Abstract
This study introduces an inverse procedure for identifying the elastic modulus (Young's modulus) of interfacial tissue around a dental implant using neural network (NN) and finite element analysis (FEA). An NN model is first trained using displacement responses obtained using FEA models with given interface properties. It is then used to identify the interface elastic modulus by feeding in measured displacements of a dental implant–bone structure whose interface elastic modulus is unknown. The results indicate that the identified elastic modulus is sufficiently close to the original one. The developed NN-FEA inverse procedure is concluded to be robust and efficient. It offers a new perspective and means for the study of the living-bone properties around dental implants, as it can be easily made in real-time.
1. Introduction
A bone anchored titanium implant, also called an osseointegration implant Citation1,Citation2, has been successfully used to replace missing teeth. Under occlusal loading, the surrounding conditions of an implant can significantly influence the state of osseointegration and the adaptive bone remodelling processes Citation3. During these processes, different material properties of the dental implant–bone interfacial tissues determine different patterns of biomechanical responses of the dental implant–bone structure. In addition, the stiffness of the interfacial tissues determines the implant stability that greatly influences the implant long-term survival. In clinical practice it is important to detect the varieties of the implant surrounding conditions. However, specific methods to obtain an accurate and effective evaluation of the implant–bone interface property have not been standardized before or after the implant replacement.
Several attempts have been described in literature to predict the properties of implant surrounding bones with in vitro or in vivo studies. Methods reported include traditional mechanical testing, nanoindentation, imaging procedures or ultrasonic techniques Citation4. However, these methods neither allow non-destructive measurement of the elastic constants nor do they allow assessment of its influence on the biomechanical behaviour of the implant–bone interface in the clinical situation.
In the past, finite element analysis (FEA) has been used extensively in an effort to predict the effects of stress on the dental implant–bone interface Citation5–9. Different biomechanical responses can be evaluated so long as the relevant different Young's moduli of the interface are inputted into the FEA models Citation10–13. Also, it is able to simulate and validate the influence of the Young's modulus on displacement responses in both implant and bone. Obviously, these responses must also encode information about the Young's moduli and hence it should be possible to decode this information from the structural behaviour of dental implant bone by means of inverse procedures.
Neural network (NN) is commonly adopted as a very useful tool to solve inverse problems related to non-destructive evaluation of material and structural systems Citation14. Due to the unique computing features of NNs, NN techniques can be applied to model the non-linear and complex relationship between the structural parameters and the dynamic characteristics of complex structures. Several studies have been devoted to the application of this technique to reconstruct material constitutive properties Citation15,Citation16. As reviewed by Sumpter and Noid Citation17, implementation of the NN technique has greatly promoted research in materials science and technology. Recently, Liu and coworkers Citation14,Citation18–22 have employed a progressive-learning NN procedure to characterize the material property of functionally graded material from its dynamic displacement response. To the best knowledge of the authors’ knowledge, no work has been done in the identification of elastic constants of bone from dental implant–bone biomechanical responses using such an inverse procedure.
It is also possible to use FEA to simulate clinical situations involving displacement response of the dental implant–bone structure with different Young's moduli of interface during the osseointegration process. The objective of this study is to inversely identify the Young's modulus of dental implant–bone interface using a progressive NN procedure from displacement responses of a dental implant–bone structure.
2. Material and methods
2.1. In vitro experimental set-up
Experiments are undertaken in a block of bovine rib of a mature specimen, obtained commercially from a butcher, and severed as a model for the edentulous human mandible. The experiment procedure strictly abided with the National Advisory Committee for Laboratory Animal Research Guidelines and General Laboratory Safety Procedure of National University of Singapore.
An implant of 13 mm in length and 4 mm in diameter (Lifecore® Biomedical, Inc., MN, USA) is used in this study. A 6 × 14 mm implant socket is prepared by using drills according to the actual surgery protocol suggested by the manufacturer. As presently defined, the mechanism of osseointegration is a very high rate of living bone modelling and remodelling within about 1 mm of the implant surface Citation23–25. To simulate the biologic change of the interfacial tissue during the osseointegration process, the drill hole is filled with self-curing resin. Within this model, a layer of 1 mm thick implant–bone interfacial tissue is thus created. The implant is assumed to be inserted into the centre of the drill hole. An aluminium rod (SmartPeg™, Osstell AB, Sweden) is screwed onto the implant. A RFA device (Osstell Mentor™, Osstell AB, Sweden) is used to measure vibration responses of the structure as shown in . The aluminium rod is excited by an electromagnetic pulse from the measurement probe. The pulse scale is from 854 to 9888 Hz.
2.2. FEA modelling
ANSYS 6.1 (ANSYS Inc., Canonsburg, PA, USA) is used to generate the three-dimensional (3-D) FEA model (). The interfaces between the cortical and cancellous bone, interfacial tissue (resin) and the bones, interfacial tissue and implant, implant and rod, are assumed to be perfectly bonded. The model is finely meshed with 10-node tetrahedral solid elements. This FEA model is constrained in all three degrees of freedom at each of the nodes located at the external bone surfaces same as the ones in the experimental model. Using traditional mechanical tensile test, the mechanical properties of the bovine rib and the cured resin are obtained. lists all of the material properties used in this FEA modelling Citation10,Citation12,Citation26.
Figure 2. (a) Overall 3-D FEM model of a dental implant–bone structure; (b) sectional view of the interface area.
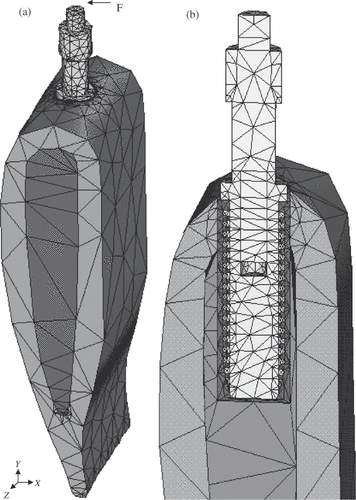
Table 1. Material properties of dental implant–bone structure used in the FEA model.
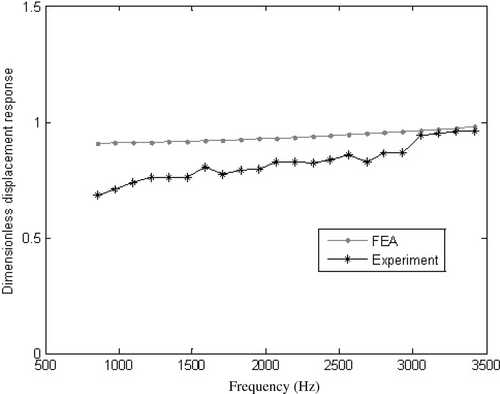
Harmonic response analysis is used to determine the vibration response of the implant-bone structure. Loading is simulated by applying a harmonic force of 1 N horizontally at a node on the top of the implant (node 779, ) in x-direction. The sweep frequency scale is same as the experimental device, i.e. from 854 to 9888 Hz. shows that the FEA results have a good agreement with the experimental measurements within the frequency range of 854–3500 Hz.
Figure 3. Comparison of experimental measurement and FEA solutions (Eactual = 2.94 × 106 (MPa)). It is shown that the FEA results have a good agreement with the experimental measurements within the frequency range of 854–3500 Hz.
The x-component displacement values are used as the inputs for training an NN model. The Young's modulus of the interface is the output of the NN model. The other elastic constants of bones are fixed as constants. After the NN is trained, it will produce this Young's modulus as an output of the NN when an input of displacement response of a dental implant–bone system is provided.
2.3. Inverse identification of Young's modulus of implant–bone interface
In this study, the NN model contains a set of neurons arranged into four layers. Two hidden layers Citation27 are used to connect the input and output neurons. The bone displacement responses as the inputs are fed into the input layer and are multiplied by interconnection weights as they are passed from the input layer to the first hidden layer. Within the first hidden layer, the interconnection weights are summed, and then processed by a non-linear hyperbolic function. As the processed data leaves the first hidden layer, again it is multiplied by interconnection weights, then summed and processed by the second hidden layer. Finally, the data is multiplied by interconnection weights then processed one last time within the output layer to produce the bone Young's moduli as the outputs. In this NN model, the neuron numbers of the input, output, first and second hidden layers are 4, 16, 8 and 1, respectively. Mathematically, the NN model represents a non-linear mapping between inputs and outputs
via the following equation:
(1)
where
is a matrix of weights corresponding to the connections between the layers, and
and
are the numbers of neurons for the i-th and j-th layers, respectively. The training process is actually to adjust the matrix of W Citation28,Citation29. Once trained, the NN model thus creates the connections of the involved parameters and can be used to on-line determination. The actual physical model can be avoided and the efficiency will be very high. The NN is trained with a modified back-propagation training algorithm Citation14.
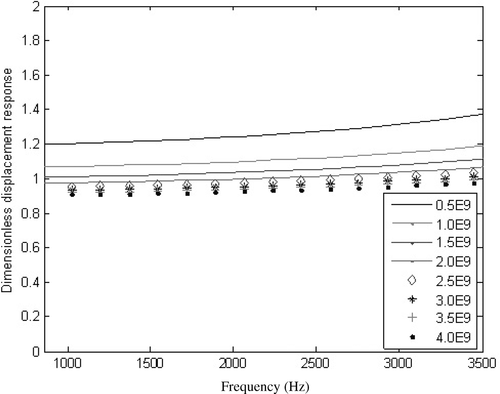
The NN model is trained using a set of initial training data of sets of Young's moduli as the outputs and their corresponding displacement responses as the inputs. In order to produce the inputs, which should sensitively reflect the change of the interface Young's modulus, the sensitive region is therefore first determined. Effects on displacement responses with respect to Young's modulus E of interfacial tissue are displayed in . It is shown that the response is quite sensitive to E. Displacement responses at four frequencies of f1 = 2929.1 Hz, f2 = 3050.4 Hz, f3 = 3173.8 Hz, f4 = 3296.2 Hz and f5 = 3416.9 are selected as inputs of our NN model. The NN model is then trained using a set of training samples.
Figure 4. Effect of Young's modulus of interface resin E on displacement responses. It is shown that the responses are very sensitive to E (MPa).
The initial training samples should cover well the possible range of the Young's modulus of the implant-bone interfacial tissue. However, it is impossible to generate all the combinations of Young's modulus, and hence a good cross-section of possible combinations is required. In this article, a method combining the orthogonal array with random selection for generating training samples is adopted Citation30. Assuming that there is no interaction among the q parameters to be identified, the number of samples required based on the orthogonal array for q parameters with p levels is q(p − 1) + 1. For the current problem, search limits of ±50% from the original values of the Young's modulus are used. To determine the initial training samples, the search range is divided evenly into six levels for the Young's modulus E. Therefore, the number of samples required based on the orthogonal array method is 6. In addition, another 10 samples are created randomly to further reinforce the sample set. Excluding the original Young's modulus, the training samples consist of 16 sets of data.
To verify our NN-FEA inverse procedure, both actual experimentally measured displacements and simulated ‘measured’ displacements are used. In order to simulate the measurement noise, noise-contaminated displacement responses are also used for the identification of the moduli. A Gauss noise with mean 0 and deviation is directly added to the computer-generated displacement responses to simulate noise contamination Citation31, where
is defined as
(2)
in which
is the calculated displacement at the i-th sample point.
The NN model requires the normalization of the input and output data. Practical experience indicates that it is better to normalize the input patterns as well as output patterns in the range between 0.1 and 0.9 Citation32. The following formulas are used for normalization.
(3)
where Ximin and Ximax are, respectively, the minimal and maximal values of the i-th input Xi in the sample data set.
and
are the scaling factors to ensure that the normalized values are not close to 0 or 1. The outputs are also normalized in exactly the same way. After the NN model is trained, the Young's modulus is identified using the trained NN model by feeding in the displacement values.
3. Results and discussion
To examine the stability of the proposed NN-FEA approach, inverse analyses are now performed using the trained NN model. In vitro experimental measurements at actual Young's modulus Eactual and fi, i = 1, …, 5 are input into the trained NN model, and the required output is inversely identified. The identified Young's modulus of interfacial tissue is given in .
Table 2. Identified Young's modulus of the dental implant–bone interface using the NN-FEA and experimental measurements.
Two additional cases of inverse analyses are also conducted in which the noise-free and 5% noise-contaminated simulated measurements are used instead of actual experimental measurements. and give estimated Young's moduli of interfacial tissues for two ‘true’ Young's moduli of Etrue1 and Etrue2. The proposed NN-FEA produces estimated results with maximum deviations of less than 1% for noise free case, and less that 5% for both 5% noise-contaminated cases and in vitro experiment case. The results interpret that our inverse procedure is very stable and reliable within the search range of ±50% off the ‘true’ Young's modulus, and this search range is sufficient in practical applications.
Table 3. Identified Young's modulus of the interface using the NN-FEA and simulated measurements with 0 and 5% noise contamination for Etrue1.
Table 4. Identified Young's modulus of the interface using the NN-FEA and simulated measurements with 0 and 5% noise contamination for Etrue2.
Several assumptions are made in the development of the models in the present study. The dental implant–bone interfaces are assumed to be perfectly bonded. This approach attempts to simulate the 100% osseointegration between the implant and bone. However, it cannot describe exactly the structure and property of interfacial bone in clinical situations Citation33. For instance, the different osseointegration degrees and patterns occur during the bone healing process. Non-destructive assessments of the dental implant interfacial tissue should be further developed to provide fundamental information about its structure and property at the level of detailed needed for biomechanical predictions. For this purpose, 3-D implant–bone FEA models at the microstructural level are required to be able to deal with anisotropic materials and simulate the biomechanical responses of implant–bone interfacial tissues during different osseointegration stages. Further enhancement of the NN model is also needed to accommodate the increase in input and output data.
The developed inverse techniques can be further applied clinically to obtain the required data for assessment of dental integrity of a patient and/or quality of a dental implant, which are extremely useful for the dentist to make a clinic decision based on sound scientific analyses.
4. Conclusion
The Young's modulus of the interfacial tissue around a dental implant is inversely identified in this study. The results of this study suggest that the computational inverse technique using a progressive NN model is applicable and accurately identified the Young's modulus by feeding in real displacement responses of the implant–bone structure. In addition, the NN model is stable to accommodate the presence of noise in the measured data, which is very critical to its practical application in clinical settings. It is concluded that the developed inverse procedure combining the 3-D FEA with the NN model provides an opportunity and means to identify multiple parameters in a complex dental implant–bone structure.
References
- Brunski, JB, 1999. In vivo bone response to biomechanical loading at the bone/dental-implant interface, Adv. Dent. Res. 13 (1999), pp. 99–119.
- Duyck, J, Rønold, HJ, Oosterwyck, HV, Naert, I, Sloten, VJ, and Ellingsen, JE, 2001. The influence of static and dynamic loading on marginal bone reactions around osseointegrated implants: An animal experimental study, Clin. Oral Implants Res. 12 (2001), pp. 207–218.
- Cowin, SC, 1986. Wolff's law of trabecular architecture at remodeling equilibrium, J. Biomech. Eng. 108 (1986), pp. 83–88.
- Turner, CH, and Burr, DB, 2001. Cowin, SC, ed. Bone Mechanics Handbook. Florida: CRC Press LLC; 2001. pp. 7.1–7.24.
- Weinstein, AM, Klawitter, JJ, Anand, SC, and Schuessler, R, 1976. Stress analysis of porous rooted dental implants, J. Dent. Res. 55 (1976), pp. 772–777.
- Geng, JP, Tan, KBC, and Liu, GR, 2001. Application of finite element analysis in implant dentistry: A review of the literature, J. Prosthet. Dent. 85 (2001), pp. 585–598.
- Geng, JP, Ma, QS, Tan, KBC, and Liu, GR, 2004. Finite element analysis of four thread-form configurations in a stepped screw implant, J. Oral Rehab. 31 (2004), pp. 233–239.
- Geng, JP, Xu, DW, Tan, KBC, and Liu, GR, 2004. Finite element analysis of an osseointegrated stepped screw dental implant, J. Oral Implantol. 30 (2004), pp. 223–233.
- Hansson, S, and Werke, M, 2003. The implant thread as a retention element in cortical bone: The effect of thread size and thread profile: A finite element study, J. Biomech. 36 (2003), pp. 1247–1258.
- O’Mahony, AM, Williams, JL, and Spencer, P, 2001. Anisotropic elasticity of cortical and cancellous bone in the posterior mandible increases peri-implant stress and strain under oblique loading, Clin. Oral Implants Res. 12 (2001), pp. 648–657.
- Natali, AN, and Pavan, PG, 2003. Natali, AN, ed. Dental Biomechanics. London: Taylor & Francis; 2003. pp. 211–213.
- Deng, B, Tan, KBC, and Liu, GR, 2008. Influence of osseointegration degree and pattern on resonance frequency in the assessment of dental implant stability using finite element analysis, Int. J. Oral Maxillofac. Implants 23 (2008), pp. 1082–1088.
- Deng, B, Tan, KBC, Liu, GR, Geng, JP, and Yan, WQ, 2008. A new numerical approach for evaluation of dental implant stability using electromagnetic impulse, Internal Chin. J. Dent. 8 (2008), pp. 1–9.
- Liu, GR, and Han, X, 2003. Computational Inverse Techniques in Nondestructive Evaluation. Florida: CRC Press LLC; 2003.
- Sribar, R, 1994. Solutions of inverse problems in elastic wave propagation with artificial neural networks.. Ph.D thesis. Ithaca, New York: Cornell University Press; 1994.
- Huber, N, and Tsakmakis, C, 1999. Determination of constitutive properties from spherical indentation data using neural networks. Part I: The case of pure kinematic hardening in plasticity laws, J. Mech. Phys. Solids 47 (1999), pp. 1569–1588.
- Sumpter, BG, and Noid, DW, 1996. On the design, analysis, and characterization of materials using computational neural networks, Annu. Rev. Mater. Sci. 26 (1996), pp. 223–277.
- Liu, GR, Han, X, and Lam, KY, 2001. Material characterization of FGM plates using elastic waves and an inverse procedure, J. Compos. Mater. 35 (2001), pp. 954–971.
- Liu, GR, Xu, YG, and Wu, ZP, 2001. Total solution for structural mechanics problems, Comput. Methods Appl. Mech. Eng. 191 (2001), pp. 989–1012.
- Liu, GR, Han, X, Xu, YG, and Lam, KY, 2001. Material characterization of functionally graded material by means of elastic waves and a progressive-learning neural network, Compos. Sci. Technol. 61 (2001), pp. 1401–1411.
- Liu, GR, Han, X, and Ohyoshi, T, 2002. Computational inverse techniques for material characterization using dynamic response, Int. J. Soc. Mater. Eng. Res. 10 (2002), pp. 26–33.
- Han, X, Xu, DL, and Liu, GR, 2003. A computational inverse technique for material characterization of a functionally graded cylinder using a progressive neural network, Neurocomputing 51 (2003), pp. 341–360.
- Albrektsson, T, Berglundh, T, and Lindhe, J, 2003. "Osseointegration: Historic background and current concepts". In: Lindhe, J, Karring, T, and Lang, NP, eds. Clinical Periodontology and Implant Dentistry. Oxford, Malden: Blackwell Science; 2003. pp. 809–820.
- Huja, SS, and Roberts, WE, 2004. Mechanism of Osseointegration: Characterization of supporting bone with indentation testing and backscattered imaging, Semin. Orthod. 10 (2004), pp. 162–173.
- Chang, MC, Ko, CC, Liu, CC, Douglas, WH, DeLong, R, Seong, WJ, Hodges, J, and An, KN, 2003. Elasticity of alveolar bone near dental implant–bone interfaces after one month's healing, J. Biomech. 36 (2003), pp. 1209–1214.
- Merz, B, Hunenbart, S, and Belser, U, 2000. Mechanics of the implant-abutment connection: An 8-degree taper compared to a butt joint connection, Int. J. Oral Maxillofac. Implants 15 (2000), pp. 519–526.
- Masri, SF, Chassiako, AG, and Ganghey, TK, 1993. Identification of nonlinear dynamic systems using neural network, J. Appl. Mech. 60 (1993), pp. 123–133.
- Han, LF, Li, GY, Han, X, and Zhong, ZH, 2006. Identification of geometric parameters of drawbead in metal forming processes, Inverse Prob. Sci. Eng. 14 (2006), pp. 233–244.
- Han, X, Jiang, C, Li, GY, Zhong, ZH, and Hu, DB, 2006. An inversion procedure for determination of variable binder force in U-shaped forming, Inverse Prob. Sci. Eng. 14 (2006), pp. 301–312.
- Park, SH, 1996. Robust Design and Analysis for Quality Engineering. London: Chapman and Hall; 1996.
- Han, X, Liu, GR, and Lam, KY, 2000. A quadratic layer element for analyzing stress waves in FGMs and its application in material characterization, J. Sound Vibrat. 236 (2000), pp. 307–321.
- Topping, BHV, and Bahreininejad, A, 1997. Neural Computing for Structural Mechanics. Edinburgh: Saxe-Coburg Publications; 1997.
- Brunski, JB, Puleo, DA, and Nanci, A, 2000. Biomaterials and biomechanics of oral and maxillofacial implants: Current status and future developments, Int. J. Oral Maxillofac. Implants 15 (2000), pp. 15–46.
