins, and phytosterol, as reported in our previous studies.
5. Conclusion
This study found that the plant extracts HSE and MSE have the potential to stabilize free radicals produced by CCl4, reduce oxidative stress and inhibit the NF kβ inflammatory pathway through COX-2, IL-17, and IL-23 downregulation. The hepatoprotective effects of the extracts were similar to that of the known standard silymarin. This study found that the free radical – scavenging activity, the enhancement of the antioxidant enzyme system, and the mild inhibition of the NF kB inflammatory pathway are all potential pathways of hepatoprotection by P. laxiflora with HSE at 250 mg/kg b.wt giving the highest protection potential. One key limitation was the inadequate of funds which prevent us from investigating into other molecular markers such as vascular endothelia growth factor and alpha smooth muscle actin. Future work may probable look into exploring other molecular markers.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Engwa GA. Free radicals and the role of plant phytochemicals as antioxidants against oxidative stress-related diseases. In: Asao T, Asaduzzaman M, editor. Phytochemicals - source of antioxidants and role in disease prevention. IntechOpen; 2018. doi:10.5772/intechopen.76719
- Govind P, Sahni YP. A review on hepatoprotective activity of silymarin. International Journal of Research in Ayur Pharm. 2011;2(1):75–79. doi:10.4254/wjh.v6.i3.144
- Bum EN, Soudi S, Ayissi ER, et al. Anxiolytic activity evaluation of four medicinal plants from Cameroon. Af J Trad, Compl Alter Med. 2011;8(5):130–139. doi:10.4314/ajtcam.v8i5S.19
- Imoro AZ, Aikins TK, Eledi JDA. Exploitation and use of medicinal plants, northern region, Ghana. J Med Plants Res. 2013;7(27):1984–1993. doi:10.5897/JMPR12.489
- Okaiyeto K, Nwodo U, Mabinya L, et al. A review on some medicinal plants with hepatoprotective effects. Pharmacogn Rev. 2018;12(24):186–199. doi:10.4103/phrev.phrev_52_17
- Sarfo-Antwi F, Larbie C, Duduyemi B. Extracts of Ageratum Conyzoides L. protects against carbon tetrachloride – induced toxicity in rats through inhibiting oxidative stress. J Adv Med Pharm Sci. 2019;19(2):1–14. doi:10.9734/JAMPS/2018/45189
- Donkor S, Larbie C, Komlaga G, et al. Evaluation of the acute hepatoprotective potential of hydroethanolic extract of Duranta erecta L. parts. J Toxicol. 2020;2020:1–10. doi:10.1155/2020/8815719
- Owojuyigbe OS, Firempong CK, Komlaga G, et al. Phytochemical, antioxidant, and safety evaluation of Hura crepitans (L.) stem bark hydroethanolic extract in animals. European J Med Plants. 2020;31(8):1–16. doi:10.9734/ejmp/2020/v31i830255
- Sarfo-Antwi F, Larbie C, Emikpe BO, et al. Ethnobotany, phytochemistry, and pharmacology of Pericopsis laxiflora (Baker) Meeuwen (Leguminosae) – a review. Int J Biochem Res Rev. 2021;30(2):1–7. doi:10.9734/IJBCRR/2021/v30i230248
- Osadebe PO, Okoye FB, Uzor PF, et al. Phytochemical analysis, the hepatoprotective and antioxidant activity of Alchornea cordifolia methanol leaf extract on carbon tetrachloride-induced hepatic damage in rats. Asian Pac J Trop Med. 2012;5(4):289–293. doi:10.1016/S1995-7645(12)60041-8
- Arthur FKN, Woode E, Terlabi E Larbie C. Evaluation of acute and subchronic toxicity of Annona muricata (Linn) aqueous extract in animal. J Exp Biol. 2011;1:115–124.
- Arthur F, Woode E, Terlabi E, et al. Evaluation of hepatoprotective effect of aqueous extract of Annona muricata (Linn.) leaf against carbon tetrachloride and Acetaminophen-induced liver damage. J Nat Pharm. 2012;3(1):25. doi:10.4103/2229-5119.96957
- Beutler E, Duron O, Kelly BM. An improved method for the determination of blood glutathione. J Lab Clin Med. 1963;61:882–888.
- Jollow DJ, Mitchell JR, Zampaglione N, et al. Bromobenzene-induced liver necrosis, the protective role of glutathione, and evidence for 3,4-bromobentene oxide as the hepatotoxic metabolite. Pharmacology. 1974;11(3):151–169. doi:10.1159/000136485
- Oyagbemi AA, Omobowale TO, Asenuga ER. Sodium fluoride induces hypertension and cardiac complications through the generation of reactive oxygen species and activation of nuclear factor-kappa beta. Environ Toxicol. 2017;32:1089–1101. doi:10.1002/tox.22306
- Genfi AKA, Larbie C, Emikpe EO. Modulation of oxidative stress and inflammatory cytokines as therapeutic mechanisms of Ocimum americanum L. extract in carbon tetrachloride and Acetaminophen-induced toxicity in rats. J Evid-Bas Int Med. 2020;25:1–13. doi:10.1177/2515690X20938002
- Casaret LJ, Klaassen CD. Casarett and toxicology the basic science of poisons (No. 615.9 C3). 2001.
- Al-Seeni MN, El Rabey HA, Zamzami MA, et al. The hepatoprotective activity of olive oil and nigella sativa oil against CCl4 induced hepatotoxicity in male rats. BMC Complement Altern Med. 2016;16(1):1–14. doi:10.1186/s12906-016-1422-4
- Jiang L, Huang J, Wang Y, et al. Metabonomic analysis reveals the CCl4-induced systems alterations for multiple rat organs. J Proteome Res. 2012;11(7):3848–3859. doi:10.1021/pr3003529
- Kotoh K, Kato M, Kohjima M, et al. Lactate dehydrogenase production in hepatocytes is increased at an early stage of acute liver failure. Exp Ther Med. 2011;2(2):195–199. doi:10.3892/etm.2011.197
- Saleem Mohamed TS, Madhusudhana Chetty C, Ramkanth SVST. Hepatoprotective herbs–a review. Int J Res Pharm Sci. 2010;1(1):1–5.
- Watson D. Bilirubin metabolism in disease. Australas Ann Med. 1963;12(1):53–69. doi:10.1111/imj.1963.12.1.53
- Palanivel MG, Rajkapoor B, Kumar RS, et al. Hepatoprotective and antioxidant effect of Pisonia aculeata L. against CCl4-induced hepatic damage in rats. Scie Pharmaceut. 2008;76(2):203–216. doi:10.3797/scipharm.0803-16
- Larbie C, Torkornoo D, Nyanor E, et al. Evaluation of the hepatoprotective potential of hydroethanolic extract of Ficus pumila L. on CCl4 induced liver damage in rats. Global J Res Med Plant Indig Med. 2016;5(7):217–225.
- Feng Y, Wang N, Ye X, et al. Hepatoprotective effect and its possible mechanism of Coptidis rhizoma aqueous extract on carbon tetrachloride-induced chronic liver hepatotoxicity in rats. J Ethnopharmacol. 2011;138:683–690. doi:10.1016/j.jep.2011.09.032
- Algandaby MM, El-Halawany AM, Abdallah HM, et al. Gingerol protects against experimental liver fibrosis in rats via suppression of pro-inflammatory and profibrogenic mediators. Naunyn-Schmiedeberg's Arch Pharmacol. 2016;389(4):419–428. doi:10.1007/s00210-016-1210-1
- Weiskirchen R, Tacke F. Cellular and molecular functions of hepatic stellate cells in inflammatory responses and liver immunology. Hepatobiliary Surg Nutr. 2014;3(6):344–363. doi:10.3978/j.issn.2304-3881.2014.11.03
- Park MH, Hong JT. Roles of NF-κB in cancer and inflammatory diseases and their therapeutic approaches. Cells. 2016;5(2):15. doi:10.3390/cells5020015
- Ramsay RG, Ciznadija D, Vanevski M, et al. Transcriptional regulation of cyclo-oxygenase expression: three pillars of control. Int J Immunopathol Pharmacol. 2003;16(2):59–67.
- Umezawa K, Kawahata N, Mizoguchi F, et al. Interleukin-23 as a therapeutic target for inflammatory myopathy. Int J Scient Rep. 2018;8:5498. doi:10.1038/s41598-018-23539-4