ABSTRACT
Introduction: Drug-eluting stents promise to reduce restenosis following endovascular treatment of diseased arteries, but technologies used in peripheral arteries of the leg have not yet achieved desired success rates.
Areas covered: The rationale behind the development of the EluviaTM Drug-Eluting Vascular Stent (Boston Scientific, Marlborough, MA) is described and current preclinical and clinical evidence related to use of the stent is reviewed.
Expert opinion: Stents remain an important endovascular treatment option for femoropopliteal lesions, especially those that are long, occluded, and calcified. Drug-eluting stent technologies show promise to improve patency rates, potentially shifting the primary treatment preference away from balloon-based treatment. The available preclinical and clinical data on treatment with EluviaTM suggest that prolonged paclitaxel elution in the femoropopliteal arteries prevents restenosis and may reduce the need for reintervention.
1. Overview of the market
Peripheral arterial disease (PAD) is estimated to affect more than 200 million people worldwide, with prevalence expected to increase [Citation1]. PAD of the lower extremities is associated with a significant clinical burden. Results from a registry initiated in 2003 showed that approximately one-third of symptomatic PAD patients had at least one vascular-related hospitalization during the 2-year follow-up period, and repeat hospitalizations are common [Citation2].
Stent placement is one of the chief endovascular approaches for treating stenotic lesions of the peripheral arteries located above the knee (i.e. superficial femoral [SFA] and proximal popliteal arteries). In the registry noted above, nearly 10% of symptomatic PAD patients had at least one hospitalization associated with peripheral angioplasty/stenting within 2 years [Citation2]. However, durability of the intervention is limited due to proliferative biological responses that promote neointimal formation, leading to restenosis or occlusion of the treated territory. Comorbidities such as diabetes and certain lesion characteristics increase risk of restenosis. Restenosis may necessitate reintervention (i.e. target lesion revascularization [TLR]) to maintain vessel patency. Drug-eluting stents provide a means to deliver an antiproliferative agent directly to the arterial tissue, thereby reducing incidence of restenosis and reintervention. In the coronary arteries, drug-eluting stent use has been highly successful, with associated TLR rates less than 2% at 1 year postprocedure [Citation3]. The technology has not transferred directly to the peripheral arteries, however, because the drug delivery technology used in the coronary arteries has not been suitably tailored to the unique requirements of the SFA. Thus, observed reintervention rates following implantation of drug-eluting stents in the femoropopliteal segment are substantially higher at 10–20% [Citation4–Citation6].
The Eluvia™ Drug-Eluting Vascular Stent (Boston Scientific, Marlborough, MA) was designed to address demands specific to treating above-the-knee peripheral artery lesions, taking into consideration the mechanical and pathological conditions unique to this anatomy. Coronary arteries and the SFA are both considered muscular arteries, but the mechanical environment of the femoropopliteal segment differs dramatically from that of the coronary arteries. As the largest unsupported artery in the body, the SFA undergoes repetitive deformations along multiple axes during movements such as walking, sitting, standing, or climbing stairs (reviewed in [Citation7]). This challenging mechanical environment highlights the need for a durable scaffold to predictably deliver an active pharmaceutical agent to the diseased vessel. Along with the differences in mechanical environments, preclinical studies indicate that the SFA has a lower ratio of collagen to elastin compared with coronary vessels [Citation8], which may contribute to differential disease progression. Additionally, bone-like osteoid metaplasia present in the calcified SFA [Citation9] mandates that the dosing of the active pharmaceutical agent is appropriate to mitigate neointimal proliferation.
Disease progression was also a consideration for the design of a purpose-built drug-eluting stent for the SFA. Restenosis after endovascular therapy follows a predictable response akin to wound healing, the time course of which is dictated by the biological milieu. In the coronary environment, this time period is 60–90 days [Citation10,Citation11]. However, the timing of restenosis in the challenging SFA environment seems to follow a longer duration [Citation12]. The implications of these differences for drug delivery are central to how coatings for drug-eluting stents are designed.
2. How the technology works
2.1. Device overview
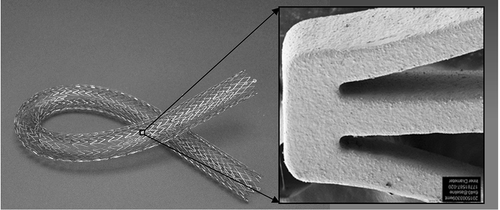
The Eluvia™ stent comprises a self-expanding nitinol stent platform with a coating composed of a polymer system with an antiproliferative active pharmaceutical agent (). The geometry of the base stent is such that it provides sufficient force and flexibility to the scaffold while ensuring that it can withstand the mechanical forces of the femoropopliteal segment, in addition to providing uniform drug coverage along the artery length and around its circumference. The stent coating consists of a primer layer, poly n-butyl methacrylate (PBMA), which promotes adhesion of the active layer. The active layer is composed of the fluoropolymer PVDF-HFP [poly(vinylidene fluoride-co-hexafluoropropylene)] and the antiproliferative agent paclitaxel [Citation13].
2.2. Antiproliferative agent and dosing strategy
Paclitaxel, a microtubule stabilizer, was chosen for its demonstrated antirestenotic effect on peripheral arteries [Citation4,Citation14,Citation15]. Although ‘-limus’ drugs (e.g. everolimus, sirolimus) are effective in coronary arteries, previous attempts to use them in the SFA have been ineffective. The failure of previous attempts with ‘-limus’ analogs in the SFA may have been confounded by design factors such as lack of durability of the base scaffold, use of polymers that lacked good biocompatibility, or selection of a drug-elution profile that did not match the restenotic cascade [Citation5,Citation6].
The dosing strategy was based on recognition that coronary and femoropopliteal arteries have similar cell biology and respond with similar antiproliferative mechanisms upon exposure to paclitaxel. However, peripheral atherosclerotic disease contains more calcium and less lipid than coronary lesions [Citation9,Citation16,Citation17], and the SFA is more prone to chronic occlusions. To counteract reduced paclitaxel bioavailability in the SFA due to these differences in tissue [Citation8] and disease composition, a dosing approach including a relatively greater total amount of released drug was adopted. Release profiles of multiple paclitaxel doses were evaluated preclinically before a dose of 0.167 µg paclitaxel/ mm2 stent surface area was chosen for clinical testing [Citation13,Citation18].
2.3. Polymer selection and elution profile
A polymeric carrier was chosen in order to provide control over the dose and duration of drug release. The base polymer (PBMA) and the fluoropolymer PVDF-HFP comprise the same polymer system as is present on the Promus Element (Boston Scientific, Marlborough, MA) and the Xience V (Abbot Vascular, Abbott Park, IL) drug-eluting coronary stents [Citation3,Citation19] and thus its clinical safety has been studied in more than 11,000 patients. Biocompatibility of the PBMA plus PVDF-HFP system was also demonstrated in preclinical studies. The polymer demonstrated a good vascular safety profile in a porcine coronary artery stenting model [Citation19] and did not inhibit endothelialization (i.e. healing) in a rabbit peripheral artery stenting model or promote thrombus formation in a bench blood loop model [Citation20]. The ability to alter the polymer to enable different concentrations of drug in a preclinical model was also a critical aspect in the polymer system selection. In addition to drug release and biocompatibility considerations, the ideal polymer coating would withstand the extreme mechanical forces of the femoropopliteal segment and not require additional forces for deployment. Researchers at Boston Scientific evaluated multiple biostable and biodegradable polymers and determined that PVDF-HFP performed within desired parameters in tests of drug release and deployment forces, and the coating demonstrated durability during deployment and fatigue testing.
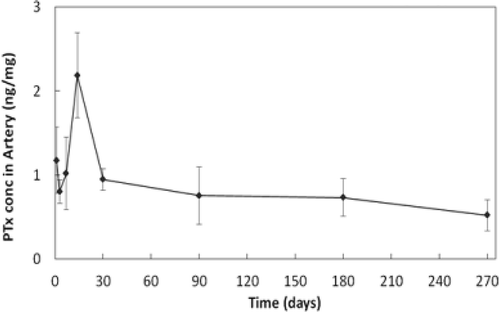
Given that restenosis incidence following stenting of the SFA peaks at about 12 months [Citation12] – later than occurs following coronary stenting – and that the underlying restenotic cascade has a multiphase time course [Citation21], an elution profile to overlap disease progression through at least 12 months was targeted. The pharmacokinetic profile of the selected dose through 6 months has been described by Hou et al. [Citation13] and is shown through 270 days in .
3. Clinical profile
Performance of the Eluvia™ stent in preclinical and clinical studies suggests that the elution profile and dose resulting from the polymer/paclitaxel combination translates into a clinically meaningful antirestenotic effect. In a porcine iliofemoral model of the vascular response to stenting, inhibition of neointimal proliferation was greater with the polymer/paclitaxel combination than with a bare metal stent through 90 days, but healing was not impeded [Citation13]. In addition, preliminary imaging (optical coherence tomography) data from a porcine model of restenosis suggest that neointimal inhibition caused by Eluvia™ may be greater than that associated with a polymer-free paclitaxel-coated stent at 90 days [Citation22]. Histopathologic analysis in this ongoing study is forthcoming.
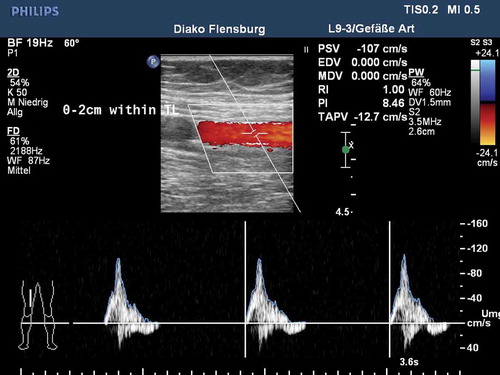
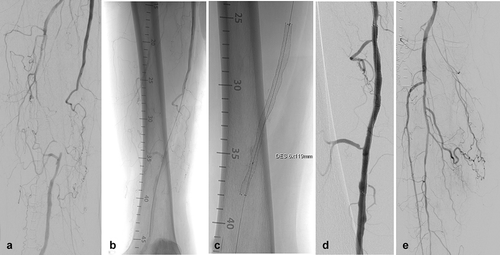
The prospective single-arm MAJESTIC study included 57 symptomatic patients with stenotic or occluded lesions of the SFA and/or proximal popliteal artery who were treated with the Eluvia™ stent. By 1 year postimplant, only two patients required a revascularization procedure to address blockage of the original target lesion, leaving 96% of patients with patent stents () [Citation18]. For comparison, reported revascularization-free rates at 1 year average approximately 87% for bare metal stents [Citation23–Citation30] and slightly higher at 90% for a drug-coated stent [Citation4,Citation31] in clinical trials. Procedural angiography images from a patient treated with an Eluvia™ stent in the MAJESTIC trial are shown in . Duplex ultrasound evaluation conducted 12 months postimplantation () confirmed patency of the treated area.
Figure 3. Kaplan-Meier estimate of primary patency at 12 months in the MAJESTIC trial. Adapted from Müller-Hülsbeck S, Keirse K, Zeller T, et al. Twelve Month Results from the MAJESTIC Trial of the EluviaTM Paclitaxel-Eluting Stent for Treatment of Obstructive Femoropopliteal Disease. J Endovasc Ther 2016, doi: 10.1177/1526602816650206.
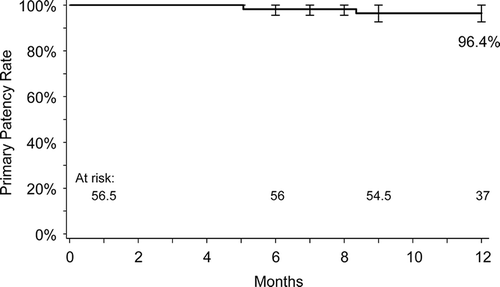
Figure 4. EluviaTM stent implantation in the MAJESTIC study. The female patient was 73 years old and a current smoker with 100% occluded lesion in the mid-SFA with severe calcification. (A) Baseline angiography of the SFA shows the occlusion of nearly 9 cm in length. (B) Unsubtracted angiography indicates an intraluminal 0.018-inch guidewire crossing. (C) Fluoroscopic view of a 6 x 119 mm EluviaTM stent. Note the vessel wall calcification located in the mid- and distal part of the successfully treated lesion. (D) Final angiography represents a good technical result without any significant residual stenosis. (E) The run-off is non-compromised and does not show any signs of distal embolization.
Treatment effectiveness with Eluvia™ is undergoing further verification in the global IMPERIAL study, which is currently enrolling patients (ClinicalTrials.gov identifier NCT02574481). Results of this study will reveal whether the encouraging findings from MAJESTIC bear out in a large (>450 patients) randomized single-blind clinical study. Details regarding alternative other technologies is included in the Alternative technologies box.
4. Conclusion
The Eluvia™ stent was designed to perform in the unique environment of the SFA. Its polymeric coating allows for paclitaxel elution over a long period of time to overlap the timeline associated with peripheral arterial restenosis. The available preclinical and clinical data on treatment with Eluvia™ suggest that prolonged paclitaxel elution in the femoropopliteal arteries prevents restenosis and may reduce the need for reintervention.
5. Expert opinion
A potential disadvantage of stent placement is the possibility of limiting options for future endovascular or surgical interventions. This ‘leave nothing behind’ philosophy garners support from studies reporting 12-month patency rates for drug-coated balloons similar to those achieved with bare metal stents (i.e. up to approximately 83% [Citation34,Citation36,Citation38]). But is this strategy carrying over into clinical studies and real-world use? Indeed, the SFA lesions treated in trials of drug-coated balloons are less complex, that is more often described as TASCII A or B [Citation39], than those typically treated with stents. Furthermore, stent use is common even in clinical studies intended to assess balloon performance. In one clinical study of a drug-coated balloon which allowed stratification to stent treatment, primary stenting was selected over balloon-only treatment for approximately 25% of patients [Citation40], and in a registry of patients treated with a drug-coated balloon, 23% of patients also had a stent implanted [Citation41]. Provisional stenting rates after acute percutaneous angioplasty (PTA) failure in the aforementioned and other recent clinical trials averaged approximately 13% [Citation34,Citation36,Citation38,Citation40,Citation42]. In a study designed to compare efficacy of balloon-based PTA with that of a drug-coated stent, 50% of those patients who had been randomly assigned to the PTA arm received provisional stents [Citation4]. The composition of these study cohorts suggests that a substantial proportion of atherosclerotic patients undergoing PTA ultimately requires a stent scaffold. In addition, availability of a stent that provides a high patency and associated very low reintervention rate would mitigate the relevance of the ‘future options’ argument against a stent-based approach [Citation43]. Together, these observations suggest that performance of drug-eluting stents such as Eluvia™ could challenge the ‘leave no implant’ philosophy if longer term use continues to demonstrate low reintervention rates.
Article highlights
Restenosis following stent placement to treat atherosclerotic lesions in the femoropopliteal segment occurs over a longer time period than that observed following coronary stenting, necessitating a prolonged drug elution profile.
A polymeric carrier was needed to control the duration and dose of drug released from the stent, and the fluoropolymer PVDF-HFP has a proven safety record.
Current clinical data involving the EluviaTM stent suggest that the polymer/drug dose combination results in an antirestenotic effect during the period when peripheral restenosis is typically observed, leading to a high clinical vessel patency rate.
Confirmatory results from ongoing preclinical and clinical studies are anticipated.
Declaration of interest
S Müller-Hülsbeck serves as a consultant for Boston Scientific, and has received consulting fees, speaker honorarium and support for accommodation and traveling when presenting BSC-related data. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. Writing assistance was utilized in the preparation of this manuscript, it was funded by Boston Scientific and carried out by Elizabeth Davis, PhD.
Acknowledgments
The author thanks Jaydeep Kokate, PhD and Elizabeth Davis, PhD, both from Boston Scientific, Maple Grove, MN, for insight into the rationale for and history of the development of the EluviaTM stent, and for medical writing assistance, respectively.
Additional information
Funding
References
- Fowkes FG, Rudan D, Rudan I, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329–1340.
- Mahoney EM, Wang K, Keo HH, et al. Vascular hospitalization rates and costs in patients with peripheral artery disease in the United States. Circ Cardiovasc Qual Outcomes. 2010;3:642–651.
- Stone GW, Teirstein PS, Meredith IT, et al. A prospective, randomized evaluation of a novel everolimus-eluting coronary stent: the PLATINUM (a Prospective, Randomized, Multicenter Trial to Assess an Everolimus-Eluting Coronary Stent System [PROMUS Element] for the Treatment of Up to Two de Novo Coronary Artery Lesions) trial. J Am Coll Cardiol. 2011;57:1700–1708.
- Dake MD, Ansel GM, Jaff MR, et al. Paclitaxel-eluting stents show superiority to balloon angioplasty and bare metal stents in femoropopliteal disease: twelve-month Zilver PTX randomized study results. Circ Cardiovasc Interv. 2011;4:495–504.
- Lammer J, Bosiers M, Zeller T, et al. First clinical trial of nitinol self-expanding everolimus-eluting stent implantation for peripheral arterial occlusive disease. J Vasc Surg. 2011;54:394–401.
- Duda SH, Bosiers M, Lammer J, et al. Drug-eluting and bare nitinol stents for the treatment of atherosclerotic lesions in the superficial femoral artery: long-term results from the SIROCCO trial. J Endovasc Ther. 2006;13:701–710.
- Laird JR. Limitations of percutaneous transluminal angioplasty and stenting for the treatment of disease of the superficial femoral and popliteal arteries. J Endovasc Ther. 2006;13(Suppl 2):II30–II40.
- Fischer GM, Llaurado JG. Collagen and elastin content in canine arteries selected from functionally different vascular beds. Circ Res. 1966;19:394–399.
- Davaine JM, Quillard T, Chatelais M, et al. Bone like arterial calcification in femoral atherosclerotic lesions: prevalence and role of osteoprotegerin and pericytes. Eur J Vasc Endovasc Surg. 2016;51:259–267.
- Kastrati A, Schömig A, Dietz R, et al. Time course of restenosis during the first year after emergency coronary stenting. Circulation. 1993;87:1498–1505.
- Schillinger M, Sabeti S, Dick P, et al. Sustained benefit at 2 years of primary femoropopliteal stenting compared with balloon angioplasty with optional stenting. Circulation. 2007;115:2745–2749.
- Iida O, Uematsu M, Soga Y, et al. Timing of the restenosis following nitinol stenting in the superficial femoral artery and the factors associated with early and late restenoses. Catheter Cardiovasc Interv. 2011;78:611–617.
- Hou D, Huibregtse BA, Eppihimer MJ, et al. Fluorocopolymer-coated nitinol self-expanding low dose paclitaxel-eluting stent: pharmacokinetics and vascular biology responses in porcine iliofemoral model. EuroIntervention. Forthcoming 2016.
- Axel DI, Kunert W, Göggelmann C, et al. Paclitaxel inhibits arterial smooth muscle cell proliferation and migration in vitro and in vivo using local drug delivery. Circulation. 1997;96(6):636–645.
- Wiskirchen J, Schöber W, Schart N, et al. The effects of paclitaxel on the three phases of restenosis: smooth muscle cell proliferation, migration, and matrix formation: an in vitro study. Invest Radiol. 2004;39:565–571.
- Matsuo Y, Takumi T, Mathew V, et al. Plaque characteristics and arterial remodeling in coronary and peripheral arterial systems. Atherosclerosis. 2012;223:365–371.
- Zacharias SK, Safian RD, Madder RD, et al. Invasive evaluation of plaque morphology of symptomatic superficial femoral artery stenoses using combined near-infrared spectroscopy and intravascular ultrasound. Vasc Med. 2016;21:337–344.
- Müller-Hülsbeck S, Keirse K, Zeller T, et al. Twelve month results from the MAJESTIC trial of the eluvia paclitaxel-eluting stent for treatment of obstructive femoropopliteal disease. J Endovasc Ther. 2016;23(5):701–707.
- Wilson GJ, Huibregtse BA, Stejskal EA, et al. Vascular response to a third generation everolimus-eluting stent. EuroIntervention. 2010;6:512–519.
- Chin-Quee SL, Hsu SH, Nguyen-Ehrenreich KL, et al. Endothelial cell recovery, acute thrombogenicity, and monocyte adhesion and activation on fluorinated copolymer and phosphorylcholine polymer stent coatings. Biomaterials. 2010;31:648–657.
- Forrester JS, Fishbein M, Helfant R, et al. A paradigm for restenosis based on cell biology: clues for the development of new preventive therapies. J Am Coll Cardiol. 1991;17:758–769.
- Kaluza G. Fluorocopolymer-coated Nitinol self-expanding Paclitaxel-eluting stent: pharmacokinetics and vascular biology responses in porcine iliofemoral model. Leipzig: LINC; 2016.
- Krankenberg H, Schlüter M, Steinkamp HJ, et al. Nitinol stent implantation versus percutaneous transluminal angioplasty in superficial femoral artery lesions up to 10 cm in length: the femoral artery stenting trial (FAST). Circulation. 2007;116:285–292.
- Bosiers M, Deloose K, Callaert J, et al. 4-French-compatible endovascular material is safe and effective in the treatment of femoropopliteal occlusive disease: results of the 4-EVER trial. J Endovasc Ther. 2013;20:746–756.
- Laird JR, Katzen BT, Scheinert D, et al. Nitinol stent implantation vs. balloon angioplasty for lesions in the superficial femoral and proximal popliteal arteries of patients with claudication: three-year follow-up from the RESILIENT randomized trial. J Endovasc Ther. 2012;19:1–9.
- IDEV. Supera Peripheral Stent System SSED (SUPERB). 2014 [cited 2016 Apr 20]. Available from: http://www.accessdata.fda.gov/cdrh_docs/pdf12/P120020b.pdf
- Laird JR, Jain A, Zeller T, et al. Nitinol stent implantation in the superficial femoral artery and proximal popliteal artery: twelve-month results from the Complete SE multicenter trial. J Endovasc Ther. 2014;21:202–212.
- Bosiers M, Torsello G, Gissler HM, et al. Nitinol stent implantation in long superficial femoral artery lesions: 12-month results of the DURABILITY I study. J Endovasc Ther. 2009;16:261–269.
- Cordis. SMART CONTROL SSED (STROLL). 2012 [cited 2016 Apr 20]. Available from: http://www.accessdata.fda.gov/cdrh_docs/pdf12/P120002b.pdf
- Matsumura JS, Yamanouchi D, Goldstein JA, et al. The United States study for evaluating endovascular treatments of lesions in the superficial femoral artery and proximal popliteal by using the protege everflex nitinol stent system II (DURABILITY II). J Vasc Surg. 2013;58(73––83):e71.
- Dake MD, Scheinert D, Tepe G, et al. Nitinol stents with polymer-free paclitaxel coating for lesions in the superficial femoral and popliteal arteries above the knee: twelve-month safety and effectiveness results from the Zilver PTX single-arm clinical study. J Endovasc Ther. 2011;18:613–623.
- Dake MD, Ansel GM, Jaff MR, et al. Sustained safety and effectiveness of paclitaxel-eluting stents for femoropopliteal lesions: 2-year follow-up from the Zilver PTX randomized and single-arm clinical studies. J Am Coll Cardiol. 2013;61:2417–2427.
- Dake MD, Ansel GM, Jaff MR, et al. Durable clinical effectiveness with paclitaxel-eluting stents in the femoropopliteal artery: 5-year results of the zilver ptx randomized trial. Circulation. 2016;133:1472–1483.
- Tepe G, Laird J, Schneider P, et al. Drug-coated balloon versus standard percutaneous transluminal angioplasty for the treatment of superficial femoral and/or popliteal peripheral artery disease: 12-month results from the IN.PACT SFA randomized trial. Circulation. 2015;131:495–502.
- Laird JR, Schneider PA, Tepe G, et al. Durability of treatment effect using a drug-coated balloon for femoropopliteal lesions: 24-month results of IN.PACT SFA. J Am Coll Cardiol. 2015;66:2329–2338.
- Micari A, Cioppa A, Vadalà G, et al. Clinical evaluation of a paclitaxel-eluting balloon for treatment of femoropopliteal arterial disease: 12-month results from a multicenter Italian registry. JACC Cardiovasc Interv. 2012;5:331–338.
- Micari A, Cioppa A, Vadalà G, et al. 2-year results of paclitaxel-eluting balloons for femoropopliteal artery disease: evidence from a multicenter registry. JACC Cardiovasc Interv. 2013;6:282–289.
- Rosenfield K, Jaff MR, White CJ, et al. Trial of a paclitaxel-coated balloon for femoropopliteal artery disease. N Engl J Med. 2015;373:145–153.
- Norgren L, Hiatt WR, Dormandy JA, et al. Inter-society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg. 2007;45(Suppl S):S5–67.
- Scheinert D, Duda S, Zeller T, et al. The LEVANT I (Lutonix paclitaxel-coated balloon for the prevention of femoropopliteal restenosis) trial for femoropopliteal revascularization: first-in-human randomized trial of low-dose drug-coated balloon versus uncoated balloon angioplasty. JACC Cardiovasc Interv. 2014;7:10–19.
- Schmidt A, Piorkowski M, Gorner H, et al. Drug-coated balloons for complex femoropopliteal lesions: 2-year results of a real-world registry. JACC Cardiovasc Interv. 2016;9:715–724.
- Werk M, Albrecht T, Meyer DR, et al. Paclitaxel-coated balloons reduce restenosis after femoro-popliteal angioplasty: evidence from the randomized PACIFIER trial. Circ Cardiovasc Interv. 2012;5:831–840.
- Zeller T, Rastan A, Macharzina R, et al. Novel approaches to the management of advanced peripheral artery disease: perspectives on drug-coated balloons, drug-eluting stents, and bioresorbable scaffolds. Curr Cardiol Rep. 2015;17:624.